Complete Genome Sequence of the Facultative Anaerobic
Magnetotactic Bacterium
Magnetospirillum
sp. strain AMB-1
TadashiMatsunaga,* YoshikoOkamura, Yorikane Fukuda, Aris TriWahyudi, YaekoMurase, and Haruko Takeyama
Department of Biotechnology, Tokyo University of Agriculture and Technology, Koganei, Tokyo, Japan
(Received 14 March 2005)
Abstract
Magnetospirillum sp. strain AMB-1 is a Gram-negative a-proteobacterium that synthesizes
nano-sized magnetites, referred to as magnetosomes, aligned intracellularly in a chain. The potential of this nano-sized material is growing and will be applicable to broad research areas. It has been expected that genome analysis would elucidate the mechanism of magnetosome formation by magnetic bacteria. Here we describe the genome ofMagnetospirillumsp. AMB-1 wild type, which consists of a single circular chromosome of 4 967 148 bp. For identification of genes required for magnetosome formation, transposon mutagenesis and determination of magnetosome membrane proteins were performed. Analysis of a non-magnetic transposon mutant library focused on three unknown genes from 2752 unknown genes and three genes from 205 signal transduction genes. Partial proteome analysis of the magnetosome membrane revealed that the membrane contains numerous oxidation/reduction proteins and a signal response regulator that may function in magnetotaxis. Thus, oxidation/reduction proteins and elaborate multidomain signaling proteins were analyzed. This comprehensive genome analysis will enable resolution of the mechanisms of magnetosome formation and provide a template to determine how magnetic bacteria maintain a species-specific, nano-sized, magnetic single domain and paramagnetic morphology.
Key words:magnetotactic bacteria; biomineralization; magnetosome
1. Introduction
Magnetic bacteria contribute to the global iron cycle by acquiring iron and converting it into magnetite (Fe3O4)1
or greigite (Fe3S4),2 which accumulates in intracellular
structures known as magnetosomes. Biominerals possess highly ordered, elaborate morphologies since many biological factors strictly control the nucleation and the assembly of single crystals into complex structures.3The
most significant physical feature of a bacterial magnetic particle is its magnetic properties. Each magnetic nano-particle synthesized by magnetic bacteria possesses a magnetic dipole moment with a single magnetic domain.4 The magnetite crystal growth and magnetic anisotropy energy must be strictly controlled by biological factors in magnetic bacteria. Contrary to artificial magnetic
particles, magnetosomes can be easily dispersed in aqueous solutions because of their enclosing membrane.5 Therefore, magnetosomes have vast potential for various technological applications, and the molecular mechanism of their formation is of particular interest. Magnetic par-ticles fromMagnetospirillum sp. AMB-1 have been util-ized as immunoassay platforms for various environmental pollutants including endocrine disruptors,6–9as a means of recovering mRNA10and DNA11,12and as a carrier for DNA13 in our previous reports. A variety of functional proteins, such as enzymes and antibodies, can be dis-played on the bacterial magnetic particles through recombination in Magnetospirillum sp. AMB-1.7,14,15
Clarification of magnetite biomineralization pathways would contribute to further biotechnological application studies inMagnetospirillumsp. AMB-1 and the potential of this material is growing and will be applicable to broad research areas. In this paper, the entire genome of
Magnetospirillum sp. AMB-1 was sequenced, annotated and analyzed.
Communicated by Masahiro Sugiura
* To whom correspondence should be addressed. Tel.þ 81-423-88-7020, Fax.þ81-423-85-7713, Email: [email protected] ÓThe Author 2005. Kazusa DNA Research Institute.
