www.elsevier.com / locate / bres
Interactive report
Towards a neuroprotective gene therapy for Parkinson’s disease: use
of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF
1
to the nigrostriatal system in the rat Parkinson model
a ,
*
a b a a c¨
A. Bjorklund
, D. Kirik , C. Rosenblad , B. Georgievska , C. Lundberg , R.J. Mandel
a
Wallenberg Neuroscience Center, Section of Neurobiology, Lund University, Solvegatan 17, S-22362 Lund, Sweden
b
NsGene A /S, Pederstrupvej 93, 2750 Ballerup, Denmark
c
Gene Therapy Center, Department of Neuroscience, University of Florida, College of Medicine, Gainesville, FL, USA
Accepted 12 September 2000
Abstract
During the last few years, recombinant viral vectors derived from adenovirus (Ad), adeno-associated virus (AAV) or lentivirus (LV) have been developed into highly effective vehicles for gene transfer to the adult central nervous system. In recent experiments, in the rat model of Parkinson’s disease, all three vector systems have been shown to be effective for long-term delivery of glial cell line-derived neurotrophic factor (GDNF) at biologically relevant levels in the nigrostriatal system. Injection of the GDNF encoding vectors into either striatum or substantia nigra thus makes it possible to obtain a regionally restricted over-expression of GDNF within the nigrostriatal system that is sufficient to block the toxin-induced degeneration of the nigral dopamine neurons. Injection of GDNF vectors in the striatum, in particular, is effective not only in rescuing the cell bodies in the substantia nigra, but also in preserving the nigrostriatal projection and a functional striatal dopamine innervation in the rat Parkinson model. Long-term experiments using AAV-GDNF and LV-GDNF vectors show, moreover, that sustained GDNF delivery over 3–6 months can promote regeneration and significant functional recovery in both 6-OHDA-lesioned rats and MPTP-lesioned monkeys. The impressive efficacy of the novel AAV and LV vectors in rodent and primate Parkinson models suggests that the time may now be ripe to explore these vector systems as tools for neuroprotective treatments in patients with Parkinson’s disease. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: Parkinson’s
Keywords: Glial cell line-derived neurotrophic factor; Neuroprotection; Parkinson’s disease; Gene-transfer; Adenovirus; Adeno-associated virus; Lentivirus
1. Introduction a concomitant decline of striatal dopamine function at a
rate of 5–10% per year [10,24]. This progressive nature of In Parkinson’s disease (PD) symptoms start to appear the disease offers opportunities for therapeutic interven-when about 70–80% of striatal dopamine is lost and about tions aimed at blocking or slowing down the ongoing 50% of the dopamine neurons in the substantia nigra have degenerative process. Indeed, recent imaging data suggest
18
degenerated. Autopsy data and neuroimaging, by F- that it may be possible to detect a decline in striatal flourodopa PET (for dopamine synthesis and storage) or dopamine function even before the onset of overt clinical
b-CIT SPECT (for dopamine uptake sites), indicate that symptoms, which would make it possible to initiate there is a progressive loss of nigral dopamine neurons and neuroprotective interventions in the very early stages of the disease, i.e. at the time when, or even before, the first symptoms appear (for a review, see Ref. [21]).
1
Published on the World Wide Web on 10 October 2000.
Neurotrophic factors are interesting candidates for *Corresponding author. Tel.: 146-46-222-0541; fax: 1
46-46-222-neuroprotective therapies since they can interfere with both 0559.
¨
E-mail address: [email protected] (A. Bjorklund). apoptotic and necrotic forms of cell death, and have been
shown to rescue injured neurons after toxic, mechanical or cells. The transferred DNA remains as a non-integrated ischemic damage in the adult nervous system. In PD, the episome in the nucleus and is, therefore, most adequate for affected dopamine neurons are likely to remain dysfunc- transient expression of transgenes in non-dividing cells. tional for long periods, perhaps years, before they are The Ad vectors used thus far in GDNF transfer experi-irreversibly lost. This suggests that neurotrophic factors ments have the disadvantage that the transduced cells may be able not only to prevent further cell loss, but also express adenoviral proteins that may cause inflammation to restore function in dysfunctional or atrophic neurons in and trigger host immune reactions towards the infected the degenerating nigrostriatal system. cells (see Wood et al. [66] and Kajiwara et al. [30] for Although a large number of growth factors can act as further discussion). This, in turn, may reduce transgene survival factors for nigral dopamine neurons, the members expression over time and contribute to the variable long-of the glial cell line-derived neurotrophic factor (GDNF) term expression of the transduced protein seen in several in family are particularly interesting because of their potent in vivo studies using first-generation Ad vectors [30,66]. The vivo effects in both rodent and primate models of PD. more recent, so-called helper-dependent, or gutless, Ad Studies using intracerebral injections of the recombinant vectors may help to solve this problem and, with all protein have shown that GDNF can provide almost com- wild-type Ad genes deleted, carries an enormous packag-plete protection of nigral dopamine neurons against 6- ing capacity of around 35 kb [27,49,62].
hydroxydopamine (6-OHDA)- or MPTP-induced damage, Intracerebral delivery of GDNF by Ad-GDNF vectors promote axonal sprouting and regrowth of lesioned dopa- has been explored in rats with 6-OHDA lesions of the mine neurons, and stimulate dopamine turnover and func- nigrostriatal dopamine system [5,12,13,15,38]. Choi-Lun-tion in neurons spared by the lesion [6,25]. Although these dberg et al. [12,13] have shown that Ad-GDNF injected toxin-induced lesion models have a weakness in that they either close to the substantia nigra or into the striatum, do not reproduce the same disease mechanism(s) and given 1 week before an intrastriatal 6-OHDA lesion, can pathophysiology as seen in human PD, the results obtained afford significant protection of the nigral dopamine neu-in animal models are sufficiently impressive to suggest that rons against the toxic insult: after intranigral Ad-GDNF GDNF, or its close relatives neurturin and artemin / neub- injection, 79% of the nigral dopamine neurons survived, lastin, may be useful as therapeutic agents for neuroprotec- compared to 31% in the controls (given a similar injection tion in PD. However, given the chronic, progressive nature of Ad-lacZ); and after intrastriatal injection, 64% of the of PD it is likely that the factor should be administered neurons survived, compared to 36% in the controls, as continuously, over months or years, in order to sustain determined 6 weeks after the 6-OHDA lesion. The size of dopamine neuron survival and function long term. More- the striatal lesion, as assessed by immunohistochemistry over, since GDNF receptors are widely distributed in the for the tyrosine hydroxylase (TH) enzyme, was unaffected nervous system, the factor may have to be delivered locally by the Ad-GDNF treatment. Thus, the intensity of TH-in order to avoid negative side effects. positive fiber staining in the striatum was reduced equally, For this reason, locally induced production of the by about 40%, in all treatment groups, suggesting that neurotrophic factor by direct in vivo or indirect ex vivo GDNF over-expression did not modify the extent of the delivery of the GDNF gene to the striatum and / or sub- acute toxic damage to the dopamine terminals in the stantia nigra may offer distinct advantages. During the last striatum.
