Excess body weight and risk of first-ever acute ischaemic non-embolic
stroke in elderly subjects
H. J. Milionis
a, T. D. Filippatos
a, C. S. Derdemezis
a, K. J. Kalantzi
a, J. Goudevenos
a,
K. Seferiadis
b, D. P. Mikhailidis
cand M. S. Elisaf
aa
Department of Internal Medicine, School of Medicine, University of Ioannina, Ioannina, Greece;bLaboratory of Biochemistry, University Hospital of Ioannina, Ioannina, Greece; andcDepartment of Clinical Biochemistry, Royal Free Hospital, London, UK
Keywords:
BMI, HDL, metabolic syndrome, obesity, stroke, waist circumference
Received 18 January 2007 Accepted 23 April 2007
In a population-based case–control study we assessed the association between obesity and acute ischaemic/non-embolic stroke. A total of 163 patients aged older than 70 years (88 men and 75 women) admitted due to a first-ever-in-a-lifetime acute ischaemic/non-embolic stroke and 166 volunteers (87 men and 79 women) without a history of cardiovascular disease were included. The association of stroke with body mass index (BMI) or waist circumference (WC) was determined by multivariate logistic regression modelling after adjusting for potential confounding factors. Over-weight and obesity were more prevalent amongst stroke patients compared to controls. Subjects with a BMI‡30 kg/m2had 2.5-times higher odds to suffer an acute ischaemic/non-embolic stroke compared to subjects within the lowest BMI category of 18.5–20.9 kg/m2. Analysis of interaction showed that in the presence of overweight and/or obesity (classified as a BMI ‡25 kg/m2 and/or a WC >102 cm in men and >88 cm in women) the inverse relationship between HDL cholesterol and ischaemic/ non-embolic stroke was negated. Excess weight is associated with an increased risk of acute ischaemic/non-embolic stroke in elderly individuals independently of concurrent metabolic derangements. Moreover, in the presence of obesity, HDL cholesterol loses its protective effect against ischaemic stroke.
Introduction
Obesity is becoming increasingly common and is recognized as a major public health problem worldwide [1]. It is a chronic condition that is associated with premature death and significant healthcare costs as it constitutes an independent risk factor for a variety of diseases, including type 2 diabetes mellitus, the meta-bolic syndrome (MetS), cardiovascular disease, gallbladder disease and some types of cancer [1].
Stroke is a major cause of mortality in the industri-alized countries leading to serious long-term physical and mental disabilities amongst survivors, especially amongst the elderly [2]. Excess weight is associated with an increased prevalence of several factors that may lead to stroke [3]. However, data on the overall association of obesity and stroke are limited and inconclusive. Several studies have suggested that a high body mass index (BMI) may increase the risk of stroke [4,5]. In the Framingham Heart Study, an association between weight and atherothrombotic stroke was found in women but not in men [4]. The Honolulu Heart Pro-gram reported that BMI was associated with increased
risk of thromboembolic stroke amongst non-smoking men in older middle age [6]. In contrast, the NursesÕ
Health Study showed that women with increased BMI had an increased risk of ischaemic stroke, but not after adjustment for hypertension, diabetes mellitus and high cholesterol [7]. Other studies have also failed to find an independent relationship between obesity (measured by BMI) and increased risk of stroke [8–12].
Evidence from studies investigating the association between abdominal obesity and stroke in middle-aged individuals indicates that the distribution of body fat predicts better stroke risk than total body fat [8,11,13]. Body fat tends to be redistributed with ageing [14]. There is limited information with regard to the associ-ation of the overall and abdominal obesity with stroke in the elderly, who comprise the fastest growing pro-portion of the population.
In a population-based case–control study, we evalu-ated potentially modifiable risk factors for acute isch-aemic/non-embolic stroke, including overweight and obesity [assessed by BMI or waist circumference (WC) measurements], amongst the elderly [15,16]. This study was conducted in the prefecture of Ioannina, Epirus (north-western Greece), a non-industrialized part of the country with 170 000 inhabitants, with those older than 70 years of age representing 18–20% of the total population [15,16].
Subjects and methods
A total of 163 elderly patients (88 men, 75 women) who were consecutively hospitalized over a 5-year period for first-ever-in-a-lifetime acute ischaemic stroke and 166 volunteers (87 men, 79 women) consecutively evaluated in the primary care setting were included in the study [15,16]. The study was based on all the available eligible consecutive patients that we could assemble over a period of 5 years.