2. Materials and Methods
2.1. Construction, isolation and sequencing of
small-insert and large-small-insert libraries
Magnetospirillumsp. AMB-1, isolated from fresh water
in Tokyo, Japan,16 is available from ATCCATCC
#700264. Genomic DNA ofMagnetospirillumsp. AMB-1
was isolated according to a standard protocol.17Isolated
DNA from Magnetospirillum sp. AMB-1 was sequenced
using a conventional whole genome shotgun strategy.18
Briefly, random 2-kb DNA fragments were isolated after mechanical shearing. These gel-extracted fragments were concentrated, end-repaired and cloned into pUC18 at the
SmaI site. Double-ended plasmid sequencing reactions
were performed using DYEnamic ETterminator chem-istry (Americium Bioscience), and sequencing ladders were resolved on MegaBACE1000 and MegaBACE4000 (Amasham Bioscience) automated DNA sequencers. One round (115 200 reads) of small-insert library sequencing generated roughly a 10-fold redundancy.
A large-insert (15 kb) Charomid library was also
con-structed by MboI partial digestion of genomic DNA
followed by cloning into the Charomid9-28 vector19 at
theBamHI site. The Charomids provided a minimal
scaf-fold to order and orient sequences across assembly gaps.
2.2. Sequence assembly and gap closure
Sequence data were converted to ESD data with Cimarron 1.53 Slim Phredify and Cimarron 3.12 Slim Phredify present on the automated DNA sequencer. Data were processed with Phred for base calling, and data quality was assessed before assembly using CAP4 (Paracel, Pasadena, CA, USA). Gaps were closed by primer walking on gap-spanning library clones (identified using linking information from forward and reverse reads). Alternatively, remaining physical gaps were closed by shotgun sequencing of PCR products using primers designed from terminal sequences of scaffolds arranged in order.
2.3. Sequence analysis and annotation
Gene modeling was performed using XanaGen
(Kawasaki, Kanagawa, Japan) software. The results were compiled, and searches of the basic local alignment search tool (BLAST) for proteins and GenBank’s non-redundant database were compared. Gene models that
overlapped by>10% of their length were flagged, giving
preference to genes with a BLASTmatch. The revised gene/protein set was searched against the XanaGenome
(incorporating COGs, SWISS-PROT, PROSITE,
PRINTS and Pfam), KEGG GENES and TC-DB (http://tcdb.ucsd.edu/tcdb/database.php). From these results, categorizations were developed using the COGs hierarchies. Initial criteria for automated functional
assignment required >80% of the length of the match
for BLASTP alignments with an E value<1 ·101.
All completed and draft genome sequences (accession numbers in Supplementary Table 1 is available at www. dnares.oxfordjournals.org) were reannotated according to this manner as well.
2.4. Nucleotide sequence accession number
The sequence of the complete genome of
Magnetospirillum sp. AMB-1 is available under DDBJ
accession number AP007255.
2.5. Analysis of transposon mutant library
Mutants were generated by using Tn5
mini-transposon.20 The target sequence of mini-Tn5 in the
genome was 50-GGC CAG GGC-30. The DNA sequences
flanking the transposon-interrupted region were obtained
by inverse PCR20using primers (R): 50
-ACA CTG ATG
AAT GTT CCG TTG-30
and (F): 50
-ACC TGC AGG
CAT GCA AGC TTC-30
. The resulting PCR product was cloned into the vector pGEM-T-easy (pGEM-T-easy Vector System, PROMEGA, WI, USA) and seq-uenced. The sequences were then aligned against the
whole genome database ofMagnetospirillum sp. AMB-1.
2.6. Two-dimensional polyacrylamide gel electrophoresis
and N-terminal amino acid sequence
Magnetosome membranes were dissolved in solubiliz-ing buffer (40 mM Tris base, 7 M urea, 2 M thiourea and 4% CHAPS). Magnetosome membrane proteins were separated in an immobilized dry strip gel (pH 3–10; 130 mm) using IPGphore (Amersham Bioscience).
