RICE BRAN PROTEIN HYDROLYSATE AND ITS
ANTIOXIDANT ACTIVITY
RICHARD
DEPARTMENT OF FOOD SCIENCE AND TECHNOLOGY
FACULTY OF AGRICULTURAL ENGINEERING AND TECHNOLOGY BOGOR AGRICULTURAL UNIVERSITY
STATEMENT LETTER OF MANUSCRIPT AND SOURCE
OF INFORMATION
I declare the truth that this manuscript entitled Rice Bran Protein Hydrolysate and Its Antioxidant Activity is my own work with guidance of the advisors and has not been submitted in any form at any college, except Bogor Agricultural University and Kasetsart University. Sources of information derived or quoted from published and unpublished works of other authors mentioned in the text and listed in the List of References at the end of this manuscript.
ABSTRACT
RICHARD. Rice Bran Protein Hydrolysate and Its Antioxidant Activity. Supervised by EKO HARI PURNOMO and WASAPORN CHANPUT
Rice bran was generally used as animal feed, while it contains many potential compounds. With hydrolysis reaction, small peptides can be obtained from rice bran protein, in which it showed higher antioxidative activity. The antioxidative activity of rice bran protein hydrolysate from fractionated rice bran was studied in this work. Albumin, globulin, glutelin and prolamin protein fractions were fractionated by its solubility and hydrolyzed with two type of enzyme, pepsin and proteaseM, at various digestion times. Albumin fraction showed high degree of hydrolysis for both enzyme hydrolysis. Protease M digestion showed different digesting ability towards different protein fractions, in which it has low impact on glutelin and prolamin fraction. Pepsin hydrolysis resulted in higher degree of hydrolysis compared to protease M digestion. After 30 minutes of hydrolysis time, hydrolysis reaction slowed down and antioxidant activity remained constant in pepsin hydrolysis. Prolamin and glutelin in pepsin digestion showed the highest activity of ABTS radical scavenging (4.02 mM and 3.93 mM TEAC) compared to the other hydrolysates. Rice bran protein hydrolysates showed much higher in antioxidant activity compared to the undigested rice bran protein which can be explained antioxidant activity derived from rice bran peptides.
Manuscript
In the partial fulfillment of the requirement for degree of Bachelor of Agricultural Technology
at
Department of Food Science and Technology
RICE BRAN PROTEIN HYDROLYSATE AND ITS
ANTIOXIDANT ACTIVITY
RICHARD
DEPARTMENT OF FOOD SCIENCE AND TECHNOLOGY
FACULTY OF AGRICULTURAL ENGINEERING AND TECHNOLOGY BOGOR AGRICULTURAL UNIVERSITY
Title : Rice Bran Protein Hydrolysate and Its Antioxidant Activity Name : Richard
Student ID : F24090083
Approved by
Dr Eko H.Purnomo, STP, MSc Academic Advisor
Acknowledged by
Dr Ir Feri Kusnandar, MSc
Head of Food Science and Technology Department
PREFACE
Praise to The Lord Jesus Christ for His mercy, bless, and love throughout the study in food science and finished this manuscript for partial fulfillment of bachelor degree.
1. I want to especially appreciate my parents, my brothers, my aunts, and all of my family for their continuing encouragement.
2. I would like to grateful thank and deeply indebted to Dr. Eko Hari Purnomo, my academic advisor in IPB, and Dr. Wasaporn Chanput, my research advisor in Kasetsart University, for the welcome, advices, encouragement, valuable times, and for my report correction.
3. And also my gratitude for Dr. Puspo Edi Giriwono and Dr. Nancy Dewi Yuliana, as my final examiner. Thanks for the time, support, help, and valuable words.
4. Grateful thank to Department of Food Science and Technology Bogor Agricultural University-Kasetsart University, who had provide the student exchange program.
5. I would like to deeply thank to Assoc. Prof. Dr. Chockchai Theerakulkait for kindly provided his laboratory to do this research work and also sincerely thank to Dr. Sashitorn Tongchitpakdee and Mr. Phu Thai who take care three of us Indonesian exchange students.
6. I want to heartfelt thank to my friends in laboratory (room 2315), P’Jin, P’ Man, P’Su, Mr. Faiq, P’Fern and especially P’ Oil, P’ Kiaw, and P’ Jip for the helping in my research work and the wonderful time together. 7. My deeply thanks to Anissa and Anan, who had become my friends and
gave me incredible moments since in Thailand. Unforgetful, Nut, Jeff, and Trung, big thanks for the unforgettable moment that we had.
8. Finally, thanks a bunch to all of my lovely friends #ITP46, the “Tampomas” group Aca, Hayyu, Seno, Sobih, Kyo, and so many others. And also special thanks to “DR33” Jody, Brian, Doni, Marcel, and Paul for the time that we pass. Thanks for the time that we had to learn together #KPA46: Stephan, Melisa, Bonita, Citra, Ira, Faithy, Gloria, Diego, and the others. Khob khun mak krub.