Criteria for inclusion in the study were: (i) subjects aged older than 70 years, (ii) residing in the prefecture of Ioannina, (iii) known to attend the Public Primary Care Health Centre facilities regularly for the last 5 years prior to enrolment and (iv) patients reaching the Emergency Department of the University Hospital of Ioannina (the referral centre for patients with cerebro-vascular disease over 65 years old in the region) within 12 h from the onset of symptoms. The diagnosis of first-in-a-lifetime acute ischaemic/non-embolic stroke was based upon history (clinical course, associated symp-toms), physical examination (including neurological and cardiac assessment) and radiological study [initial non-contrast brain computed tomography (CT) scan]. Further confirmation involved full cardiac evaluation (history and physical, electrocardiogram, and a trans-thoracic echocardiogram) and carotid Doppler ultra-sound to exclude a cardiac and/or carotid artery source of emboli, and a new brain CT scan where appropriate [15,16].
Subjects with a history of vascular disease [previous stroke (including those with old infarcts on imaging), angina, myocardial infarction, revascularizations, per-ipheral artery disease], active infections, neoplasia, re-nal or liver disease, thyroid dysfunction, chronic obstructive pulmonary disease, chronic inflammatory bowel disease and excessive alcohol consumption were excluded. Stroke patients and controls with a known or possible cardiac source of emboli (atrial fibrillation, heart valve disease, patients receiving anticoagulant treatment) were also excluded. None of the participants was receiving specific lipid lowering treatment (i.e. a statin or a fibrate). All subjects gave informed consent and the study protocol was approved by the Institu-tional Ethics Committee.
BMI and WC assessments
Body weight was measured using a digital scale (Seca Personal Floor Scale 861; Seca, Marsden, UK) with an accuracy of ±100 g. Subjects were weighed without shoes, in the minimum clothing possible. Standing height was measured without shoes to the nearest 0.1 cm with the Portable Leister height measure (Seca)
with the shoulders in relaxed position and arms hanging freely. BMI was calculated by dividing weight (kg) by height squared (m2). We evaluated BMI on the basis of the World Health Organization (WHO) cat-egories of <25.0 (normal weight), 25.0–29.9 (over-weight) and 30.0 kg/m2or greater (obese), as well as a continuous term. We further categorized BMI into a priori categories (so as to include subjects of under-, normal, over-weight, as well as obese) of <18.5, 18.5– 20.9, 21–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, and
‡30.0 kg/m2.
Waist circumference was measured (whilst the sub-jects were in the standing position at the end of gentle expiration) to the nearest centimetre with tape-measure (Seca 200; Seca).
Ascertainment of hypertension, diabetes mellitus and the MetS
Hypertension in patients was recorded according to medical history and relevant drug treatment. More-over, blood pressure measurements (for patients dur-ing the acute episode as well as for controls) were obtained in the sitting position in triplicate on the subject’s non-dominant arm and following a 10-min rest using a validated mercury sphygmomanometer. Diabetes mellitus was coded as present according to the medical records and/or relevant treatment. The diagnosis of MetS was based on the National Cholesterol Education Program (NCEP)/Adult Treatment Panel (ATP) III criteria [17] and was made when three or more of the following risk determi-nants were present: abdominal obesity [WC (standing position) >102 cm for men; >88 cm for women], triglycerides ‡1.7 mmol/l, low HDL cholesterol (i.e. <1.0 mmol/l for men and <1.3 mmol/l for women)], blood pressure over 130/85 or drug treatment for elevated blood pressure, and fasting gluco-se‡6.1 mmol/l or drug treatment for elevated blood glucose [15,16].
Laboratory measurements
Statistical analysis
Values were expressed as mean ± SD. Comparison of continuous normally distributed variables was per-formed using the one-way analysis of variance (ANOVA). The inflation in Type-I error due to multiple post hoc comparisons was corrected using the Bonferroni rule. For non-normally distributed variables (even though several transformations were made) nonparametric tests (Kruskal–Wallis and Mann–Whitney) were used. Chi-squared tests were used for categorical variables. Relationships between variables were assessed by the Pearson’s correlation coefficient, and Spearman corre-lation coefficients for parametric and nonparametric variables.
The strength of associations between parameters of central obesity (i.e. BMI and WC) and acute ischaemic/ non-embolic stroke was assessed by means of logistic regression analysis comparing stroke and control sub-jects. This association was first tested univariately. Multivariate analysis was performed by binary logistic regression analysis, which allows adjustment for con-founding factors, such as age, sex, the presence of hypertension, diabetes mellitus, smoking and serum
lipids [total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C), HDL-C].