After rehydration at 20C for 12 h, the strips were run
with a previously described program.21 The strips were
subjected to 2D- electrophoresis on a homogeneous SDS–polyacrylamide gel (12.5%). After gel electrophor-esis, the gel was electroblotted onto PDVF membrane,
Immobilon-PSQ (Millipore Corp.). The membrane was
stained with Coomassie brilliant blue R 250 and visible protein spots were excised. N-terminal amino acid sequen-cing was performed by automated Edman degradation
using a PPSQ-1 amino acid sequencing system
(Shimadzu, Kyoto, Japan). Resulting sequences were
aligned against the whole genome database of
Magnetospirillum sp. AMB-1.
3. Results and Discussion
3.1. General features of the genome of
Magnetospirillumsp. strain AMB-1
The genome ofMagnetospirillumsp. AMB-1 consists of
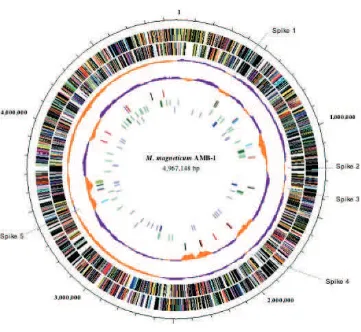
a single circular chromosome of 4 967 148 bp. Table 1 shows the general features of the genome. The physical map is shown in Figure 1. The GC content of
Magnetospirillum sp. AMB-1 genome is 65.1%. The GC
and several transposases were found in Spike 4. The GC content (Fig. 1, the fourth circle) further suggests hori-zontal gene transfer (HGT) because regions with below average GC content correspond to insertion sequence (IS) elements (Fig. 1, the fifth circle) or phage regions.
3.2. Repeat sequences
The genome contains 33 ISs, which consist of four multicopy and seven single-copy elements. IS elements are intensively localized in three specific regions of the genome (Fig. 1, the fifth circle, positions nt 429 860–
444 817; nt 691 208–692 355; and nt 3 526 739–
3 608 663). The IS-concentrated regions have a lower GC content than the average of the whole genome, sug-gestive of gene transfer from other bacteria or phage. The inversions of GC content, or spikes, are observed mainly in IS insertion positions.
There are nine regions that encode phage-related pro-teins, two of which lack a capsid gene. The GC content of these regions was as low as that of IS elements. Twenty-three proteins encoded within these regions were identified as integrase XerC or functional homologs. XerC is known to promote several DNA deletion
reac-tions. Interestingly, one XerC homolog (amb0926) is
located 2 kb and 102 kb upstream (positions nt 997 403–998 535 and nt 1 095 895–1 097 027, respectively) of two identical 1132-bp sequences. Both 1132-bp repet-itive sequences include truncated incomplete IS elements. The 100-kb region between the two repetitive sequences encodes magnetosome-specific proteins (see below). It is likely that this genomic island would be deleted via the XerC integrase and the two 1132-bp direct repeat, not via an IS element.
An 80-kb cluster encoding magnetosome-specific proteins, such as Mms6 and mamAB, was deficient in
a spontaneous non-magnetic mutant of M.
gryphyswal-dens MSR-1.22 Schubbe et al. determined that 35 kb
of the sequence of this 80-kb cluster contains one of a pair of IS 66 elements and suggested that the region
was removed because of IS element.22We obtained
spon-taneous non-magnetic mutant lacking the 100-kb region. The complete sequence in this study can explain that integrase recognized the 1132-bp direct repeats and deleted the DNA segment between the two sites.
3.3. Disrupted genes by transposon mutagenesis in
non-magnetic mutants
To date, genome sequence analyses have tended toward prediction only. To annotate genes with high reliability,
the analysis was performed with 1·101of E-value in
this study and half of the total genes were not annotated. Through usual analysis, only annotated genes would be subject to prediction. Transposon (Tn) mutagenesis can spotlight those of unknown genes function for
magneto-some formation. An Hþ
/Fe(II) antiporter, magA, was
isolated from a magnetosome depleted Tn5 mutant.23
Isolation and characterization of the genes that mediate magnetite formation in bacteria are prerequisites for determining the mechanisms of magnetic particle biosyn-thesis. To identify specific genes involved in magnetite synthesis, transposon mutagenesis was conducted in
Table 1.General features of theMagnetospirillumsp. AMB-1 genome.