TABLE OF CONTENT
LIST OF TABLE viii
LIST OF FIGURE viii
LIST OF APPENDIXES viii
INTRODUCTION
Background 1
Research Objectives 3
METHOD
Materials 3
Equipments 3
Procedure 3
RESULT AND DISCUSSION
Protein Extraction 8
Degree of Hydrolysis 8
Protein Content 9
Total Phenolic Compound 10
Antioxidant Activity 11
CONCLUSION AND RECOMMENDATION
Conclusion 12
Recommendation 13
REFERENCES 13
APPENDICES 15
LIST OF TABLE
1 Protein content and protein yield of fractionated rice bran proteins,
crude rice bran protein and defatted rice brans 8
LIST OF FIGURE
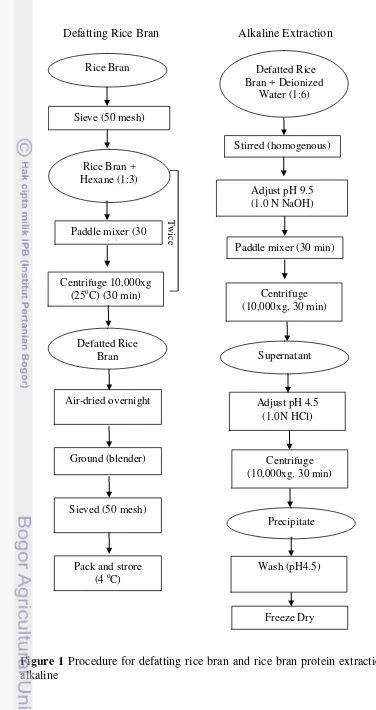
1 Procedure for defatting rice bran and rice bran protein extraction using
alkaline 5
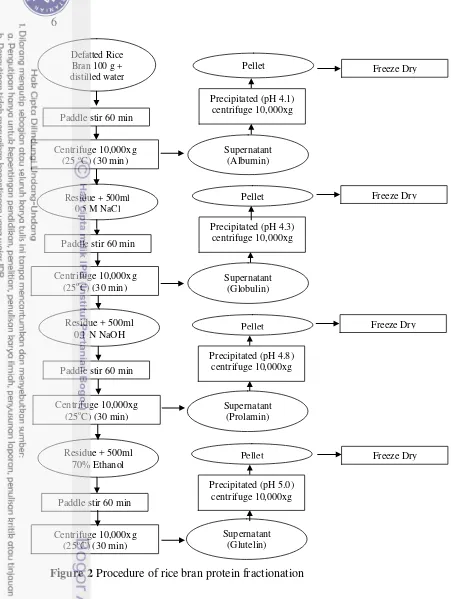
2 Procedure for rice bran protein fractionation 6
3 Degree of hydrolysis of RBP and the fraction hydrolysate with control 8 4 Protein content (Biuret) of RBP and the fraction hydrolysate with
control 9
5 Total phenolic compoud of RBP and the fraction hydrolysate with
control 10
6 Antioxidative activity of RBP and the fraction hydrolysate with control 11
LIST OF APPENDICES
1 Standard curve of Bovine Serum Albumin (BSA) by Biuret assay 15 2 Standard curve of Galic Acid by total phenolic content assay 15
INTRODUCTION
Background
Rice is one of the dominant staple food which has been commonly consumed in Asia. There is about 650 million metric tons of paddies produced annually among Asian countries (IRRI 2012). Nearly one-third of the paddy production comes from Southeast Asia countries, especially Indonesia, Thailand, and Vietnam. One of the by products from milling process is rice bran which is approximately 3-8% of rice grain weight (Amissah et al. 2003).
Rice bran is generally used for animal feed, while it contains many bioactive compounds, for instance, lipid, protein, diet-fibers, and antioxidant compounds, like tocopherol, tocotrienol, and oryzanol. Rice bran contains 15-22% lipids, 34.1-52.3% carbohydrates, 7-11.4% fiber, 6.6-9.9% ash, 8-12% moisture, and 10-16% protein (Fabian and Ju 2011). Due to high amount of fat in rice bran, thus it can undergo to rancid relatively fast by lipase activity (Xia et al. 2012). Rice bran oil have been extracted and commercialized as high quality cooking oil, which is high in poly- and mono-saturated fatty acids. Such fatty acids are good for human consumption and very heat stable (Amarasinghe et al. 2009).