The ÔinteractionÕ of obesity indices (BMI and WC) with lipid risk factors (TC, TG, HDL-C and LDL-C) as well as with the presence of MetS was evaluated by creating newÔproductÕvariables and adding them to the logistic regression model. The effect ofÔinteractionÕwas tested using theF-statistic for the improved fit. In the interaction terms, BMI and WC were expressed both as continuous and binary variables (i.e. BMI ‡30 kg/m2, and WC >102 cm in men and >88 cm in women or less), lipid variables were expressed as continuous var-iables, whilst MetS was expressed as a binary variable. All statistical analyses were carried out using the SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA) package for personal computers. In all analyses, P< 0.05 was used to indicate statistical significance.
Results
The clinical and laboratory characteristics of the study population with regard to weight status are shown in Table 1. No subjects with a BMI lower than 18.5 kg/m2 were identified in either group. BMI was highly
Table 1 Clinical and laboratory characteristics of the study population according to weight status
Stroke patients Control population
Normal (n¼55)
Overweight (n¼92)
Obese (n¼16)
Normal (n¼72)
Overweight (n¼73)
Obese (n¼11)
Age (years) 78.0 ± 4.7 77.2 ± 4.7 78.2 ± 6.0 77.8 ± 5.2 77.6 ± 4.7 78.1 ± 5.9
Sex
Men 27 53 8 40 41 6
Women 28 39 8 42 32 5
Body mass index (kg/m2) 23.1 ± 1.3a,b 27.1 ± 1.3a 33.4 ± 3.1b 22.8 ± 1.2c,d 27.0 ± 1.2c 31.4 ± 1.0d Men 23.4 ± 1.2a,b 27.0 ± 1.2a 34.4 ± 5.6b 22.8 ± 1.1c,d 27.1 ± 1.2c 31.8 ± 1.1d Women 22.7 ± 1.4a,b 27.3 ± 1.4a 33.0 ± 2.0b 22.9 ± 1.3c,d 26.8 ± 1.1c 31.0 ± 0.8d Waist circumference (cm) 77.9 ± 8.5a,b 92.9 ± 9.4a 103.1 ± 8.2b 77.2 ± 6.8c,d 92.3 ± 10.2c 107.3 ± 8.2d
Men 83.4 ± 6.8a,b 96.9 ± 8.8a 113.7 ± 7.2b 81.9 ± 3.7c,d 99.3 ± 8.5c 113.6 ± 1.4d Women 71.7 ± 5.4a,b 87.3 ± 7.0a 99.1 ± 3.9b 71.2 ± 4.7c,d 85.3 ± 6.1c 98.8 ± 3.0d
Current smokers 15 (27.2%) 25 (27.2%) 3 (18.7%) 24 (33.3%) 19 (26%) 2 (18.2%)
Diabetes mellitus 13 (23.6%) 28 (30.4%) 5 (31.2%) 15 (20.8%) 16 (21.9%) 3 (27.3%)
Arterial hypertension 27 (49.1%) 43 (46.7%) 7 (43.7%) 18 (25.0%) 39 (53.4%) 4 (36.4%) Metabolic syndrome 18 (32.7%)a,b 48 (52.2%)a 9 (56.2%)b 5(6.9%) 17 (23.3%) 4 (36.4%) Total cholesterol (mmol/l) 5.3 ± 1.3 5.4 ± 1.1 5.4 ± 2.1 5.5 ± 1.1 5.3 ± 1.1 5.7 ± 1.2 LDL cholesterol (mmol/l) 3.4 ± 1.1 3.4 ± 0.9 3.7 ± 1.9 3.5 ± 1.0 3.3 ± 1.2 3.7 ± 1.0 HDL cholesterol (mmol/l) 1.0 ± 0.3 1.0 ± 0.3 0.9 ± 0.3 1.3 ± 0.3 1.3 ± 0.3 1.3 ± 0.2 Triglycerides (mmol/l) 2.0 ± 0.9 1.9 ± 0.8 1.8 ± 0.8 1.4 ± 0.5 1.3 ± 0.5 1.4 ± 0.6
Apolipoprotein A-I (mg/dl) 127.8 ± 23.8 132.0 ± 24.1 124.2 ± 28.5 150.9 ± 23.7 150.0 ± 21.3 144.3 ± 16.9 Apolipoprotein B (mg/dl) 132.5 ± 40.4 131.1 ± 30.7 131.1 ± 33.8 129.2 ± 22.9 124.6 ± 25.1 134.7 ± 48.6 Lipoprotein (a) (mg/dl) 15.7 (0.8–65.3) 15.5 (0.8–62.0) 10.6 (1.8–50.0) 5.4 (0.8–48.0) 7.7 (0.8–48.0) 11.8(3.5–25.0) Fibrinogen (g/l) 4.0 (1.3–6.3) 4.4 (1.8–5.6) 3.5 (2.1–5.3) 2.9 (1.1–6.1) 3.1 (1.5–5.6) 2.4 (1.5–2.9) Uric acid (lmol/l) 315.2 ± 95.2 349.1 ± 102.3 306.9 ± 74.9 274.2 ± 77.3 292.6 ± 84.5 304.0 ± 95.2
correlated with WC (Spearman correlation coefficient, r¼0.81,P< 0.001). The prevalence of the MetS was
higher in subjects with excess weight both in the patient group (P < 0.01) and in the control group (P¼0.048)
(Table 1). The distribution of BMI in cases and con-trols is shown in Table 2. Stroke patients were more frequently categorized as ÔoverweightÕ and ÔobeseÕ
(according to WHO guidelines and to pre-specified BMI categories), whilst controls tended to be classified as normal (Table 2a,b). Compared to stroke patients, a higher proportion of controls had a BMI within normal limits [72 (43.4%) vs. 55 (33.7%),P¼0.07], 92 patients
vs. 73 controls were classified as overweight (P ¼0.02),
whilst a BMI‡30 kg/m2 was noted in 16 (9.8%)
pa-tients versus 11 (6.6%) controls (P¼NS, not
signifi-cant) (Table 2a).