Table 1
a
Levels of GDNF expression in the basal ganglia obtained by three viral vector systems
Vector Host Injection Site of GDNF content GDNF content in
[Ref.] volume injection in striatum substantia nigra
(ml) (ng / mg tissue) (ng / mg tissue)
Adenovirus Rat [13] 2 str (one site) 1–2* ND
2 sn (one site) ND 0.5–1.7*
†
Monkey [9] 30 str (one site) 0.09 ND
rAAV Rat [34] 9 str (three sites) 0.22 0.1
Rat [34] 2 sn (two sites) 1.58 1.41
Rat [45] 2 sn (two sites) ND 0.2–0.6*
Lentivirus Rat 3 str (three sites) 2.3–6.3 0.2–0.9
Mouse [4] 1 sn (one site) ND 0.5–0.6*
†
Monkey [37] 45 str (five sites) 0.22–0.35 ND
5 sn (one site)
a
Approximate levels of GDNF from the indicated anatomical areas and species are presented for purposes of a rough comparison rather than a precise quantitative analysis. For example, the adenovirus injections were made in both anatomical structures as were the lentivirus injections in the monkey, but the rAAV injections were made in the individual structures. Different vector particle concentrations were used in each study and are not taken into account in this comparison because of the difficulty in comparing this parameter between different vectors. Vector titers can be found in the cited references. In addition, the survival times varied widely between the studies. Some of the values are estimated as detailed below. An asterisk (*) indicates that the ng / mg tissue was calculated from a reported ng per punch value by a rough estimate of the mg per punch. The dagger (†) indicates that the reported value’s units were originally ng / mg protein. Therefore, we estimated that protein constitutes approximately 10% of the tissue weight and calculated the values in the table accordingly (ND, not done; str, striatum; sn, substantia nigra). The rat LV data are from our own unpublished study (Georgievska et al., to be published).
In the second study, Choi-Lundberg et al. [13] moni- higher than those used in the Choi-Lundberg et al. [12,13]
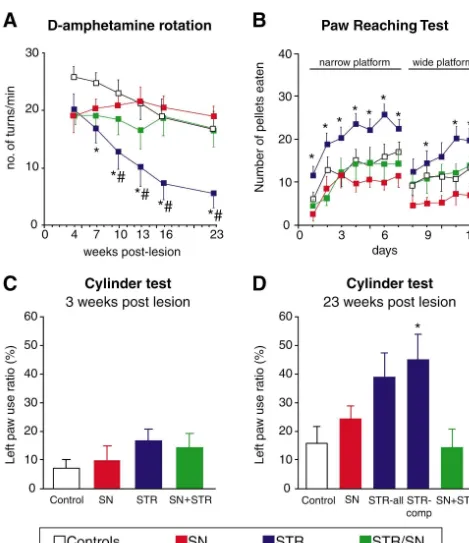
7
tored changes in nigrostriatal function in tests of drug- experiments (3.2–3.9310 pfu). The level of GDNF induced rotation and spontaneous forelimb use. However, expression in the Ad-GDNF-injected animals was not with the type of lesion used (16mg of 6-OHDA in a single determined in the Bilang-Bleuel et al. [5] study, but the striatal deposit) the extent of striatal denervation (about multiple injection protocol used suggests that GDNF may 40%) is insufficient to induce any consistent functional have been expressed more widely and at higher levels effects that are stable over time (see Ref. [32] for further throughout the striatum. This would explain why Bilang-discussion). Thus, although the Ad-GDNF vector-injected Bleuel et al. [5] observed not only partial protection of the animals displayed less motor asymmetry acutely after the TH-positive nigral cell bodies (to a mean of 62% of lesion (at 4–12 days after a unilateral 6-OHDA injection), normal) but also partial sparing of the striatal TH-positive these differences were not maintained at longer survival innervation, which was semi-quantitatively estimated to be times, which at least in part was due to spontaneous in the range of 75%. In line with these morphological data, recovery in the control groups. In a follow-up study, amphetamine-induced rotation was reduced by 80–90% in Connor et al. [15] used the same lesion parameters, but this the Ad-GDNF-injected animals at 1–3 weeks after 6-time in aged rats. In 20-month-old rats, the lesion-induced OHDA treatment. Although no observations on sponta-deficits, albeit small, were more stable over time. Signifi- neous (i.e. non-drug-induced) motor behaviors were made cant improvement in side bias and amphetamine-induced in this study, the data suggest partial sparing of a func-rotation was observed when the Ad-GDNF vector was tional nigrostriatal pathway in the Ad-GDNF-injected injected into the striatum. Vector injection into the sub- animals. The interpretation of the results, however, is stantia nigra, by contrast, had a detrimental, rather than somewhat complicated by the toxicity induced by the large
8
positive, effect on the rats’ behavioral performance. amounts of Ad vectors used (1.5310 pfu in 9ml). Both Bilang-Bleuel et al. [5] used the same intrastriatal 6- Ad vectors, containing GDNF or lacZ, caused both inflam-OHDA lesion model, but employed a more severe type of mation and atrophy of the injected striatum. The control lesion (20mg of 6-OHDA distributed over three injection Ad-lacZ vector, in addition, induced a non-specific 37% sites). This lesion resulted in an approx. 75% reduction in loss of dopamine neurons in the ipsilateral substantia nigra TH-positive fiber staining in the striatum and a 70% loss of (i.e. in the absence of any 6-OHDA lesion).
TH-positive neurons in the substantia nigra at 3 weeks Clearly, with the current E1, E3 deleted Ad vectors used after 6-OHDA injection, which is compatible with more so far, the magnitude of the inflammatory response and the pronounced and consistent motor impairments [32]. To toxicity increases with increasing titers of the injected maximize the Ad-GDNF effect, the vector was injected at vector. These host responses, which are likely to be multiple sites in the striatum (nine injections), and the total triggered by the expression of viral proteins on the surface
8
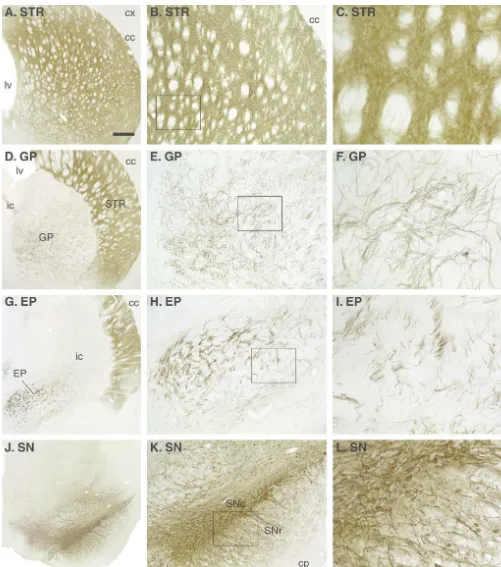
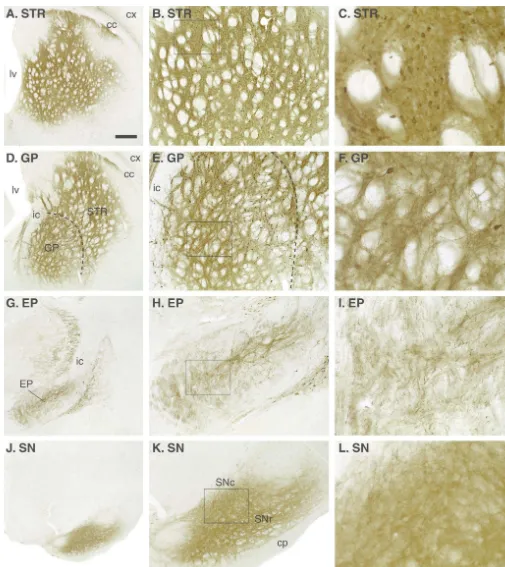
of the transgene or even kill the transduced cells [66]. This absence of such receptor molecules on the cell surface may is particularly well illustrated in the experiments of Bohn determine the efficiency by which the rAAV vectors are et al. [8] and Lawrence et al. [40], using injections of internalized into different types of neurons [3,54,61]. Ad-lacZ vector in the caudate nucleus of intact monkeys. In the nigrostriatal system, rAAV vectors have been In a group of 10 monkeys, all given the Ad-lacZ vector in shown to be effective in transducing neurons in both the same dose, the number ofb-gal-expressing cells in the substantia nigra, globus pallidus and striatum, and high caudate varied from 0 to 600 000. Staining with inflamma- levels of transgene expression have been observed over at tory markers revealed an inverse correlation between least 6 months after vector injection [34,35,41,42] (Figs. 1 transgene expression and extent of the inflammatory and 2). Over 90% of the transduced cells can, by mor-response, including demyelination [40]. Injection of vary- phological criteria, be classified as neurons. The high
7 9
ing titers of the vector (from 5310 to 2310 pfu) showed affinity of the rAAV vector for the pars compacta of the that the intensity of the host’s immune response increased substantia nigra makes it possible to express the transgene with increasing titers. At 1 month after vector injection, no in a high proportion of the nigral dopamine neurons with a
b-gal expression was seen in the animal given the highest single 1–2 ml injection of the vector (Fig. 1J–L). The titer [8]. These data clearly support the view that the host’s tranduction efficiency is, by comparison, lower in the immune response is a major limitation for high-level, striatum. In our recent study [34], using a single 3 ml stable transgene expression in the brain from the currently injection of the rAAV-GFP vector in the striatum, we used Ad vectors, and is probably a major reason why observed GFP-positive cells around the injection site GDNF expression from intracerebrally injected Ad vectors within a radius of about 0.3 mm, compared to a radius of declines and becomes more variable over time. In the about 1.5 mm in the substantia nigra after a 1ml injection. recent study of Connor et al. [15], for example, only 10 of To reach larger areas of the striatum, therefore, we have 14 animals showed GDNF expression at 6 weeks after had to use multiple injections of the vector (333 ml), Ad-GDNF injection into striatum or nigra of aged rats, as spaced with a distance of about 1 mm between the revealed by immunohistochemistry. Ad-GDNF expression injection sites.