Rice bran is also a good source of protein. Rice bran protein (RBP) contains approximately 3-4% lysine, an essential amino acid, which is highest among other cereal proteins (Shih et al. 1999). Protein digestibility of rice bran protein is greater than 90% and the protein efficiency ratio (PER) values of rice bran concentrate is relatively high, around 2.0 to 2.5, whereas casein is 2.5 (Wang et al. 1999). The RBP can be used as a source for protein ingredient, such as in infant food formula and restricted food for children with allergies, due to its hypoallergenicity. As a functional food, rice bran protein has been reported to show antioxidative activity. After hydrolyze rice bran protein with protease enzyme, small peptides can be obtained, in which they showed higher antioxidative activity (Adebiyi et al. 2008). Rice bran protein contained a lot of important amino acids like lysine, histidine, arginine, threonine, glycine, cystine, valine, methionine, isoleucine, leucine, tyrosine, and phenylalanine (Fabian and Ju 2011). Amino acids such as histidine, tyrosine, triptofan, methionine, and lysine had know potent to showed antioxidant activity (Saito et al. 2003). Therefore, rice bran protein hydrolysate (RBPH) can be possibly used as a new source of antioxidant-derived from food, as well as a good source of an inexpensive quality protein for functional food ingredient (Fabian and Ju 2011).
2
Bioactive peptides are protein fragments which have a positive impact on functions and conditions of living organism (Korhonen and Pihlanto 2006). Bioactive peptides can function as hormones or drugs, thus can modulate physiological function through binding to specific receptors leading to induction of physiological responses (Fitzgerald and Murray 2006). According to their bioactivities, bioactive peptides can function as antimicrobial, antithrombotic, antihypertensive, opioid, immunomodulatory, mineral binding, and antioxidative compound (Sharma et al. 2012). These peptides are inactive within the sequence of parent protein but can be released during proteolytic enzyme digestion, gastrointestinal digestion or food processing (Korhonen et al. 1998).
Protein hydrolysate, containing short peptides, is a product from protein hydrolysis either by enzymatic or by chemical hydrolysis. Protein hydrolysis cleaves peptide bond and break down protein into peptides and free amino acids depending on enzyme used in the reaction and hydrolysis time. Bioactive peptides usually contain 3-20 amino acid residues, and their activities are based on their amino acid composition and the peptide sequence (Pihlanto-Leppala 2001). It has been reported that peptides and protein hydrolysates both from plant and animal origins possess antioxidant activity, for example, peptides derived from milk protein (Pihlanto-Lepalla 2001), soybean (Chen et al. 1996), fish Alaska Pollack (Je et al. 2005), hen egg yolk (Xu et al. 2007), and many others. The antioxidant mechanism of the peptide has been proven to be metal chelation or free radical scavenging (Chen et al. 1996). The presence of these bioactive peptides may give a potential for human health promotion and disease risk reduction.
Rice bran contains four protein fractions i.e. albumin (39%), globulin (27%), glutelin (33%), and prolamin (1%) (Adebiyi et al. 2009). Globulin fraction from rice bran protein showed the strongest antioxidant activity compare to other fractions, whereas albumin fraction showed the best in reducing activity by FRAP assay (Chanput et al. 2009). The antioxidative activities either of amino acids or peptides have been investigated to gain insight into the antioxidative mechanism of protein hydrolysates. Nineteen antioxidative peptides isolated from rice bran globulin protein had been determined. These peptides were composed of 6–30 amino acid residues with molecular masses ranging from 670–3,611 Da, in which Tyr-Leu-Ala-Gly-Met-Asn sequence showed the highest antioxidative activity (Adebiyi et al. 2009). Hydrolysis of RBP using pepsin and protease M established high degree of hydrolysis value (Adebiyi et al. 2008). The use of α-amylase in oat bran displayed the highest antioxidant activity with ORAC assay (Jodayree et al. 2012).
3 disease (Young and Mine 2010). The whole part of the research project is aimed to solve this problem. The present study is the beginning of the whole project on the study anti-inflammatory effect of rice bran protein hydrolysates.
Research Objectives
This present study aims to produce rice bran protein hydrolysate (RBPH) using two different protease enzymes at different digesting time and determine degree of hydrolysis of RBPH and their antioxidative activity.
METHOD
Materials
Rice bran was obtained from Thailand commercial rice cultivar, Khao Dawk Mali 105. The chemical used for the sample preparation and analysis were: hexane, 1.0 N NaOH, 1.0 N HCl, α-amylase, NaCl, ethanol, pepsin (1120 units/mg) , protease M (500 units/g), o-phthaldialdehyde (OPA), sodium dodecyl sulfate (SDS), β-mercaptoethanol, sodium tetraborat, methanol, galic acid, Folin– Ciocalteau reagent, sodium carbonat, KH2PO4, Na2HPO4, KCl, K2S4O8, (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylicacid) Trolox, and 2,2'-azino-bis(3-ethylbenzo thiazoline-6-sulphonic acid) ABTS. There were also other chemical reagents needed to perform for protein determination using Kjeldhal and Biuret method.
Equipments
The equipments for this research are 50 mesh sieve, analytical balance, pipettes, micropipettes, paddle mixer, centrifuge, blender, waterbath shaker, microplate, microplate reader (TECAN Infinite 200 PRO), freeze drier, glassware, and equipments for Kjeldhal and moisture content assay.