Waist circumference values did not significantly differ between patients and controls (92.7 ± 11.0 cm vs. 92.9 ± 11.7 cm, respectively, in men and 82.7 ± 11.1 cm vs. 80.8 ± 9.4 cm, respectively, in women; P¼NS for both comparisons). The proportion of
subjects with abdominal obesity evaluated by WC (i.e. WC >102 cm for men; >88 cm for women) was similar in cases and controls [56 (34%; 21 women) vs. 53 (32%; 19 women) respectively;P¼NS].
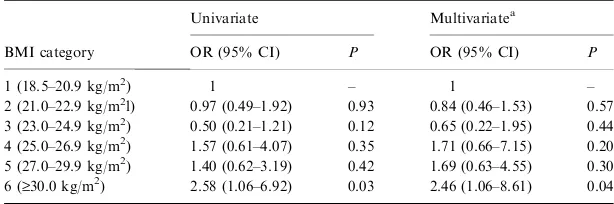
Compared to subjects with BMI values in the lowest category, those within the highest category (i.e. BMI
‡30 kg/m2) had 2.5-times higher odds to suffer an acute ischaemic/non-embolic stroke [adjusted odds ratio (OR) 2.46; 95% confidence interval (CI), 1.06–8.61; P¼0.04) (Table 3). Moreover, those ÔoverweightÕ
(according to WHO guidelines) had a 1.5-fold higher odds (adjusted OR, 1.53; 95% CI, 0.76–3.10;P¼0.23)
and those ÔobeseÕ a 1.3-fold (adjusted OR, 1.27; 95% CI, 0.79–2.00;P¼0.34) higher odds of having an acute
ischaemic/non-embolic stroke compared to subjects with ÔnormalÕ BMI. Likewise, an increased WC (>102 cm for men; >88 cm for women) was associated with a twofold higher odds of suffering an acute isch-aemic stroke (adjusted OR, 1.99; 95% CI 0.94–4.23; P¼0.07) compared to subjects with a WC £102 cm
(men) and£88 cm (women).
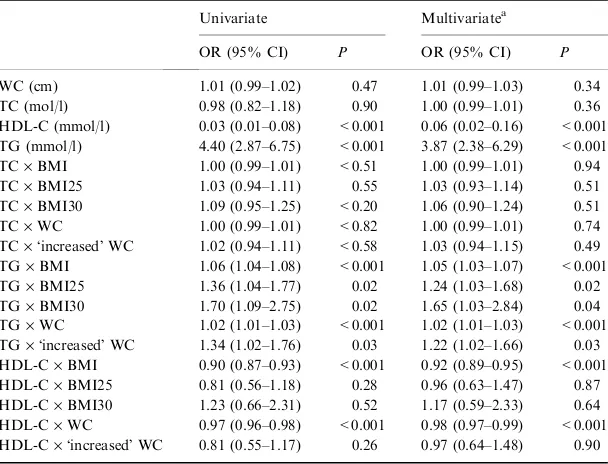
In logistic regression analysis, crude OR for TG was 4.40 per 1 mmol/l increase (95% CI, 2.87–6.85; P< 0.001) and for HDL-C 0.03 per 1 mmol/l increase (95% CI, 0.01–0.08; P< 0.001); this association was evident after adjusting for potential confounders (Table 4).ÔInteraction analysisÕ between MetS and either WC or BMI produced additional interesting results. In the presence of the MetS, abdominal obesity (manifested as WC >102 cm in men and >88 cm in women) was associated with an increased risk of stroke (adjusted OR, 2.74; 95% CI, 1.15–6.50; P ¼0.02).