at survival times longer than 6–7 weeks has so far not Recent improvements in rAAV vector production has been reported in the rat PD model. resulted in 100–10 000-fold higher titers, a higher propor-tion of infectious particles (relative to empty ones), as well as completely Ad helper virus free vector preparations 3. Adeno-associated viral (AAV) vectors [67,68]. The new vector we are exploring now (provided by the University of Florida vector core) includes, in The recombinant AAV vectors have 96% of the viral addition, a modified promoter construct, i.e. a hybrid genome removed, leaving only the two short inverted CMV/ chicken b-actin (CBA) promoter with the wood-terminal repeats (ITRs) which are sufficient for packaging chuck hepatitis virus posttranscriptional element (WPRE) and integration. The advantage of these vectors is that they [18,69], instead of the MD promoter [53] used in our can integrate and stably express their transgene product in earlier studies [34,43,45]. The new vector has resulted in a non-diving cells, including neurons, and that the absence substantial increase in transduction efficiency in both of viral genes minimizes the expression of foreign proteins striatum and substantia nigra, both in terms of the number and hence the risk of triggering host immune responses of GFP-expressing cells and the level of GFP per cell. The [50]. The disadvantage is that the rAAV DNA packaging transduced protein is transported intra-axonally, from the capacity is small, less than 5 kb, which limits the size of nigra along the nigrostriatal pathway to the terminals in the the gene constructs that can be delivered with the rAAV striatum (Fig. 1), and from the striatum along the system. Another limiting factor is that the transgene is striatonigral pathway to the terminals in the globus pal-expressed with a delay of several days, and increases lidus, entopeduncular nucleus and pars reticulata of the gradually over the first 2–3 weeks, probably due to the fact substantia nigra (Fig. 2). Indeed, with the new vector that a second strand of DNA needs to be synthesized in the construct, the level of expression is such that the axonal transduced cells before the transgene can be expressed (for and dendritic projections of the transduced nigral and recent reviews, see Refs. [48,54,61]). striatal neurons appear to be completely filled by the
Studies using b-gal or GFP as reporter genes have transduced GFP.
shown that the rAAV vector is efficient in transducing Intracerebral delivery of GDNF by means of AAV-non-dividing cells, mainly neurons, in the adult CNS GDNF vectors has so far been explored in the intrastriatal [3,31,35,43,55]. However, not all types of neurons are 6-OHDA lesion model in three studies [34,44,45], using a
12
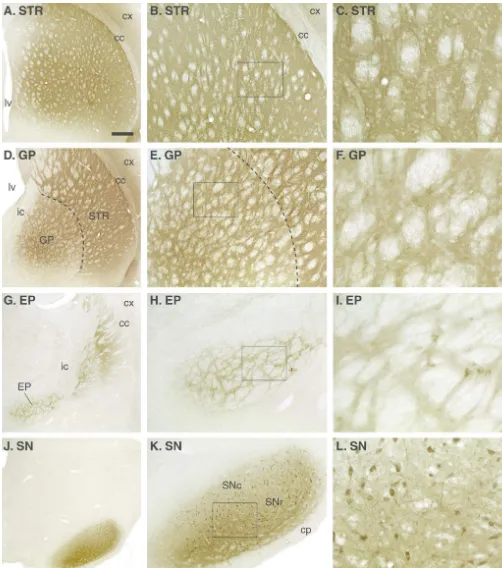
Fig. 1. Distribution of the GFP protein in an intact animal receiving an injection of the AAV-GFP vector into the substantia nigra (SN) (2ml, 5 weeks survival, CBA promoter). The transgene is highly expressed within the nigral dopamine neurons (J–L) and transported anterogradely in the axons to fill out virtually the entire nigrostriatal pathway and its axonal branches in the entopeduncular nucleus (EP; G–I), globus pallidus (GP; D–F) and the striatum (STR; A–C). Also, the dendrites in the substantia nigra, pars reticulata contain high levels of the transduced GFP protein (L).
Fig. 2. Injection of the AAV-GFP vector in the striatum (333ml, 5 weeks survival, CBA promoter) will transduce a large number of cells (almost exclusively neurons) in both striatum and globus pallidus (B,C,E,F). The transduced protein is effectively transported anterogradely along the striatanigral pathway to the globus pallidus (GP, D–F), entopeduncular nucleus (EP, G–I) and substantia nigra (SN, J–L).
surviv-al in the AAV-lacZ-injected groups. The extent of denerva- These results are consistent with previous studies using tion of the TH-positive fibers in the striatum, however, was injections or infusions of recombinant GDNF protein, unaffected by the AAV-GDNF treatment. which have shown that survival of the nigral dopamine The level of GDNF expression, as determined in 2-mm neurons in the absence of a functional nigrostriatal projec-diameter punches from the transduced substantia nigra, tion is insufficient for functional sparing or functional remained fairly stable over the 10-week observation recovery in the intrastriatal 6-OHDA lesion model period, at a level of 0.7–1.2 ng / punch (approximately [33,59,65].