Procedure
Defatting Rice Bran (Wang et.al 1999)
4
Alkaline Protein Extraction (Gnanasambandam and Hettiarachchy 1995)
Defatted rice bran (100 g) was dispersed in distilled deionized water with a ratio 1:6 (w/v). The pH was adjusted to 9.5 using 1.0 N NaOH and stirred with a paddle stirrer for 30 min, after that centrifuged at 10,000xg for 30 min at 25oC. The supernatant was adjusted to pH 4.5 with 1.0N HCl and was centrifuged again at 10,000xg for 30min at 25oC. The precipitate was washed with distilled deionized water (pH 4.5) and dispersed in a small amount of distilled water (pH 7.0) before freeze dry and store at -20 oC.
Fractionation Rice Bran Protein (Agboola et al. 2005)
The defatted rice bran (100 g) was fractionated by extracting with 500 ml of distilled water for 60 min and centrifuge at 10,000xg for 30 min to obtain the albumin fraction (supernatant). The residue (precipitate) from this step was extracted with 500 ml of 0.5 M NaCl to recover the globulin fraction (supernatant). The residue after extraction of globulin was further extracted with 0.1 N NaOH to obtain the glutelin fraction (supernatant), while the residue was extracted again with 70% ethanol to yield the prolamin fraction. The supernatants from albumin, globulin, glutelin, and prolamin fractions are adjusted with 0.1 M HCl to their isoelectric pH (4.1, 4.3, 4.8, and 5.0, respectively) to precipitate the proteins. The precipitated proteins were collected by centrifugation at 10,000xg for 30 min. The precipitates were washed with distilled water. All fractions were evaporated to remove either water or ethanol by freeze-dried and keep at -20 oC. Figure 2 shows the scheme of rice bran protein fractionation. Protein content in extracted RBP and FRBP was determined using Kjeldahl method (AOAC Official Method, 960.52, 1995) with conversion factor 5.95. The protein yield was obtained by multiplied the freeze dried protein isolate weight with the protein content.
Hydrolysis of Rice Bran Protein (Chanput et al. 2009)
The freeze-dried rice bran protein and its fractions (1 g) were suspended at 1% (w/v) protein in distilled water and adjusted to pH 2 for pepsin digestion and at pH 3 for protease M digestion. The protein solutions were mixed with enzyme where the pepsin to protein ratio was 1:100 (w/w) and 1:25 (w/w) of protease M. The pepsin mixtures and protease M mixtures were incubated at 37 oC and 50 oC for 0.5, 1, 2, and 4 hr. The enzymes were inactivated by heating in water bath 95 o
5
Figure 1 Procedure for defatting rice bran and rice bran protein extraction using alkaline
T
w
ic
e
Rice Bran + Hexane (1:3)
Rice Bran
Centrifuge 10,000xg (25oC) (30 min)
Sieve (50 mesh)
Paddle mixer (30 min)
Air-dried overnight Defatted Rice
Bran
Ground (blender)
Sieved (50 mesh)
Pack and strore (4 oC)
Defatting Rice Bran Alkaline Extraction
Paddle mixer (30 min) Stirred (homogenous)
Defatted Rice Bran + Deionized
Water (1:6)
Adjust pH 9.5 (1.0 N NaOH)
Centrifuge (10,000xg, 30 min)
Adjust pH 4.5 (1.0N HCl)
Precipitate
Wash (pH4.5)
Freeze Dry Centrifuge (10,000xg, 30 min)
6
Figure 2 Procedure of rice bran protein fractionation
Degree of Hydrolysis (Adebiyi 2008)
The o-phthaldialdehyde (OPA) reagent was prepared by combining the following reagents. The 25 mL of 100 mM sodium tetraborate, 2.5 mL of 20%
SDS (w/w) and 40 mg of OPA (dissolved in 1 mL of methanol) and 100 µL of β
7 hydrolysates (50 µL) was added directly to 2 mL of OPA reagent in a cuvette, the solution mixed briefly and incubated for 2 min at ambient temperature. The absorbance was measured at 340 nm using a spectrometer. The percentage of degree hydrolysis was calculated using the equation:
Degree of hydrolysis = (Mw.Δ340nm)/ (d.ε .p) x100
Where Mw is the averaged molecular weight of amino acids (120), Δ340nm is the absorbance at 340 nm, d the dilution factor, 1/41, ε the constant value (6000 m-1 cm-1), p is the mg/mL (10).
Determination of Protein Content (Chanput et al. 2009)
Biuret reagent consisting of 2.25 g sodium potassium tartrate, 0.75 g copper sulfate (CuSO4 .5H2O) and 1.25 g potassium iodine, was prepared by dissolving in the above order in 100 ml of 0.2 M NaOH. The volume of the solution was dilute up to 250 ml with distilled water. A 5 ml portion of biuret reagent was then mixed with 1 ml of standard protein or sample and left at room temperature for 30 min. Standard protein solution was prepared by using bovine serum albumin (BSA) at a concentration of 1–10 mg/ml. The absorbance was read at 540 nm against the reagent blank
Total Phenolic Compound (TPC) (Zhou and Yu 2004)
Standard solution was prepared using galic acid solution at 10 concentrations ranged from 200–2000 µg/L. The reaction mixture contained 50 µ l of either standard or sample solution, 250 µl of freshly prepared Folin–Ciocalteau reagent, and 3 ml of distilled water. The reaction mixture left at room temperature for 10 min, thereafter 750 µL of 20% sodium carbonate was added. The solution was mixed and incubated in for 2 hours at 25 oC. The absorbance at 765 nm was measured by spectrometer and distilled water was used as a blank.