When BMI was used in place of WC, subjects with a BMI‡30.0 kg/m2showed fourfold higher odds to suffer
an acute ischaemic/non-embolic stroke (adjusted OR, 4.10; 95% CI, 1.33–12.65;P¼0.01).
Further analysis of ÔinteractionÕ between obesity parameters and serum lipids revealed that an increasing BMI or WC attenuated theÔprotective effectÕof HDL-C levels in the prediction of stroke, whereas the associ-ation of stroke with serum triglycerides remained significant (Table 4). Of note, in the presence of overweight and/or abdominal obesity (classified as a BMI ‡25 kg/m2 and/or a WC >102 cm in men and >88 cm in women) the strong inverse relationship Table 2Prevalence of overweight and obesity in the study population
and frequency of subjects across body mass index (BMI) categories
Stroke patients
Table 3Odds ratios (95% CI) for first-ever-in-a-lifetime acute ischaemic
non-embolic stroke across body mass index
(BMI) categories BMI category
Univariate Multivariatea
OR (95% CI) P OR (95% CI) P
1 (18.5–20.9 kg/m2) 1 – 1 –
2 (21.0–22.9 kg/m2l) 0.97 (0.49–1.92) 0.93 0.84 (0.46–1.53) 0.57 3 (23.0–24.9 kg/m2) 0.50 (0.21–1.21) 0.12 0.65 (0.22–1.95) 0.44 4 (25.0–26.9 kg/m2) 1.57 (0.61–4.07) 0.35 1.71 (0.66–7.15) 0.20 5 (27.0–29.9 kg/m2) 1.40 (0.62–3.19) 0.42 1.69 (0.63–4.55) 0.30 6 (‡30.0 kg/m2) 2.58 (1.06–6.92) 0.03 2.46 (1.06–8.61) 0.04
aAdjusted for age, sex, smoking, the presence of hypertension and diabetes mellitus, and levels
between HDL-C and acute ischaemic/non-embolic stroke was negated (Table 4).
Discussion
In line with previous studies, this case–control study is strongly in favour of an association between excess weight and a first-ever-in-a-lifetime ischaemic/non-embolic stroke in elderly individuals, which was not attenuated after adjustment for the presence of estab-lished cerebrovascular risk factors, including hyperten-sion, diabetes mellitus and dyslipidaemia.
It has been argued that BMI might be a poor indi-cator of risk in elderly individuals because it does not reflect regional fat distribution [18–20]. However, a re-cent report from the British Regional Heart study showed that BMI is strongly associated with morbidity and cardiovascular disease risk factors in elderly men [21]. The underlying mechanisms explaining the addit-ive risk of high BMI in stroke risk, independently of conventional risk factors, such as hypertension, diabe-tes mellitus smoking or lipid levels, are not fully understood.
Obese patients in our study presented with the Ô ath-erogenic dyslipidaemiaÕ characterized by elevated TG and low HDL-C levels. There is a controversy regarding the association between serum TG levels and stroke [22]. It has been shown that postprandial
hypertri-glyceridaemia is associated with carotid artery athero-sclerosis [23]. In the Copenhagen City Heart Study, a log-linear association between serum TG levels and non-haemorrhagic stroke was found, which was inde-pendent of age and sex [10]. In general, in the majority of studies, an inverse association between HDL-C and stroke risk has been documented [22]. In the Northern Manhattan Stroke Study, increased levels of HDL-C were associated with a reduced risk of ischaemic stroke in elderly subjects and amongst different racial or ethnic groups [24]. In our cohort, HDL-C levels were inversely related to the risk of acute ischaemic stroke in elderly subjects. However, in the face of significant overweight and obesity the Ôprotective effectÕ of HDL-C was negated. This finding needs to be further tested in future studies.
Increased levels of inflammatory markers, (especially C-reactive protein) [25–27], endothelial dysfunction [28], haemorrheological disorders [29] (including blood hyperviscosity and hyperfibrinogenaemia), and platelet activation [30] in overweight and obese individuals have also been proposed to play a role in the increased risk of ischaemic cardiovascular events, including stroke [31,32].