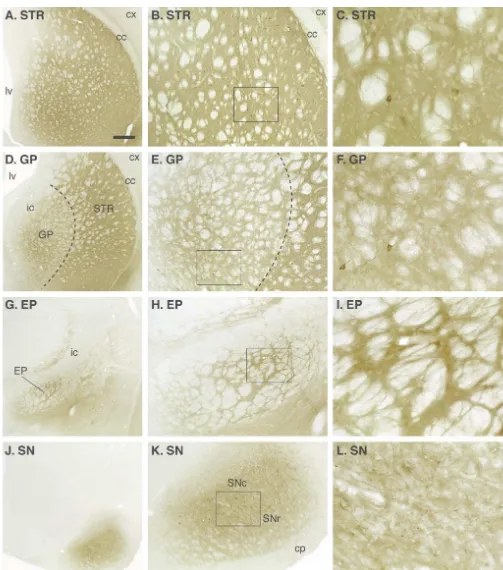
equivalent to 0.3–0.6 ng / mg tissue; see Table 1). In the The animals receiving AAV-GDNF injections into the Choi-Lundberg et al. [12] study, using Ad-GDNF in- striatum were as impaired as the control lesion rats acutely jections into the substantia nigra, the protection of the after the lesion (Fig. 4A,C), indicating that the over-nigral cell bodies was incomplete (79%) despite several- expression of GDNF, at the levels obtained, was not fold higher GDNF tissue levels (see Table 1). This sufficient to protect the striatal dopamine terminals against difference may be explained by the fact that the Ad vector the toxic insult. However, the TH-positive axons along the is expressed mainly outside the dopamine neurons. Thus, nigrostriatal pathway were partly preserved and sprouting over-expression of GDNF within the dopamine neurons fibers were abundant in the globus pallidus and in the themselves, as obtained with the AAV-GDNF vector, may caudal and ventral parts of the striatum (Fig. 5C). The be particularly efficient for rescue of the axotomised nigral efficient striatal reinnervation seen in these animals thus cell bodies. With the new generation of AAV vector now appeared to be caused by a combination of protection of available (see above) the level of GDNF obtained after lesioned nigrostriatal axons followed by regeneration injection in either nigra or striatum is 7–50-fold higher that towards and into the region of high GDNF expression. that achieved with the previous vector (Kirik et al., This sequence of axonal damage followed by a protracted unpublished data). remodelling of the nigrostriatal projection is consistent In the Kirik et al. [34] study, the AAV-GDNF vector was with the slow and progressive functional recovery that injected at multiple sites in the striatum, in the nigra, or in developed during the first months after the lesion. both striatum and nigra, 4 weeks before the 6-OHDA It is notable that over-expression of GDNF in the nigra injection. Expression of GDNF, as observed by immuno- failed to preserve the TH-positive axons along the nigros-histochemistry, was maintained at high levels in both sites triatal pathway. In these animals, there was massive axonal throughout the 6-month experimental period. In animals sprouting in and around the medial forebrain bundle, close receiving vector injection into the striatum, the GDNF to the rescued cell bodies. These sprouting fibers were seen protein was widely distributed throughout the striatum and to extend up to the border of the globus pallidus, but not transported along the striatonigral pathway to the globus further rostrally (Fig. 5D). Indeed, the area containing pallidus (Fig. 3D–F), the entopeduncular nucleus (Fig. dense sprouting fibers coincided with the area of high 3G–I) and the substantia nigra (Fig. 3J–L). In this GDNF-immunoreactivity, as seen in sections stained with experiment, a four-site intrastriatal 6-OHDA lesion was the GDNF antibody.
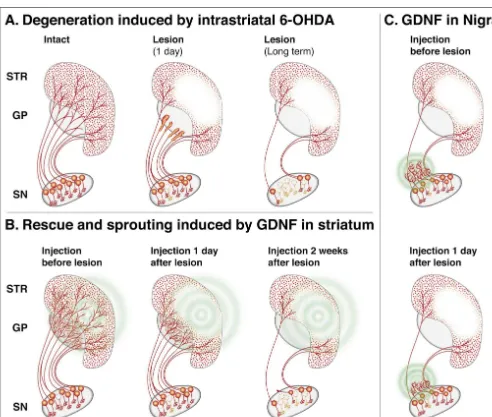
used (437 mg) which gives a substantial, 80–90%, These data are in agreement with results obtained with denervation of the striatum, sufficient to induce marked direct intracerebral injections of recombinant GDNF pro-motor impairments that are stable over time [32,34]. Vector tein [33,60]. As illustrated in Fig. 7, GDNF injected into injection into the nigra was more efficient in protecting the the striatum either 6 h before or 1 day after the intrastriatal nigral dopamine cell bodies: 91% cell survival in the nigral 6-OHDA injection is efficient in preserving the cell bodies injection group, compared to 57% in the striatal injection and the axons of the nigrostriatal pathway, as well as group and 12% in the lesion-only controls. However, only inducing axonal sprouting in the globus pallidus and the rats receiving AAV-GDNF injections into the striatum regeneration of TH-positive fibers in the striatum, while showed behavioral recovery, and this was accompanied by GDNF over the substantia nigra induces sprouting locally partial sparing of the nigrostriatal projection and reinnerva- around the injection site. In both cases, sprouting of tion of the lesioned striatum (see Fig. 5C). As illustrated in TH-positive fibers occurs in the area reached by high Fig. 4, the recovery developed gradually over 4–5 months concentrations of the GDNF protein. Consistent with these after the lesion and was observed in both drug-induced morphological data, significant sparing or recovery of rotation (Fig. 4A) and in tests of spontaneous motor motor functions was obtained only in the animals receiving behavior, i.e. forelimb use in the so-called cylinder and GDNF injection into the striatum, i.e. in those animals staircase tests (Fig. 4B–D). No functional sparing or which had significant sparing of the nigrostriatal projection recovery was seen in the animals receiving AAV-GDNF in combination with GDNF-induced sprouting of TH-injections into the substantia nigra, despite near-complete positive axons in the lesioned striatum [33].
Fig. 4. Functional recovery in drug-induced rotation (A), and forelimb use in the paw-reaching (B) and cylinder tests (C and D) in 6-OHDA lesioned rats that had received AAV-GDNF injections into either striatum (STR), substantia nigra (SN) or both striatum and nigra (STR / SN). The vector injection was made 3 weeks before the intrastriatal 6-OHDA injection. Note that functional recovery is seen only in animals with intrastriatal vector injections (modified after Kirik et al. [34]).
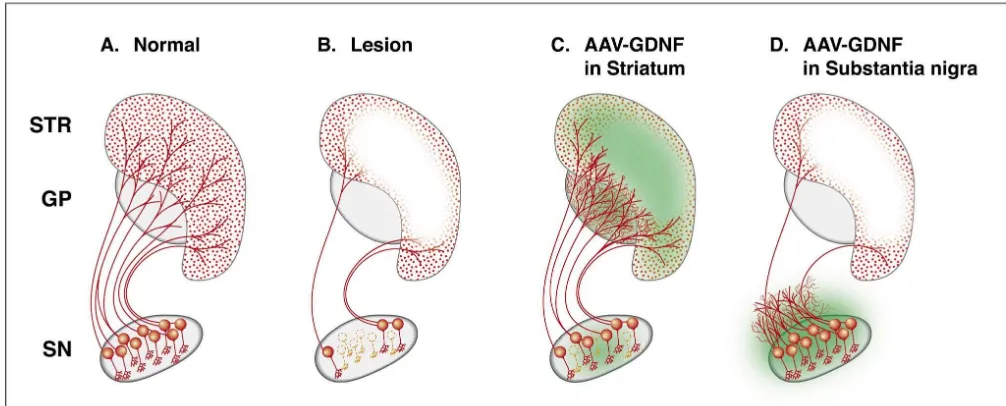
Fig. 5. Over-expression of GDNF in the striatum by intrastriatal injections of the AAV-GDNF vector can block dopamine neuron degeneration induced by the intrastriatal 6-OHDA lesion, as illustrated in B and C. Importantly, the axons along the nigrostriatal pathway are partially preserved. These rescued axons provide the substrate for sprouting and regrowth into the area of high GDNF expression (indicated by green color in C), which in turn is accompanied by a gradual functional recovery (as illustrated in Fig. 4). Injection of the vector into the substantia nigra (D), by contrast, protects the nigral cell bodies but is unable to preserve the axonal projection to the striatum. Instead, there is extensive local sprouting of TH-positive fibers in regions close to the rescued cell bodies, i.e. into the area of high GDNF expression (green color in D). (Based on data from Ref [34].)