Antioxidant Activity (Maruffo-estrada et al. 2012)
The 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was dissolved at a 2 mM concentration in 50 mL phosphate buffered saline (prepared from 4.0908 g NaCl, 0.1347 g KH2PO4, 0.7098 g Na2HPO4, and 0.0749 g KCl dissolved in 500 mL distilled water). The pH was adjusted to 7.4 with NaOH. Seventy mM K2S4O8 solution in distilled water was prepared. ABTS radical cation was produced by reacting 10 mL of ABTS stock solution with 40 µL K2S4O8 solution and stood the mixture in the dark at room temperature for 16–17 hr before use. The radical stability in this form lasts for more than 2 days when stored in dark at room temperature.
8
All analyses were performed in duplicate. The percentage decrease in absorbance at 734 nm was calculated and plotted as a function of the Trolox concentration for the standard reference data. The antioxidant activity of the tested samples was expressed as inhibition percentage (%Is) by calculation using the below equation: %Is = (AB - AA)/AB x100 %
where AB is absorbance of the blank sample (t = 0), and AA is absorbance of the sample with antioxidant after 6 min.
Statistical Analysis
All analyses were performed in duplicate and data were report as means and SD. Error bars represent standard deviations.
RESULT AND DISCUSSION
Protein Extraction
Fractionation of rice bran proteins was followed by Osborne method (Agboola 2005), in which four rice bran proteins fractions were obtained base on its solubility. The defatted rice bran from Khao Dawk Mali 105 variety contains 18.76% protein. Protein content in each fraction and its yield on dry basis are showed in Table 1. Glutelin showed the highest yield of protein that can be extracted, while prolamin showed the lowest yield.
Table 1 Protein content and protein yield of fractionated rice bran proteins, crude rice bran protein and defatted rice brans
Fraction Protein
Content (% db)
Yield (g/100g DRB)
Albumin 73.48 1.61
Globulin 92.57 1.97
Glutelin 77.78 5.36
Prolamin 59.08 0.36
Crude rice bran protein (CRBP) 74.29 8.80
Defatted rice bran (DRB) 18.76 -
Degree of Hydrolysis
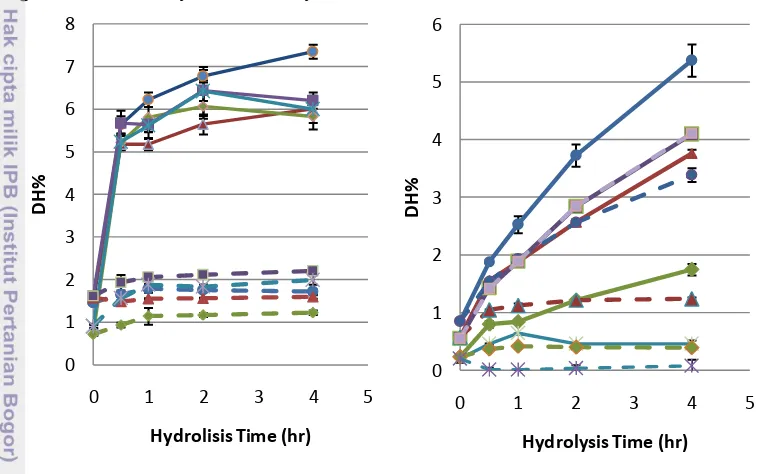
9 defined higher degree of hydrolysis compared to the control sample which was undigest by pepsin enzyme. Protease M digestion showed a selective activity for each protein fraction, especially the low impact to glutelin and prolamin fraction. Adebiyi et al. (2009) also reported glutelin and prolamin rice bran protein fraction from Hitomebore rice variety were more resistant for hydrolysis. Crude rice bran protein and its control sample showed a similar result in degree of hydrolysis. The CRBP were not affected by protease M digestion. Its degree of hydrolysis increase may be caused by the incubation condition rather than enzyme activity. From Figure 3, that could be stated albumin from rice bran protein was the most digested fraction by both of enzymes.