Genome length (bp) 4 967 148
Plasmids none
Protein coding sequences (bp) 4 384 030 (88.26%) Average length of ORFs (bp) 961 GC contents (%)
Total Number of ORFs 4559 COG functional category*
Categorized number of genes 2290 Information storage and processing
Translation, ribosomal structure
Cell division and chromosome partitioning
27
Cell envelope biogenesis, outer membrane
186
Cell motility and secretion 83 Posttranslational modification,
protein turnover, chaperones
117
Inorganic ion transport and metabolism 171 Signal transduction 205 Metabolism
Energy production and conversion 226 Amino acid transport and metabolism 234 Nucleotide transport and metabolism 51 Carbohydrate transport and metabolism 123 Coenzyme metabolism 109
General function prediction only 315 Function unknown 168 Uncategorized number of genes 2269
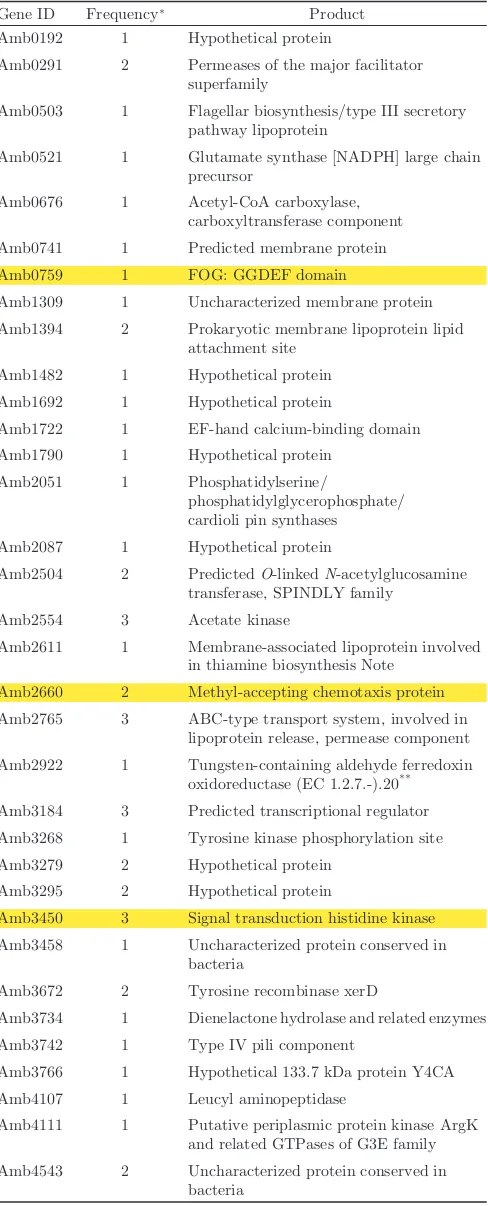
strain AMB-1.20Of 5762 Tn mutants, 69 were found to be defective for magnetosomes. The list of mutants and dis-rupted genes is shown in Table 2. Mapping results (Fig. 1, seventh circle) suggest that the genes required for mag-netosome formation are distributed throughout the genome. Based on the COG database, protein functions encoded by disrupted genes were categorized as signal transduction (six mutants), energy production and conversion (four mutants), cell envelope biogenesis and outer membrane (three mutants) or cell motility and secretion (three mutants). However, unknown genes or genes of unknown function were disrupted in most mutants. Therefore, these genes should be focused
among 2752 unknown genes. Interestingly, amb2554
(acetate kinase),amb2765(ABC-type transport system)
andamb3450(signal transduction histidine kinase) were
disrupted individually in three mutants. Moreover, among 205 genes categorized as having signal transduction
functions (Table 1), amb0759 (two mutants), amb2660
(two mutants) and amb3450 (three mutants) genes
were disrupted in two or three mutants. Therefore, these genes are likely to be magnetosome-related signal transduction genes.