4. Lentiviral (LV) vectors In the current version of LV vectors, the particles are pseudotyped with the G envelope protein of the vesicular LV vectors are derived from a group of highly patho- stomatitis virus (VSV-G). This gives the vector the capaci-genic retroviruses, which includes the HIV viruses. They ty to infect a broad range of tissues, including nervous share the useful properties of the commonly used oncoret- tissues, and is probably responsible for their high affinity roviral vectors, with the additional advantage that the LV for fully differentiated neurons within the CNS vectors can integrate also into non-dividing cells. They [7,14,47,51,52]. The level of expression in the brain is have a large cloning capacity, at least 9 kb, and are stably further increased by the introduction of the woodchuck integrated into the genome of the target cells, i.e. prop- regulatory element (WPRE) into the vector construct erties that are highly favorable for long-term expression of [19,70].
transgenes in the nervous system (for recent reviews, see The VSV-G pseudotyped LV vectors are highly efficient Refs. [11,64]). Most of the efforts so far have been focused in transducing cells in both striatum and substantia nigra, on the development of efficient vector systems based on and in both sites the majority of the transduced cells are the HIV-1 virus. This raises a number of important safety neurons [7,19,20,36,52]. Deglon et al. [19] and Bensadoun issues that have to be solved before these vectors can be et al. [4] reported a total of 21 800 and 38 000 cells considered for clinical use. In this respect, the alternative expressing b-gal after injection of 1–2 ml of a high titer vector systems based on equine or feline lentiviruses, LV-lacZ vector in the substantia nigra in rat and mouse, which appear to be non-pathogenic for humans, may be respectively. Up to 50% of the TH-positive neurons were particularly attractive candidates for clinical use labelled when the most efficient SIN vector with the [17,28,46,56]. In the current versions of the HIV-1-based WPRE was used. In the monkey, using the same efficient LV vector, up to 60% of the viral genome has been vector, Kordower et al. [36] reported an average of eliminated and only three or four of the nine genes of 187 000 b-gal-positive cells in the nigra and up to 1.5 HIV-1 are retained [20,71]. The viral particles are gener- million positive cells in the striatum at 3 months post-ated by transient transfection of 293T cells with three or injection. In the striatum, stable expression of reporter four different plasmids, which further reduces the risk of genes,b-gal or GFP has been observed for up to 6 months such recombination events that may lead to the generation without any signs of toxicity or adverse inflammatory of an infectious, replication-competent retrovirus reaction in the host tissue ([7], Kirik et al., unpublished [20,51,52,64,71]. The introduction of the so-called self- data).
pub-lished studies [4,19,37,58]. In the Deglon et al. [19] study, the striatum, and transport in the striatal projection neurons 2 ml of the LV-GDNF vector was injected unilaterally down to the globus pallidus, entopeduncular nucleus and above the substantia nigra, followed 1 week later by a substantia nigra (Fig. 6). Indeed, the GDNF level mea-knife transection of the ipsilateral medial forebrain bundle sured in the nigra in the striatum-injected animals was (MFB). At 1 week after the lesion, 56% of the TH-positive quite high, 0.4 ng / mg tissue, which is similar to that neurons remained in the LV-GDNF-injected animals, com- obtained by Deglon et al. [19] in the nigra after intranigal pared to 24% in the LV-LacZ-injected lesion controls. In injection of the LV-GDNF vector.
the Bensadoun et al. [4] study, which was carried out in The most compelling data on the efficacy of the LV-mice, 1 ml of the LV-GDNF vector was injected over the GDNF vector have been obtained by Kordower et al. [37] nigra, followed 2 weeks later by an intrastriatal injection of in their recent study in aged and MPTP-treated monkeys. 6-OHDA on the same side. At 4 weeks after the lesion, In their experiment, LV-GDNF was injected into both 70% of the nigral TH-positive neurons survived compared caudate nucleus (1015 ml), putamen (1011015ml), and to 33% in the controls. Striatal dopamine levels were substantia nigra (5ml) 1 week after an intracarotid MPTP reduced by 90% in both groups, indicating that the striatal injection. As in our AAV-GDNF experiment in the rat dopamine innervation was not spared in the LV-GDNF- (Kirik et al. [34], see above) the acute behavioral impair-injected animals. The level of GDNF, as determined by ments were as severe in the LV-GDNF-treated animals as ELISA in dissected pieces containing the whole nigral in the controls. However, over the subsequent 3 months, region, was 2.6 and 1.95 ng at 1–6 weeks post-injection in the motor deficits seen in the Parkinson rating scale and an the two studies. Assuming that the weight of the dissected operant hand-reaching task were reversed in the LV-piece was in the range of 4–6 mg, this is equivalent to GDNF-treated animals, in three of the four animals to near 0.4–0.6 ng / mg, i.e. similar to the tissue levels obtained in normal levels. The loss of TH-positive neurons in the the rat substantia nigra with the old version of the AAV- nigra, which was 289% in the LV-bGal-treated lesion GDNF vector [34,44,45] (see Table 1). controls, was completely prevented, and the TH-positive In our own study [58], the LV-GDNF vector was innervation in the striatum was significantly preserved injected both over the nigra (1 ml) and in the striatum (70–80% of normal, compared to about 25% in the
LV-18
(231 ml). One week later, the rats received a single bGal controls). Consistent with this, striatal F-flourodopa injection of 20 mg 6-OHDA into the ipsilateral striatum. uptake (as assessed by PET prior to sacrifice) was in-To help in the assessment of cell survival, the nigrostriatal creased by 300% in the LV-GDNF-treated striatum. Inter-neurons were labeled retrogradely by an injection of estingly, sparing of striatal TH-positive fibers, and increase
18
fluoro-gold in the striatum 2 weeks before the vector in F-flourodopa uptake, was best in those monkeys injection. With this combined vector injection there was showing the most pronounced recovery in the behavioral almost complete rescue of the nigrostriatal neurons: 87% tests, suggesting that a functional nigrostriatal projection of the fluoro-gold labeled cells and 81% of the TH-positive had been preserved in the LV-GDNF-treated animals. cell bodies, compared to 17–24% survival in the LV-GFP- In a second experiment, the LV-GDNF vector was injected lesion controls. In addition, there was a significant injected in striatum and nigra in aged (25 years old) partial preservation of the TH-positive innervation in the monkeys [37]. Three months later the LV-GDNF-treated striatum, suggesting that over-expression of GDNF in the animals displayed an increase in the number of TH-posi-striatum had afforded partial protection of the nigrostriatal tive neurons in the ipsilateral substantia nigra, as well as an dopamine terminals against the toxic insult. Interestingly, a increase in the size and the expression of TH-mRNA in similar level of neuroprotection and sparing of the striatal individual nigral TH-positive neurons. Striatal TH-im-TH-positive innervation was obtained with a vector ex- munoreactivity was increased by about 40%, striatal tissue pressing artemin / neublastin, suggesting that this newly levels of dopamine and HVA by 47–207%, and striatal
18
discovered member of the GDNF family has prominent F-flourodopa uptake by 27%. Since aged monkeys show neurotrophic actions on the nigrostriatal dopamine neurons a decline in these parameters with increasing age [23], the
[58]. likely interpretation of these data is that over-expression of
Table 1). Consistent with the rodent data, efficient antero- efficient in preserving a functional striatal dopamine grade transport of the transduced GDNF was observed innervation. And, only intrastriatal GDNF administration is from the striatum to both globus pallidus and substantia efficient in promoting regeneration or sprouting into the nigra in the LV-GDNF-injected monkeys. striatal target (Fig. 7). However, not only the site of injection but also the timing, i.e. the degenerative state of the lesioned nigrostriatal dopamine neurons, is important: 5. How should GDNF be applied for optimal regardless of how GDNF is administered — as recombi-therapeutic effect? nant protein or via viral vectors — a substantial level of functional sparing or recovery is obtained only when both The results obtained in the rat PD model show that the the cell bodies and the axons along the nigrostriatal ability of GDNF to preserve or restore nigrostriatal func- pathway remain intact up into, or very close to, the tion depends on the site of administration of the trophic striatum. If administered early in the degenerative process, factor (or the vector). Thus, in the intrastriatal 6-OHDA GDNF can prevent further die-back of the lesioned axons lesion model where the initial insult is at the level of the and induce regrowth and sprouting into the area of high axon terminals in the striatum, administration of GDNF GDNF expression. Clearly, GDNF has to be delivered at into the striatum — but not into substantia nigra — is the level of the axonal stumps in order to elicit the
sprouting in the striatum; administration of GDNF at the nigra but the dopaminergic innervation in the striatum is level of the cell bodies does not induce any sprouting in largely gone, particularly in the putamen where less than the striatum (Fig. 7C). When the degenerative process has 10% of the innervation may remain in severe cases. progressed further, intrastriatal GDNF is no longer capable
of rescuing either axons or cell bodies of lesioned nigral
neurons, as indicated in Fig. 7B. In this case, sprouting in 6. Clinical perspective the striatum is much less pronounced. This suggests that
GDNF is able to induce sprouting primarily from lesioned The techniques for direct intracerebral gene delivery axons or axon terminals. Intact axons, either the ones using recombinant viral vectors are still highly experimen-spared by a 6-OHDA lesion or those in non-lesioned tal. However, the efficiency and safety of the vector animals, appear to respond primarily by a functional up- systems have during the last few years been improved to regulation of the transmitter machinery, i.e. by increased such an extent that they are now seriously considered for dopamine synthesis or turnover [39]. clinical application in conditions of CNS disorders. PD is In the rat PD model, where the primary insult is in the likely to be one of the neurodegenerative diseases in which striatum, intrastriatal delivery is clearly the preferred route this technique will be first tested. The reason for this is that of administration of GDNF. In this acute lesion model the underlying neuropathology is well known, and that administration of GDNF in the nigra induces extensive there is a well-defined and anatomically restricted target sprouting locally in the midbrain. Such aberrant sprouting for intervention, i.e. the slowly degenerating nigrostriatal appears to impair rather than promote functional recovery. dopamine system. With all vector systems discussed here, Whether these observations are relevant for PD may localized deposits of microliter amounts of vector are depend on the nature of the degenerative process in the sufficient for efficient tranduction of the substantia nigra or human disease: if nigrostriatal degeneration in PD starts in striatum not only in rodents, as demonstrated for both Ad, the terminals and progresses towards the cell bodies, then AAV and LV vectors, but also in non-human primates as intrastriatal application is the obvious choice. If degenera- shown for the LV vector [36,37].