Figure 3 Degree of Hydrolysis of RBP and the fraction hydrolysate by pepsin (left) and protease M (right). Albumin ( ), Globulin ( ), Glutelin ( ), CRBP ( ), and Prolamin ( ). Dashed line showed the control for ever y fraction of rice bran protein. Error bars represent standard deviations
Protein Content using Biuret Method
10
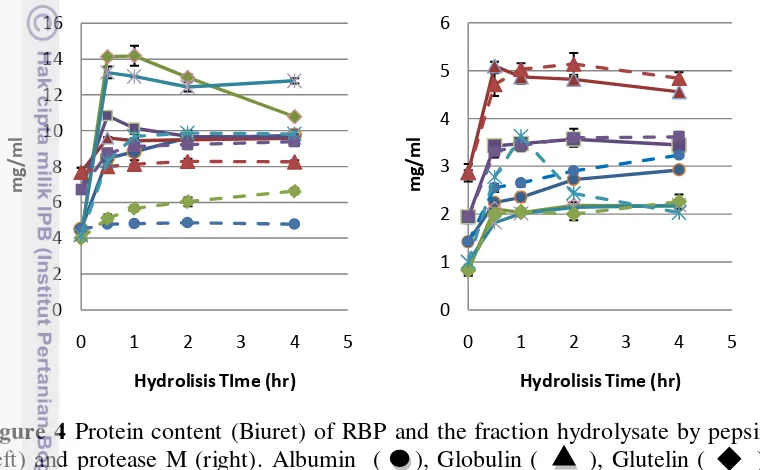
caused by hydrolysate from protease M was break down to amino acid and less containing peptide bonds. Adebiyi et al. (2009) reported that, protease M hydrolyze protein into large numbers of small peptides. Protease M contains both endoprotease and exoprotease activities which can cleave protein to peptides and free amino acid, while pepsin was endoprotease type-enzyme which only cleaves protein into peptides. However, long digestion time can cleave proteins into free amino acid resulted in lower detected protein content (peptide bonds).
Figure 4 Protein content (Biuret) of RBP and the fraction hydrolysate by pepsin (left) and protease M (right). Albumin ( ), Globulin ( ), Glutelin ( ), CRBP ( ), and Prolamin ( ). Dashed line showed the control for every fraction of rice bran protein. Error bars represent standard deviations
Total Phenolic Content
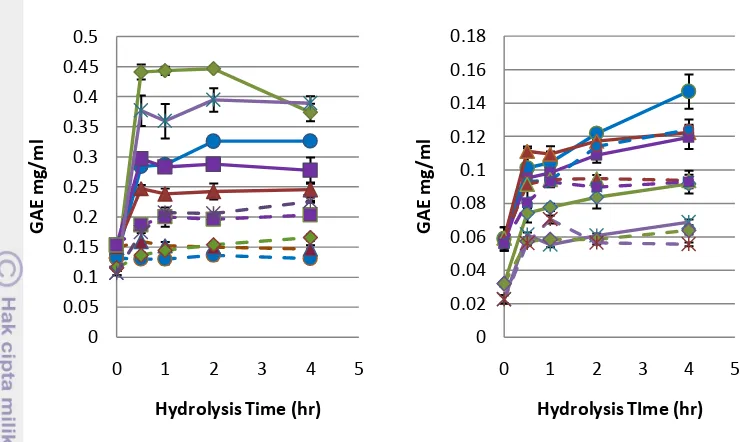
Total phenolic compound (TPC) showed a correlation with degree of hydrolysis. When %DH increases, the total phenolic compound also increases as showed in Figure 5. It can be explained that protease hydrolysis caused the phenolic compounds which have been trapped inside protein matrix was release. Glutelin fraction in pepsin hydrolysis showed the highest total phenolic compound, followed by prolamin, albumin, CRBP, and globulin. On the other hand, the highest gallic acid equivalent value in protease M was albumin while prolamin showed the lowest amount. The phenolic content in hydrolysate from protease M was lower than pepsin hydrolysate. This was because protease M contains exopetidase and endopeptidase which has low activity in releasing phenolic compounds inside the protein structure. Exopeptidase cleaves single amino acids from the end of polypeptide chain while endopeptidase cleaves peptide bonds in the interior of polypeptide chain producing various peptides (Hamada 2000). The temperature used for enzymatic digestion using protease M and pepsin were 50 oC and 37 oC respectively. Higher digestion temperature of protease M might cause degradation of heat-sensitive phenolic compounds.