Body mass index and WC were highly correlated in our study. Few direct comparisons exist between BMI and WC as predictors of metabolic abnormalities in elderly subjects. In the third National Health and Table 4 Univariate and multivariate logistic regression analyses for the prediction of acute ischaemic non-embolic stroke in association with abdominal obesity (evaluated by BMI or waist circumference) and lipidaemic parameters
Univariate Multivariatea
OR (95% CI) P OR (95% CI) P
WC (cm) 1.01 (0.99–1.02) 0.47 1.01 (0.99–1.03) 0.34
TC (mol/l) 0.98 (0.82–1.18) 0.90 1.00 (0.99–1.01) 0.36
HDL-C (mmol/l) 0.03 (0.01–0.08) <0.001 0.06 (0.02–0.16) <0.001 TG (mmol/l) 4.40 (2.87–6.75) <0.001 3.87 (2.38–6.29) <0.001 TC·BMI 1.00 (0.99–1.01) <0.51 1.00 (0.99–1.01) 0.94
TC·BMI25 1.03 (0.94–1.11) 0.55 1.03 (0.93–1.14) 0.51
TC·BMI30 1.09 (0.95–1.25) <0.20 1.06 (0.90–1.24) 0.51 TC·WC 1.00 (0.99–1.01) <0.82 1.00 (0.99–1.01) 0.74 TC·ÔincreasedÕWC 1.02 (0.94–1.11) <0.58 1.03 (0.94–1.15) 0.49 TG·BMI 1.06 (1.04–1.08) <0.001 1.05 (1.03–1.07) <0.001
TG·BMI25 1.36 (1.04–1.77) 0.02 1.24 (1.03–1.68) 0.02
TG·BMI30 1.70 (1.09–2.75) 0.02 1.65 (1.03–2.84) 0.04
TG·WC 1.02 (1.01–1.03) <0.001 1.02 (1.01–1.03) <0.001 TG·ÔincreasedÕWC 1.34 (1.02–1.76) 0.03 1.22 (1.02–1.66) 0.03 HDL-C·BMI 0.90 (0.87–0.93) <0.001 0.92 (0.89–0.95) <0.001 HDL-C·BMI25 0.81 (0.56–1.18) 0.28 0.96 (0.63–1.47) 0.87 HDL-C·BMI30 1.23 (0.66–2.31) 0.52 1.17 (0.59–2.33) 0.64 HDL-C·WC 0.97 (0.96–0.98) <0.001 0.98 (0.97–0.99) <0.001 HDL-C·ÔincreasedÕWC 0.81 (0.55–1.17) 0.26 0.97 (0.64–1.48) 0.90
Nutrition Examination Study and in population studies from Canada, Hong Kong and Japan, WC was more closely related to metabolic risk factors than was BMI [33–36]. However, the age range in these study popu-lations was broad and included subjects as young as 20 years. In contrast, several studies with broad age ranges reported similar associations between BMI and WC and metabolic risk factors in men [37–39]. Indeed, in the Baltimore Longitudinal Study, WC showed stronger correlations with coronary heart disease risk factors than did BMI in younger subjects (<65 years of age) but not in older subjects [40]. According to our findings, an increased WC is independently associated with acute ischaemic/non-embolic stroke in elderly subjects, and thus, measurement of WC could be a useful tool in the evaluation of abdominal obesity in this population.
Several studies have shown the association of central obesity with the MetS, which represents aÔconstellationÕ of lipid and non-lipid risk factors for cardiovascular disease and has been recognized as a secondary target for increased behavioural therapy in the NCEP/ATP III [17]. The presence of the MetS appears to provide incremental predictive value on top of the Framingham risk score in predicting future cardiovascular events [4]. A recent meta-analysis of the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/Tex-CAPS), which included only those patients who re-ceived placebo, showed that patients with the MetS were at increased risk of major coronary events irres-pective of their Framingham-calculated 10-year risk score category [41]. The association of MetS presence with stroke risk in older patients has also been recog-nized [16,42–45]. In the present study, interaction ana-lysis indicated that abdominal obesity as a component of the MetS (coded either by increased BMI or in-creased WC) contributed significantly to the prediction for stroke dependent upon the presence of the MetS. Therefore, it stands to reason that the clustering of risk factors comprising the MetS is worsening under the excess weight influence.
The results of our study must be interpreted in the light of certain limitations. This was a case–control study including only subjects of Caucasian origin within a certain geographical area; the selection criteria for cases and controls were strict in the present study, excluding patients with any history of vascular disease and/or potential source of emboli. This was decided in order to attenuate the heterogeneity of the sample studied and to increase the chances of correctly identi-fying any associations present. However, this process represents a study limitation and reduces the general-izability of the results.