[3] J.S. Bartlett, R.J. Samulski, T.J. McCown, Selective and rapid the cases where the vector is integrated into the genome, is
uptake of adeno-associated virus type 2 in brain, Hum. Gene Ther. 9 also a concern that needs to be taken seriously.
(1998) 1181–1186.
There are also important issues related to the level and [4] J.C. Bensadoun, N. Deglon, J.L. Tseng, J.L. Ridet, A.D. Zurn, P. site of the expression of the transgene, in this case GDNF, Aebischer, Lentiviral vectors as a gene delivery system in the mouse as well as the possible negative or adverse side effects that midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson’s disease using GDNF, Exp. Neurol. 164 (2000) might be induced by sustained, high-level expression of
15–24. this biologically active molecule in the human brain. Since
[5] A. Bilang-Bleuel, F. Revah, P. Colin, I. Locquet, J.J. Robert, J. we do not yet have any relevant human data to guide us on Mallet, P. Horellou, Intrastriatal injection of an adenoviral vector these important safety issues, it appears essential to expressing glial-cell-line-derived neurotrophic factor prevents dopa-develop vector constructs that are regulatable, most im- minergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease, Proc. Natl. Acad. Sci. USA 94 (1997) portantly so that the GDNF production can be substantially
8818–8823. reduced, or completely switched-off, if necessary.
Regulat-¨
[6] A. Bjorklund, C. Rosenblad, C. Winkler, D. Kirik, Studies on able promoters, such as the tetracycline-based systems, are neuroprotective and regenerative effects of GDNF in a partial lesion currently being explored in both the Ad, AAV and LV model of Parkinson’s disease, Neurobiol. Dis. 4 (1997) 186–200. vector systems [16,26,29,57]. [7] U. Blomer, L. Naldini, T. Kafri, D. Trono, I.M. Verma, F.H. Gage,
Highly efficient and sustained gene transfer in adult neurons with a Of the three vector systems discussed here, the AAV and
lentivirus vector, J. Virol. 71 (1997) 6641–6649. LV vectors seem to be the most promising ones for
long-[8] M.C. Bohn, D.L. Choi-Lundberg, B.L. Davidson, C. Leranth, D.A. term high-level transgene expression in the CNS. From
Kozlowski, J.C. Smith, M.K. O’Banion, D.E. Redmond Jr., Adeno-available data, it seems that these vector systems are virus-mediated transgene expression in nonhuman primate brain, essentially non-toxic, even when injected in high titers, and Hum. Gene Ther. 10 (1999) 1175–1184.
[9] M.C. Bohn, D.A. Kozlowski, D. George, E. Bremer, B.L. Davidson, that they induce, at most, limited inflammation or cellular
D.E. Redmond Jr., Quantitation of glial cell line-derived neuro-immune responses in the brain. The AAV-GDNF and
trophic factor (GDNF) expression in the monkey CNS after LV-GDNF vectors are thus interesting candidates for
injection of an Adenoviral vector (AdGDNF), Abstr. Soc. Neurosci. intracerebral gene delivery in clinical trials in PD patients. 25 (1999) 2055.
However, before going to the clinic, it will be essential to [10] D.J. Brooks, The early diagnosis of Parkinson’s disease, Ann. Neurol. 44 (1998) S10–S18.
demonstrate not only safety but also efficacy, both
ana-[11] G.L. Buchschacher Jr., F. Wong-Staal, Development of lentiviral tomically and functionally, in long-term experiments in the
vectors for gene therapy for human diseases, Blood 95 (2000) monkey MPTP model; preferably with vectors that allow
2499–2504.
external regulation of GDNF transgene expression. The [12] D.L. Choi-Lundberg, Q. Lin, Y.N. Chang, Y.L. Chiang, C.M. Hay, recent study of Kordower et al. [37], demonstrating H. Mohajeri, B.L. Davidson, M.C. Bohn, Dopaminergic neurons efficacy of the LV-GDNF vector in MPTP-treated mon- protected from degeneration by GDNF gene therapy, Science 275
(1997) 838–841. keys, is an important first step in this direction.
[13] D.L. Choi-Lundberg, Q. Lin, T. Schallert, D. Crippens, B.L. Davidson, Y.N. Chang, Y.L. Chiang, J. Qian, L. Bardwaj, M.C. Bohn, Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived Acknowledgements
neurotrophic factor, Exp. Neurol. 154 (1998) 261–275.
[14] C. Cisterni, C.E. Henderson, P. Aebischer, B. Pettmann, N. Deglon, The studies reported here were supported by grants from Efficient gene transfer and expression of biologically active glial cell the Swedish MRC (Gene Therapy Program 99XG-13285), line-derived neurotrophic factor in rat motoneurons transduced with the Biotech program of the European Commission (BI04- lentiviral vectors, J. Neurochem. 74 (2000) 1820–1828.
[15] B. Connor, D.A. Kozlowski, T. Schallert, J.L. Tillerson, B.L. CT98-0530) and the Parkinson’s Disease Foundation. We
Davidson, M.C. Bohn, Differential effects of glial cell line-derived ¨
thank Tomas Bjorklund for expert assistance in the
prepa-neurotrophic factor (GDNF) in the striatum and substantia nigra of ration of the illustrations of this review. the aged Parkinsonian rat, Gene Ther. 6 (1999) 1936–1951.
[16] O. Corti, O. Sabate, P. Horellou, P. Colin, S. Dumas, D. Buchet, M.H. Buc-Caron, J. Mallet, A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain
References grafts of human neural progenitors, Nat. Biotechnol. 17 (1999)
349–354.