11
Figure 5 Total phenolic compoud of RBP and the fraction hydrolysate by pepsin (left) and protease M (right). Albumin ( ), Globulin ( ), Glutelin ( ), CRBP ( ), and Prolamin ( ). Dashed line showed the control for ever y fraction of rice bran protein. Error bars represent standard deviations
Antioxidant activity
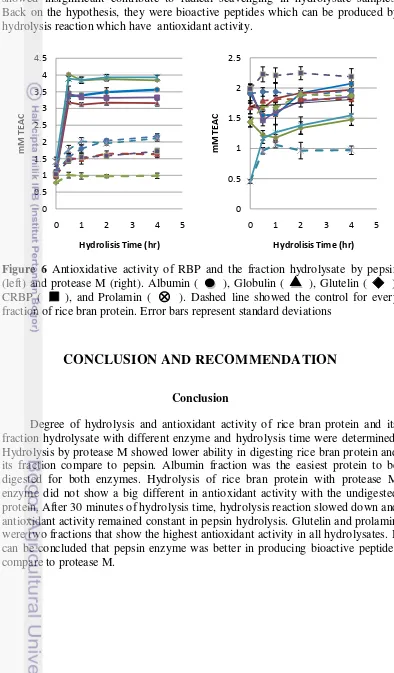
Antioxidant activity was determined by ABTS radical scavenging activity and expressed by trolox equivalent antioxidant capacity (TEAC). Protein hydrolysate obtained from pepsin digestion showed higher antioxidant capacity as compared to protease M digestion. Prolamin and glutelin in pepsin digestion showed the highest antioxidative capacity. The antioxidant activity of pepsin digestion did not show an obvious difference after 30 minutes of hydrolysis for every protein fraction. This was correlated to the sharp increase of hydrolysis reaction in the first 30 minutes. Hydrolysates obtained by protease M did not show a consistent trend between antioxidant capacity and hydrolysis time. Exopeptidase in protease M showed strong activity and the result in big amount of amino acids which did not contribute to antioxidant activity. In the other hand, pepsin contain endopeptidase which produce peptides, not amide. Crude rice bran protein and glutelin fraction hydrolysates showed lower antioxidant activity than undigested protein. There were free antioxidative peptides in the protein sample digested by protease M to become amino acids. This caused decrease of antioxidant activity after hydrolysis. Adebiyi et al. (2009) reported protease M digestion in rice bran protein caused high degree of hydrolysis resulting in low antioxidative activity.
Zhou and Yu (2004) found that total phenolic compound from Akron wheat bran extracted with 50% acetone contained 2.63 ± s and antioxidant activity measured by ABTS radical scavenging was 15.26 ± 0.29 µmol/g bran. Awika et.al (2003) showed phenolic compound in white bran of sorghum was 5 mg GAE/g and the antioxidant activity measured by ABTS method in was 28 µ mol/g sample. Rice bran protein hydrolysates that produced in this research contained between 0.1-0.45 GAE mg/ml for pepsin and 0.02-0.15 GAE mg/ml for protease M. The antioxidant activity measured by ABTS was 1.0-4.0 mMol TEAC for pepsin and
12
0.5-2.0 mMol TEAC for protease M. This means there are a lot of non-phenolic antioxidants that contribute to scavenging of ABTS radical. Phenolic compound showed insignificant contribute to radical scavenging in hydrolysate samples. Back on the hypothesis, they were bioactive peptides which can be produced by hydrolysis reaction which have antioxidant activity.
Figure 6 Antioxidative activity of RBP and the fraction hydrolysate by pepsin (left) and protease M (right). Albumin ( ), Globulin ( ), Glutelin ( ), CRBP ( ), and Prolamin ( ). Dashed line showed the control for every fraction of rice bran protein. Error bars represent standard deviations
CONCLUSION AND RECOMMENDATION
Conclusion
13 Recommendation
Further research was needed to determine the peptides in hydrolysates by using SDS-PAGE. There was also a need to know other compounds contained in the hydrolysates which may contribute to antioxidant activity. The Pepsin-hydrolysates can be choosen for further study.
REFERENCE
Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K. 2008. Purification and characterization of antioxidative peptides from unfractionated rice bran protein hydrolysates. Int. J. of Food Sci. Technol. 43: 35–43
Adebiyi AP, Adebiyi, AO, Ogawa T, Muramoto K. 2009. Isolation and characterization of protein fractions from deoiled rice bran. Eur Food Res Technol. 228:553–563
Agboola S, Darren NG, Mills D. 2005.Characterisation and functional properties of Australian rice protein isolates. Journal of Cereal Science. 41: 283–290. Ajila CM, Naidu KA, Bhat SG, Prasada-Rao UJS. 2007. Bioactive compounds
and antioxidant potential of mango peel extract. Food Chemistry.105 :982–988. Amarasinghe BMWPK, Kumarasiri MPM, Gangodavilage NC. 2009. Effect of method of stabilization on aqueous extraction of rice bran oil.Food and Bioproducts Processing. 87(2): 108-114.
Amissah JGN, Ellis WO, Oduro I, Manful JT. 2003. Nutrient composition of bran from new rice varieties under study in Ghana. Food Control. 14: 21–24.
Awika JM, Rooney LW, Wu XL, Prior RL, Zevallos LC. 2003. Screening Methods To Measure Antioxidant Activity of Sorghum (Sorghum bicolor) and Sorghum Products. J. Agric. Food Chem. 51; 6657-6662.
Chanput W, Theerakulkait C, Nakai S. 2009. Antioxidative properties of partially purified barley hordein, rice bran protein fractions and their hydrolysates. J Cereal Sci. 49: 422-428
Chen HM, Muramoto K, Yamauchi F, Nokihara K. 1996. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. Journal of Agricultural and Food Chemistry, 44(9): 2619-2623.