3.4. Magnetosome membrane proteins
Magnetosome membrane proteins were identified
because a number of proteins expressed in situ were
expected to play a direct role in magnetite formation. Protein fractions prepared from magnetosome
mem-branes were separated by 2D-electrophoresis and >100
protein spots were analyzed by amino acid sequencing.
Based on the protein database of Magnetospirillum sp.
AMB-1, the genes and annotations were identified from determined sequences. Table 3 provides a list of these genes, and Figure 1 indicates the distribution of
Figure 1. Circular representation of the 4 967 148-bp genome ofMagnetospirillumsp. AMB-1. The outer and second circles represent predicted
ORFs on the plus and minus strands, respectively (salmon: translation, ribosomal structure and biogenesis; light blue: transcription; cyan: DNA replication, recombination and repair; turquoise: cell division; deep pink: post-translational modification, protein turnover and chaperones; olive drab: cell envelope biogenesis; purple: cell motility and secretion; forest green: inorganic ion transport and metabolism; magenta: signal transduction; red: energy production; sienna: carbohydrate transport and metabolism; yellow: amino acid transport; orange: nucleotide trans-port and metabolism; gold: co-enzyme transtrans-port and metabolism; dark blue: lipid metabolism; blue: secondary metabolites, transtrans-port and catabolism; gray: general function prediction only; black: function unclassified or unknown). The third circle represents GC skew: purple indicates>0, orange indicates<0. The fourth circle further represents GC content: purple indicates higher than average, orange indicates
magnetosome membrane (MM) proteins throughout the entire genome.
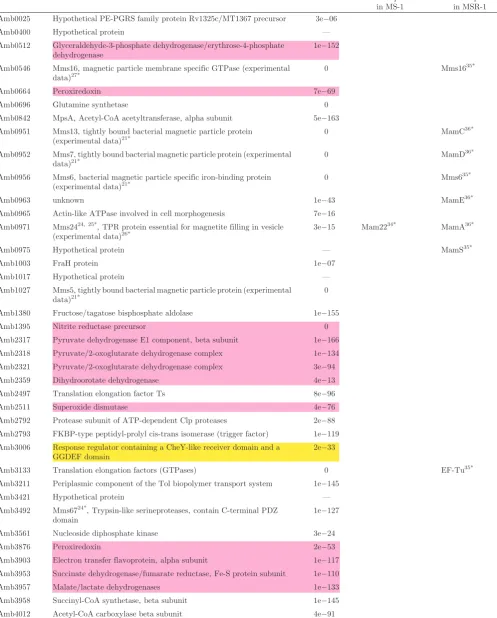
To date, eight proteins specific to the magnetosome
membrane in Magnetospirillum sp. AMB-1 have been
identified and reported.21,24–27A 24-kDa protein,
desig-nated Mms2424,25[corresponding to MamA in (26)], and
four proteins tightly bound to the magnetite crystal,
des-ignated Mms5, Mms6, Mms7 and Mms13,21were encoded
within the 100-kb region between the two repetitive sequences. Other magnetosome-specific proteins, such
as MpsA,24 a 67-kDa protein24 designated Mms67 and
Mms16,27are located at different loci.
Forty-eight proteins were identified as individual mag-netosome membrane proteins. Proteins related to oxida-tion/reduction were particularly prominent in this group,
comprising33% of the total. These proteins were similar
to respiratory chain components. They might be a part of the respiratory electron transfer chain, because the mag-netosome membrane would be derived from the cytoplas-mic membrane. Otherwise, an alternative electron transfer involved in iron oxidation/reduction would exist in the magnetosome membrane. Other unknown genes were also recognized as candidate functional factors.