[1] P. Aebischer, N.A. Pochon, B. Heyd, N. Deglon, J.M. Joseph, A.D. [17] M.A. Curran, S.M. Kaiser, P.L. Achacoso, G.P. Nolan, Efficient Zurn, E.E. Baetge, J.P. Hammang, M. Goddard, M. Lysaght, F. transduction of nondividing cells by optimized feline immuno-Kaplan, A.C. Kato, M. Schluep, L. Hirt, F. Regli, F. Porchet, N. De deficiency virus vectors, Mol. Ther. 1 (2000) 31–38.
Tribolet, Gene therapy for amyotrophic lateral sclerosis (ALS) using [18] T.M. Daly, T. Okuyama, C. Vogler, M.E. Haskins, N. Muzyczka, a polymer encapsulated xenogenic cell line engineered to secrete M.S. Sands, Neonatal intramuscular injection with recombinant hCNTF, Hum. Gene Ther. 7 (1996) 851–860. adeno-associated virus results in prolonged beta-glucuronidase [2] P. Aebischer, M. Schluep, N. Deglon, J.M. Joseph, L. Hirt, B. Heyd, expression in situ and correction of liver pathology in
potential gene transfer system in Parkinson’s disease, Hum. Gene [38] P.A. Lapchak, D.M. Araujo, D.C. Hilt, J. Sheng, S. Jiao, Adenoviral Ther. 11 (2000) 179–190. vector-mediated GDNF gene therapy in a rodent lesion model of late [20] T. Dull, R. Zufferey, M. Kelly, R.J. Mandel, M. Nguyen, D. Trono, stage Parkinson’s disease, Brain Res. 777 (1997) 153–160.
L. Naldini, A third-generation lentivirus vector with a conditional [39] P.A. Lapchak, P.J. Miller, S. Jiao, Glial cell line-derived neuro-packaging system, J. Virol. 72 (1998) 8463–8471. trophic factor induces the dopaminergic and cholinergic phenotype
¨
[21] S.B. Dunnett, A. Bjorklund, Prospects for new restorative and and increases locomotor activity in aged Fischer 344 rats, Neuro-neuroprotective treatments in Parkinson’s disease, Nature 399 science 77 (1997) 745–752.
(1999) A32–A39. [40] M.S. Lawrence, H.G. Foellmer, J.D. Elsworth, J.H. Kim, C. Leranth, [22] J.L. Eberling, K.S. Bankiewicz, S. Jordan, H.F. VanBrocklin, W.J. D.A. Kozlowski, A.L. Bothwell, B.L. Davidson, M.C. Bohn, D.E. Jagust, PET studies of functional compensation in a primate model Redmond Jr., Inflammatory responses and their impact on beta-of Parkinson’s disease, Neuroreport 8 (1997) 2727–2733. galactosidase transgene expression following adenovirus vector [23] M.E. Emborg, S.Y. Ma, E.J. Mufson, A.I. Levey, M.D. Taylor, W.D. delivery to the primate caudate nucleus [see comments], Gene Ther.
Brown, J.E. Holden, J.H. Kordower, Age-related declines in nigral 6 (1999) 1368–1379.
neuronal function correlate with motor impairments in rhesus [41] S.E. Leff, S.K. Spratt, R.O. Snyder, R.J. Mandel, Long-term monkeys, J. Comp. Neurol. 401 (1998) 253–265. restoration of striatalL-aromatic amino acid decarboxylase activity [24] J.M. Fearnley, A.J. Lees, Ageing and Parkinson’s disease: substantia
using recombinant adeno-associated viral vector gene transfer in a nigra regional selectivity, Brain 114 (1991) 2283–2301.
rodent model of Parkinson’s disease, Neuroscience 92 (1999) 185– [25] D.M. Gash, G.A. Gerhardt, B.J. Hoffer, Effects of glial cell
line-196. derived neurotrophic factor on the nigrostriatal dopamine system in
[42] W.D. Lo, G. Qu, T.J. Sferra, R. Clark, R. Chen, P.R. Johnson, rodents and nonhuman primates, Adv. Pharmacol. 42 (1998) 911–
Adeno-associated virus-mediated gene transfer to the brain: duration 915.
and modulation of expression, Hum. Gene Ther. 10 (1999) 201– [26] M. Gossen, H. Bujard, Tight control of gene expression in
mam-213. malian cells by tetracycline-responsive promoters, Proc. Natl. Acad.
[43] R.J. Mandel, K.G. Rendahl, S.K. Spratt, R.O. Snyder, L.K. Cohen, Sci. USA 89 (1992) 5547–5551.
S.E. Leff, Characterization of intrastriatal recombinant adeno-associ-[27] S. Hardy, M. Kitamura, T. Harris-Stansil, Y. Dai, M.L. Phipps,
ated virus-mediated gene transfer of human tyrosine hydroxylase Construction of adenovirus vectors through Cre-lox recombination,
and human GTP-cyclohydrolase I in a rat model of Parkinson’s J. Virol. 71 (1997) 1842–1849.
disease, J. Neurosci. 18 (1998) 4271–4284. [28] J.C. Johnston, M. Gasmi, L.E. Lim, J.H. Elder, J.K. Yee, D.J. Jolly,
[44] R.J. Mandel, R.O. Snyder, S.E. Leff, Recombinant adeno-associated K.P. Campbell, B.L. Davidson, S.L. Sauter, Minimum requirements
viral vector-mediated glial cell line-derived neurotrophic factor gene for efficient transduction of dividing and nondividing cells by feline
transfer protects nigral dopamine neurons after onset of progressive immunodeficiency virus vectors, J. Virol. 73 (1999) 4991–5000.
degeneration in a rat model of Parkinson’s disease, Exp. Neurol. 160 [29] T. Kafri, H. van Praag, F.H. Gage, I.M. Verma, Lentiviral vectors:
(1999) 205–214. regulated gene expression, Mol. Ther. 1 (2000) 516–521.
[45] R.J. Mandel, S.K. Spratt, R.O. Snyder, S.E. Leff, Midbrain injection [30] K. Kajiwara, A.P. Byrnes, H.M. Charlton, M.J. Wood, K.J. Wood,
of recombinant adeno-associated virus encoding rat glial cell line-Immune responses to adenoviral vectors during gene transfer in the
derived neurotrophic factor protects nigral neurons in a progressive brain, Hum. Gene Ther. 8 (1997) 253–265.
6-hydroxydopamine-induced degeneration model of Parkinson’s [31] M.G. Kaplitt, P. Leone, R.J. Samulski, X. Xiao, D.W. Pfaff, K.L.
disease in rats, Proc. Natl. Acad. Sci. USA 94 (1997) 14083–14088. O’Malley, M.J. During, Long-term gene expression and phenotypic
correction using adeno-associated virus vectors in the mammalian [46] K. Mitrophanous, S. Yoon, J. Rohll, D. Patil, F. Wilkes, V. Kim, S. brain, Nat. Genet. 8 (1994) 148–154. Kingsman, A. Kingsman, N. Mazarakis, Stable gene transfer to the
¨
[32] D. Kirik, C. Rosenblad, A. Bjorklund, Characterization of behavioral nervous system using a non-primate lentiviral vector, Gene Ther. 6 and neurodegenerative changes following partial lesions of the (1999) 1808–1818.
nigrostriatal dopamine system induced by intrastriatal 6-hydroxy- [47] H. Miyoshi, U. Blomer, M. Takahashi, F.H. Gage, I.M. Verma, dopamine in the rat, Exp. Neurol. 152 (1998) 259–277. Development of a self-inactivating lentivirus vector, J. Virol. 72
¨
[33] D. Kirik, C. Rosenblad, A. Bjorklund, Preservation of a functional (1998) 8150–8157.
nigrostriatal dopamine pathway by GDNF in the intrastriatal 6- [48] P.E. Monahan, R.J. Samulski, AAV vectors: is clinical success on the OHDA lesion model depends on site of administration of the trophic horizon?, Gene Ther. 7 (2000) 24–30.