Data from large population studies show that mean body weight and BMI in both men and women gradually increase during most of the adult life, reach peak values at 50–59 years of age and tend to decrease after the age of 60 years [46]. However, these obser-vations were obtained from cross-sectional studies and may be affected by survival bias, as obese subjects have higher mortality rates at younger ages [47]. Therefore, premature mortality of obese young and middle-aged adults would tend to decrease mean body weight and BMI in surviving older adults. The present study was held in a non-industrialized, rural region of the country, where at least elderly individuals lead their lives in the so-called Ôtraditional wayÕ. Eating habits in the elderly population remained unchanged for years (Mediterranean diet, including olive oil and minimal saturated fat consumption), whilst smoking is restricted to men [15,16]. This is in contrast to a more westernized way of living followed by urban younger inhabitants. Although we performed adjustments for several characteristics, including sex, age, smoking, obesity, hypertension, diabetes and dyslipidaemia, the data might not be applicable to the general population.
In conclusion, our study indicates that excess weight is associated with increased risk of acute ischaemic/non-embolic stroke in elderly individuals independently of conventional risk factors. Considering the remarkable growth in the proportion of the elderly population throughout the industrialized world, it is becoming increasingly important for societies to reduce the bur-den of illness in their ageing populations. Preventing obesity may lead to a substantial reduction in the occurrence of ischaemic stroke amongst elderly individuals.
References
1. Le Gales-Camus C, Waxman A. Fighting obesity:
clari-fication from World Health Organization.British Medical
Journal2004;329:53–54.
2. Hankey GJ. Stroke: how large a public health problem,
and how can the neurologist help?Archives of Neurology
1999;56:748–754.
3. Jood K, Jern C, Wilhelmsen L, Rosengren A. Body mass index in mid-life is associated with a first stroke in men: a
prospective population study over 28 years.Stroke2004;
35:2764–2769.
4. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the
Fra-mingham Heart Study.Circulation1983;67:968–977.
5. Rhoads GG, Kagan A. The relation of coronary disease, stroke, and mortality to weight in youth and in middle age.Lancet1983;1:492–495.
6. Abbott RD, Behrens GR, Sharp DS et al. Body mass
older middle age. The Honolulu Heart Program.Stroke
1994;25:2370–2376.
7. Rexrode KM, Hennekens CH, Willett WCet al.A
pros-pective study of body mass index, weight change, and risk
of stroke in women.Journal of American Association1997;
277:1539–1545.
8. Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in
Goth-enburg, Sweden. British Medical Journal (Clinical
Re-search Edition)1984;289:1257–1261.
9. Kitamura A, Iso H, Naito Yet al.High-density
lipopro-tein cholesterol and premature coronary heart disease in
urban Japanese men.Circulation1994;89:2533–2539.
10. Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and tri-glycerides on risk of cerebrovascular disease: the
Copen-hagen City Heart Study. British Medical Journal 1994;
309:11–15.
11. Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distri-bution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of
men born in 1913. British Medical Journal (Clinical
Research Edition)1984;288:1401–1404.
12. Walker SP, Rimm EB, Ascherio A, Kawachi I, Stampfer MJ, Willett WC. Body size and fat distribution as
pre-dictors of stroke among US men. American Journal
Epidemiology1996;144:1143–1150.
13. Welin L, Svardsudd K, Wilhelmsen L, Larsson B, Tibblin G. Analysis of risk factors for stroke in a cohort of men
born in 1913.New England Journal of Medicine1987;317:
521–526.
14. Kannel WB, Cupples LA, Ramaswami R, Stokes J 3rd, Kreger BE, Higgins M. Regional obesity and risk of
cardiovascular disease; the Framingham Study.Journal of
Clinical Epidemiology1991;44:183–190.
15. Milionis HJ, Liberopoulos E, Goudevenos J, Bairaktari ET, Seferiadis K, Elisaf MS. Risk factors for first-ever acute ischemic non-embolic stroke in elderly individuals. International Journal of Cardiology2005;99:269–275. 16. Milionis HJ, Rizos E, Goudevenos J, Seferiadis K,
Mikhailidis DP, Elisaf MS. Components of the metabolic syndrome and risk for first-ever acute ischemic nonembolic
stroke in elderly subjects.Stroke2005;36:1372–1376.
17. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood
cho-lesterol in adults (Adult Treatment Panel III).Journal of
American Medical Association2001;285:2486–2497. 18. Seidell JC, Visscher TL. Body weight and weight change
and their health implications for the elderly. European
Journal of Clinical Nutrition2000;54(Suppl. 3):S33–S39. 19. Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic
dis-ease.Obesity Research1995;3:73–95.
20. Lean ME, Han TS, Seidell JC. Impairment of health and quality of life in people with large waist circumference. Lancet1998;351:853–856.
21. Wannamethee SG, Shaper AG, Whincup PH, Walker M. Overweight and obesity and the burden of disease and
disability in elderly men.International Journal of Obesity
and Related Metabolic Disorders2004;28:1374–1382.