A signal response regulator (amb3006) was identified on
the magnetosome membrane in this study. This protein might receive sensor signals related to magnetotaxis ori-ginating from the interaction between magnetosomes and the magnetic field. This response regulator will be described in the following section.
3.5. Iron oxidation/reduction
AlthoughMagnetospirillumsp. AMB-1 is a facultative
anaerobic bacterium, respiratory nitrate reduction allows the oxidation of a substrate under anaerobic conditions. The terminal electron acceptor may be Fe(III) via membrane-bound ferric reductase (amb3335). This enzyme is encoded in only 18 other eubacterial genomes,
including microaerobic magnetic bacteriumM.
magneto-tacticumMS-1. Specifically, ferric reductase activity has
been measured in strain MS-1.28Moreover, iron reduction
was coupled with nitrate reduction in both strains.29,30It
is suggested that electron flow branching from quinone reduced iron. The membrane potential derived from
electron transfer can be used in iron oxidoreduction.29
The number of oxidation/reduction proteins expressed on the magnetosome membranes is remarkable so that the genes encoding ferredoxin and cytochrome were analyzed. Strain AMB-1 possesses the most ferredoxin and related genes compared with 165 other eubacteria (Supplementary Table 2 is available at www.dnaresearch. oxfordjournals.org). Additionally, the number of cyto-chrome genes is comparable with that found in other bacteria containing multiple cytochrome genes among the 165 strains, with completely sequenced genomes (Supplementary Table 3 is available at www.dnaresearch. oxfordjournals.org). These enrichment genes would
Table 2.Disrupted genes by Transposon mutagenesis in
Magneto-spirillumsp. AMB-1.
Gene ID Frequency* Product
Amb0192 1 Hypothetical protein
Amb0291 2 Permeases of the major facilitator superfamily
Amb0503 1 Flagellar biosynthesis/type III secretory pathway lipoprotein
Amb0521 1 Glutamate synthase [NADPH] large chain precursor
Amb0676 1 Acetyl-CoA carboxylase, carboxyltransferase component Amb0741 1 Predicted membrane protein Amb0759 1 FOG: GGDEF domain
Amb1309 1 Uncharacterized membrane protein Amb1394 2 Prokaryotic membrane lipoprotein lipid
attachment site Amb1482 1 Hypothetical protein Amb1692 1 Hypothetical protein
Amb1722 1 EF-hand calcium-binding domain Amb1790 1 Hypothetical protein
Amb2051 1 Phosphatidylserine/
phosphatidylglycerophosphate/ cardioli pin synthases
Amb2087 1 Hypothetical protein
Amb2504 2 PredictedO-linkedN-acetylglucosamine transferase, SPINDLY family
Amb2554 3 Acetate kinase
Amb2611 1 Membrane-associated lipoprotein involved in thiamine biosynthesis Note
Amb2660 2 Methyl-accepting chemotaxis protein Amb2765 3 ABC-type transport system, involved in
lipoprotein release, permease component Amb2922 1 Tungsten-containing aldehyde ferredoxin
oxidoreductase (EC 1.2.7.-).20** Amb3184 3 Predicted transcriptional regulator Amb3268 1 Tyrosine kinase phosphorylation site Amb3279 2 Hypothetical protein
Amb3295 2 Hypothetical protein
Amb3450 3 Signal transduction histidine kinase Amb3458 1 Uncharacterized protein conserved in
bacteria
Amb3672 2 Tyrosine recombinase xerD
Amb3734 1 Dienelactone hydrolase and related enzymes Amb3742 1 Type IV pili component
Amb3766 1 Hypothetical 133.7 kDa protein Y4CA Amb4107 1 Leucyl aminopeptidase
Amb4111 1 Putative periplasmic protein kinase ArgK and related GTPases of G3E family Amb4543 2 Uncharacterized protein conserved in
bacteria
* Frequency is the disrupting number. ** Indicates reference number.