factor, Eur. J. Neurosci. (in press). [49] N. Morral, W. O’Neal, K. Rice, M. Leland, J. Kaplan, P.A. Piedra, ¨
[34] D. Kirik, C. Rosenblad, A. Bjorklund, R.J. Mandel, Long-term H. Zhou, R.J. Parks, R. Velji, E. Aguilar-Cordova, S. Wadsworth, rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: F.L. Graham, S. Kochanek, K.D. Carey, A.L. Beaudet, Administra-intrastriatal but not intranigral transduction promotes functional tion of helper-dependent adenoviral vectors and sequential delivery regeneration in the lesioned nigrostriatal system, J. Neurosci. 20 of different vector serotype for long-term liver-directed gene transfer (2000) 4686–4700. in baboons, Proc. Natl. Acad. Sci. USA 96 (1999) 12816–12821. [35] R.L. Klein, E.M. Meyer, A.L. Peel, S. Zolotukhin, C. Meyers, N. [50] N. Muzyczka, Use of adeno-associated virus as a general
transduc-Muzyczka, M.A. King, Neuron-specific transduction in the rat tion vector for mammalian cells, Curr. Top. Microbiol. Immunol. septohippocampal or nigrostriatal pathway by recombinant adeno- 158 (1992) 97–129.
associated virus vectors, Exp. Neurol. 150 (1998) 183–194. [51] L. Naldini, U. Blomer, F.H. Gage, D. Trono, I.M. Verma, Efficient [36] J.H. Kordower, J. Bloch, S.Y. Ma, Y. Chu, S. Palfi, B.Z. Roitberg, transfer, integration, and sustained long-term expression of the M. Emborg, P. Hantraye, N. Deglon, P. Aebischer, Lentiviral gene transgene in adult rat brains injected with a lentiviral vector, Proc. transfer to the nonhuman primate brain, Exp. Neurol. 160 (1999) Natl. Acad. Sci. USA 93 (1996) 11382–11388.
1–16. [52] L. Naldini, U. Blomer, P. Gallay, D. Ory, R. Mulligan, F.H. Gage, [37] J.H. Kordower, M. Emborg, J. Bloch, S.Y. Ma, Y. Chu, L. Leventhal, I.M. Verma, D. Trono, In vivo gene delivery and stable transduction J. McBride, E.-Y. Chen, S. Palfi, B.Z. Roitberg, W.D. Brown, J.E. of nondividing cells by a lentiviral vector, Science 272 (1996) Holden, R. Pyzalski, M.D. Taylor, P. Carvey, Z. Ling, D. Trono, P. 263–267.
Hantraye, N. Deglon, P. Aebischer, Lentiviral vector-mediated [53] D.S. Ory, B.A. Neugeboren, R.C. Mulligan, A stable human-derived expression of GDNF prevents motor deficits and nigrostriatal packaging cell line for production of high titer retrovirus / vesicular degeneration in nonhuman primate models of Parkinson’s disease, stomatitis virus G pseudotypes, Proc. Natl. Acad. Sci. USA 93
[54] A.L. Peel, R.L. Klein, Adeno-associated virus vectors: activity and F. Wang, M.L. Metzker, R. Savino, C.T. Caskey, Optimization of applications in the CNS, J. Neurosci. Methods 98 (2000) 95–104. the helper-dependent adenovirus system for production and potency [55] A.L. Peel, S. Zolotukhin, G.W. Schrimsher, N. Muzyczka, P.J. Reier, in vivo, Proc. Natl. Acad. Sci. USA 97 (2000) 1002–1007.
Efficient transduction of green fluorescent protein in spinal cord [63] H. Sauer, W.H. Oertel, Progressive degeneration of nigrostriatal neurons using adeno-associated virus vectors containing cell type- dopamine neurons following intrastriatal terminal lesions with 6-specific promoters, Gene Ther. 4 (1997) 16–24. hydroxydopamine: a combined retrograde and immunocytochemical [56] E.M. Poeschla, F. Wong-Staal, D.J. Looney, Efficient transduction of study in the rat, Neuroscience 59 (1994) 401–415.
nondividing human cells by feline immunodeficiency virus lentiviral [64] D. Trono, Lentiviral vectors: turning a deadly foe into a therapeutic vectors, Nat. Med. 4 (1998) 354–357. agent, Gene Ther. 7 (2000) 20–23.
¨
[57] K.G. Rendahl, S.E. Leff, G.R. Otten, S.K. Spratt, D. Bohl, M. Van [65] C. Winkler, H. Sauer, C.S. Lee, A. Bjorklund, Short-term GDNF Roey, B.A. Donahue, L.K. Cohen, R.J. Mandel, O. Danos, R.O. treatment provides long-term rescue of lesioned nigral dopaminergic Snyder, Regulation of gene expression in vivo following transduc- neurons in a rat model of Parkinson’s disease, J. Neurosci. 16 tion by two separate rAAV vectors, Nat. Biotechnol. 16 (1998) (1996) 7206–7215.
757–761. [66] M.J. Wood, H.M. Charlton, K.J. Wood, K. Kajiwara, A.P. Byrnes, [58] C. Rosenblad, M. Gronborg, C. Hansen, N. Blom, M. Meyer, J. Immune responses to adenovirus vectors in the nervous system,
Johansen, L. Dago, D. Kirik, U.A. Patel, C. Lundberg, D. Trono, A. Trends Neurosci. 19 (1996) 497–501. ¨
Bjorklund, T.E. Johansen, In vivo protection of nigral dopamine [67] X. Xiao, J. Li, R.J. Samulski, Production of high-titer recombinant neurons by lentiviral gene transfer of the novel GDNF-family adeno-associated virus vectors in the absence of helper adenovirus, member Neublastin /Artemin, Mol. Cell. Neurosci. 15 (2000) 199– J. Virol. 72 (1998) 2224–2232.
214. [68] S. Zolotukhin, B.J. Byrne, E. Mason, I. Zolotukhin, M. Potter, K. ¨
[59] C. Rosenblad, D. Kirik, A. Bjorklund, Sequential administration of Chesnut, C. Summerford, R.J. Samulski, N. Muzyczka, Recombi-GDNF into the substantia nigra and striatum promotes dopamine nant adeno-associated virus purification using novel methods im-neuron survival and axonal sprouting but not striatal reinnervation or proves infectious titer and yield, Gene Ther. 6 (1999) 973–985. functional recovery in the partial 6-OHDA lesion model, Exp. [69] R. Zufferey, J.E. Donello, D. Trono, T.J. Hope, Woodchuck hepatitis Neurol. 161 (2000) 503–516. virus posttranscriptional regulatory element enhances expression of [60] C. Rosenblad, D. Kirik, B. Devaux, B. Moffat, H.S. Phillips, A. transgenes delivered by retroviral vectors, J. Virol. 73 (1999) 2886–
¨
Bjorklund, Protection and regeneration of nigral dopaminergic 2892.
neurons by neurturin or GDNF in a partial lesion model of [70] R. Zufferey, T. Dull, R.J. Mandel, A. Bukovsky, D. Quiroz, L. Parkinson’s disease after administration into the striatum or the Naldini, D. Trono, Self-inactivating lentivirus vector for safe and lateral ventricle, Eur. J. Neurosci. 11 (1999) 1554–1566. efficient in vivo gene delivery, J. Virol. 72 (1998) 9873–9880. [61] R.J. Samulski, M. Sally, N. Muzyczka, Adeno-associated viral [71] R. Zufferey, D. Nagy, R.J. Mandel, L. Naldini, D. Trono, Multiply
vectors, in: T. Friedmann (Ed.), The Development of Human Gene attenuated lentiviral vector achieves efficient gene delivery in vivo, Therapy, Cold Spring Harbor, New York, 1999, pp. 131–172. Nat. Biotechnol. 15 (1997) 871–875.