22. Rizos E, Mikhailidis DP. Are high density lipoprotein (HDL) and triglyceride levels relevant in stroke
preven-tion?Cardiovascular Research2001;52:199–207.
23. Ryu JE, Howard G, Craven TE, Bond MG, Hagaman AP, Crouse JR 3rd. Postprandial triglyceridemia and
carotid atherosclerosis in middle-aged subjects. Stroke
1992;23:823–828.
24. Sacco RL, Benson RT, Kargman DEet al.High-density
lipoprotein cholesterol and ischemic stroke in the
elderly: the Northern Manhattan Stroke Study. The
Journal of the American Medical Association 2001; 285: 2729–2735.
25. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in
over-weight and obese adults. The Journal of the American
Medical Association1999;282:2131–2135.
26. Maseri A. Inflammation in acute coronary syndromes. Haematologica2001;86:1–2.
27. Abbott RD, Sharp DS, Burchfiel CM et al.
Cross-sec-tional and longitudinal changes in total and high-density-lipoprotein cholesterol levels over a 20-year period in
elderly men: the Honolulu Heart Program. Annals of
Epidemiology1997;7:417–424.
28. Arcaro G, Zamboni M, Rossi Let al.Body fat
distribu-tion predicts the degree of endothelial dysfuncdistribu-tion in
uncomplicated obesity. International Journal of Obesity
and Related Metabolic Disorders1999;23:936–942.
29. Solerte SB, Fioravanti M, Pezza N et al.Hyperviscosity
and microproteinuria in central obesity: relevance to
cardiovascular risk. International Journal of Obesity and
Related Metabolic Disorders1997;21:417–423.
30. Davi G, Guagnano MT, Ciabattoni G et al. Platelet
activation in obese women: role of inflammation and
oxidant stress. The Journal of the American Medical
Association2002;288:2008–2014.
31. Di Napoli M. C reactive protein and acute phase of
ischaemic stroke. British Medical School 2001; 322:
1605–1606.
32. Rost NS, Wolf PA, Kase CSet al.Plasma concentration
of C-reactive protein and risk of ischemic stroke and
transient ischemic attack: the Framingham study.Stroke
2001;32:2575–2579.
33. Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk fac-tors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. American Journal of Clinical Nutrition2002;76:743–749. 34. Ho SY, Lam TH, Janus ED. Waist to stature ratio is more strongly associated with cardiovascular risk factors than
other simple anthropometric indices.Annals of
Epidemi-ology2003;13:683–691.
35. Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health
Surveys. International Journal of Obesity and Related
Metabolic Disorders2001;25:652–661.
36. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health
risk. American Journal of Clinical Nutrition 2004; 79:
379–384.
37. Dalton M, Cameron AJ, Zimmet PZ et al. Waist
Australian adults.Journal of Internal Medicine2003;254: 555–563.
38. Ho SC, Chen YM, Woo JL, Leung SS, Lam TH, Janus ED. Association between simple anthropometric indices
and cardiovascular risk factors. International Journal of
Obesity and Related Metabolic Disorders2001;25:1689– 1697.
39. Weir CJ, Sattar N, Walters MR, Lees KR. Low trigly-ceride, not low cholesterol concentration, independently
predicts poor outcome following acute stroke.
Cerebro-vascular Diseases2003;16:76–82.
40. Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Does waist circumference add to the predictive
power of the body mass index for coronary risk?Obesity
Research2001;9:685–695.
41. Girman CJ, Rhodes T, Mercuri Met al. The metabolic
syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study
(AFCAPS/TexCAPS). American Journal of Cardiology
2004;93:136–141.
42. Ruland S, Hung E, Richardson D, Misra S, Gorelick PB. Impact of obesity and the metabolic syndrome on risk factors in African American stroke survivors: a report
from the AAASPS.Archives of Neurology2005;62:386–
390.
43. Athyros VG, Mikhailidis DP, Papageorgiou AA et al.
Prevalence of atherosclerotic vascular disease among subjects with the metabolic syndrome with or without
diabetes mellitus: the METS-GREECE Multicentre
Study. Current Medical Research and Opinion2004; 20:
1691–1701.
44. Brown WV. Metabolic syndrome and risk of stroke. Clinical Cornerstone2004;6(Suppl. 3):S30–S34. 45. Ford ES. The metabolic syndrome and mortality from
cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II
Mortality Study.Atherosclerosis2004;173:309–314.
46. Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and
NAASO, The Obesity Society. American Journal of
Clinical Nutrition2005;82:923–934.
47. Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phosp-holipase A(2) levels and the risk of future cardiovascular
events in women. Journal of the American College of