Table 3.List of the proteins and coding genes expressed on the magnetosome membrane.
Gene ID Product evalue Identical protein in MS-1
Identical protein in MSR-1 Amb0025 Hypothetical PE-PGRS family protein Rv1325c/MT1367 precursor 3e06
Amb0400 Hypothetical protein — Amb0512 Glyceraldehyde-3-phosphate dehydrogenase/erythrose-4-phosphate
dehydrogenase
1e152
Amb0546 Mms16, magnetic particle membrane specific GTPase (experimental data)27*
0 Mms1635*
Amb0664 Peroxiredoxin 7e69 Amb0696 Glutamine synthetase 0 Amb0842 MpsA, Acetyl-CoA acetyltransferase, alpha subunit 5e163 Amb0951 Mms13, tightly bound bacterial magnetic particle protein
(experimental data)21*
0 MamC36*
Amb0952 Mms7, tightly bound bacterial magnetic particle protein (experimental
data)21* 0 MamD
36*
Amb0956 Mms6, bacterial magnetic particle specific iron-binding protein
(experimental data)21* 0 Mms6
35*
Amb0963 unknown 1e43 MamE36*
Amb0965 Actin-like ATPase involved in cell morphogenesis 7e16 Amb0971 Mms2424, 25*, TPR protein essential for magnetite filling in vesicle
(experimental data)26* 3e
15 Mam2234* MamA36*
Amb0975 Hypothetical protein — MamS35*
Amb1003 FraH protein 1e07
Amb1017 Hypothetical protein — Amb1027 Mms5, tightly bound bacterial magnetic particle protein (experimental
data)21* 0
Amb1380 Fructose/tagatose bisphosphate aldolase 1e155 Amb1395 Nitrite reductase precursor 0 Amb2317 Pyruvate dehydrogenase E1 component, beta subunit 1e166 Amb2318 Pyruvate/2-oxoglutarate dehydrogenase complex 1e134 Amb2321 Pyruvate/2-oxoglutarate dehydrogenase complex 3e94 Amb2359 Dihydroorotate dehydrogenase 4e13 Amb2497 Translation elongation factor Ts 8e96 Amb2511 Superoxide dismutase 4e76 Amb2792 Protease subunit of ATP-dependent Clp proteases 2e88 Amb2793 FKBP-type peptidyl-prolyl cis-trans isomerase (trigger factor) 1e119 Amb3006 Response regulator containing a CheY-like receiver domain and a
GGDEF domain
2e33
Amb3133 Translation elongation factors (GTPases) 0 EF-Tu35*
Amb3211 Periplasmic component of the Tol biopolymer transport system 1e145 Amb3421 Hypothetical protein — Amb3492 Mms6724*, Trypsin-like serineproteases, contain C-terminal PDZ
domain
1e127
contribute to their membrane potential and a considerable amount of iron reduction.
3.6. Regulation and signal transduction
In response to several environmental conditions,
Magnetospirillum sp. AMB-1 alters the respiratory
pathway and magnetosome formation. Therefore, it should appropriately regulate gene expression. It must also integrate its metabolism and distribute intracellular iron pools, which can be toxic to the cells. Several signal-ing genes were identified with relation to magnetosome formation from Tn mutant library.
As shown in Table 4,Magnetospirillumsp. AMB-1
tains numerous regulatory and signaling genes that con-serve multiple domains of bacterial signal transduction systems. Remarkably, the sensor module histidine kinase, corresponding to HisKA and HATPase, is encoded in 105 genes, 77 of which contain both histidine kinases (Table 4 and Supplementary Table 4 is available at www. dnaresearch.oxfordjournals.org). This redundancy is
much higher than that of other bacteria, such as M.