Fabian C, and Ju YH. 2011. A review on rice bran protein: its properties and extraction methods. Critical reviews in food science and nutrition. 51(9): 816-827.
Fitzgerald RJ and Murray BA. 2006. Bioactive peptides and lactic fermentations. International Journal of Dairy Technology. 59:118–125.
Gnanasambandam R and Heltiarachchy NS. 1995. Protein concentrates from unstabilized and stabilized rice bran: Preparation and properties. Journal of food science, 60(5): 1066-1069.
14
Je JY, Park PJ, Kim SK. 2005. Antioxidant activity of a peptide isolated from Alaska Pollack (Theragra chalcogrammaframe) protein hydrolysate. Food Research International. 38(1): 45-50.
Jodayree S, Smith JC, Tsopmo A. 2012. Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Research International. 46: 69–75
Korhonen H and Pihlanto A. 2006. Bioactive peptides: Production and functionality. Int.Dairy J.16 : 945-960.
Korhonen H, Pihlanto-Leppala A, Rantamaki P, Tupasela T. 1998. Impact of processing on bioactive proteins and peptides. Trends in Food Science and Technology. 9: 307–319.
Marrufo-Estrada DM, Segura-Campos MR, Chel-Guerrero LA, Betancur-Ancona DA. 2012. Defatted Jatrophacurcas flour and protein isolate as materials for protein hydrolysates with biological activity. Food chemistry. 138 :77–83 Pihlanto-Leppala A. 2001. Bioactive peptides derived from bovine proteins:
Opiood and ACE-inhibitory peptides. Trends Food Sci. Technol. 11: 347-356 Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. 1995. Free radicals and
phagocyticcells. FASEB J. 9 ( 2 ): 200 –209.
Sapan CV, Lundblad RL, Price NC. 1999. Colorimetric protein assay techniques. BiotechnolApplBiochem 29 ; 99 -108.
Saito K, Jin DH, Ogawa T, Muramoto K, Hatakeyama E, Yasuhara T, Nokihara K. 2003. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J Agric Food Chem 51 ( 12 ): 3668 – 3674.
Sharma S, Singh R, Rana S. 2011. Bioactive peptides: A review. Int. J. Bioautomation. 15(4) :223-250.
Shih FF, Champagne ET, Daigle K, Zarins Z. 1999. Use of enzymes in the processing of protein products from rice bran and rice flour. Food/Nahrung. 43(1): 14-18.
Young D and Mine Y. 2010. Anti-inflammatory/Oxidative Stress Proteins and Peptides, in Bioactive Proteins and Peptides as Functional Foods and Nutraceuticals (eds Y. Mine, E. Li-Chan and B. Jiang).Wiley-Blackwell, Oxford, UK. doi: 10.1002/9780813811048.ch2
Wang M, Hettiarachchy NS, Qi M, Burks W, Siebenmorgen T. 1999. Preparation and functional properties of rice bran protein isolate. Journal of Agricultural and Food Chemistry. 47: 411–416.
[IRRI] International Rice Research Institute. 2012. World Rice Statistics [internet]. [referenced 2013 July 15]. Available from: http://ricestat.irri.org:8080/wrs2/ entrypoint.htm
Xu X, Katayama S, Mine Y. 2007. Antioxidant activity of tryptic digests of hen egg yolk phosvitin. J. Sci. Food Agric. 87: 2604–2608
Xia N, Wang J, Yang X, Yin X, Qi J, Hu L, Zhou X. 2012. Preparation and characterization of protein from heat-stabilized rice bran using hydrothermal cooking combined with amylase pretreatment. J. Food Eng. 110 :95–101 Zhou K and Yu L. 2004. Effect of extraction solvent on wheat bran antioxidant
15
APPENDICES
Appendix 1 Standard Curve of Bovine Serum Albumin (BSA) by Biuret assay
Appendix 2 Standard curve of Galic Acid by total phenolic compound assay
y = 0.024x + 0.074 R² = 0.999
0 0.1 0.2 0.3 0.4 0.5
0 5 10 15 20
A
b
so
rb
a
n
ce
BSA mg/ml
y = 1.415x + 0.059 R² = 0.999
0 0.5 1 1.5 2 2.5 3 3.5
0 0.5 1 1.5 2 2.5
A
b
so
rb
a
n
ce
16
Appendix 3 Standard curve of Trolox by ABTS assay
y = 0.205x + 0.002 R² = 0.999
0 0.2 0.4 0.6 0.8 1 1.2
0 1 2 3 4 5 6
A
b
so
rb
a
n
ce
17
AUTHOR BIOGRAPHY
Richard was born on 4th December 1990 in Medan, North Sumatra, Indonesia. He is the third son with two elder brothers from Mr. Sanjaya Lawer and Mrs. Rose Eny Henny. He finished his elementary school, junior high school, and senior high school in Methodist-2 Christian School Medan. In 2009, the author continued his further study in Bogor Agricultural University as a Food Science and Technology student.