loti, Pseudomonas aeruginosa and Caulobacter
crescentus.31 Moreover, GGDEF, EAL and HD-GYP
domains were maintained more frequently than in typical
free-living bacteria.31 The response regulator involving
the GGDEF domain was characterized as a typical two-component signal transduction system like a CheY domain. Numerous bacterial signaling proteins show multidomain structures involving response domains (not only CheY-like but also GGDEF, EAL and HD-GYP) with ligand-binding sensor domains (PAS and GAF). These multiple domains in the signaling proteins reflect the mechanism of signal transduction, from an N-terminal sensor domain to a C-N-terminal response domain, and suggest that the novel domains comprise a distinct system that provides an additional output module and a means of feedback control (Supplementary Table 4 is
available at www.dnaresearch.oxfordjournals.org).31
The frequent occurrence of typical regulator receiver domains containing CheY and other modules (GGDEF,
EAL and HD-GYP) in Magnetospirillum sp. AMB-1
suggests that they provide strict specificity to various
environments, especially for switching between
magnet/non-magnet synthesis and magnetotaxis.
Alexandre et al.32 hypothesized that large numbers of
chemoreceptors inM. magnetotacticumMS-1 are related
to its energy taxis functions. Therefore, it would be required to monitor changes in the cellular energy genesis and to seek an environment that provides efficient energy generation. Tn-mutants led us to focus on three genes
(amb0759, amb2660 and amb3450) classified in signal
transduction. Histidine kinase (sensor signal) and methyl-accepting chemotaxis proteins are encoded in
amb3450andamb2660, respectively. The amb0759gene
encodes a conserved GGDEF protein domain. The results provided several genes among hundreds that should be analyzed, but it is still unclear where they function in the signaling cascade. Proteome analysis identified a response regulator containing a CheY-like receiver and a GGDEF
domain (amb3006) that was expressed on the
magneto-some membrane. This protein probably functions in
mag-netotaxis.33A cell capable of magnetotaxis must be able
to sense a geomagnetic field line by using a magnetosome chain, to transmit the information to flagella, and to move flagella to propel the organism in the appropriate direction.
3.7. Conclusions
For the process of magnetosome formation, we hypothesized four major stages: (i) invagination of the cytoplasmic membrane and vesicle formation for the magnetosome membrane precursor, (ii) accumulation of ferrous/ferric ions in the cell and the vesicles, (iii) strictly controlled iron oxidation–reduction and (iv) magnetite
crystal nucleation and morphology regulation.25
Moreover, signaling pathways are important for
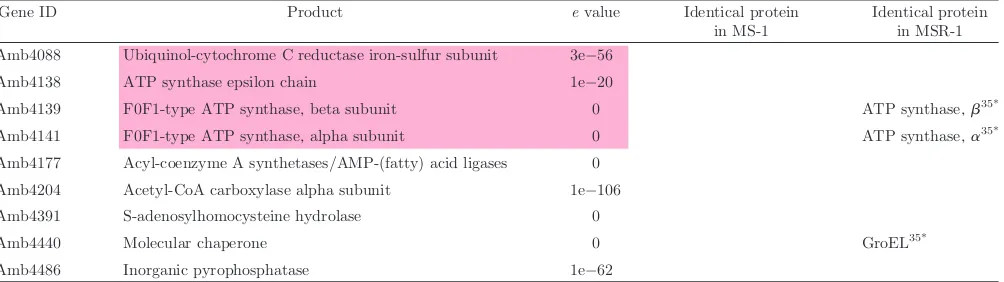
Table 3.Continued.
Gene ID Product evalue Identical protein in MS-1
Identical protein in MSR-1 Amb4088 Ubiquinol-cytochrome C reductase iron-sulfur subunit 3e56
Amb4138 ATP synthase epsilon chain 1e20
Amb4139 F0F1-type ATP synthase, beta subunit 0 ATP synthase,b35*
Amb4141 F0F1-type ATP synthase, alpha subunit 0 ATP synthase,a35*
Amb4177 Acyl-coenzyme A synthetases/AMP-(fatty) acid ligases 0 Amb4204 Acetyl-CoA carboxylase alpha subunit 1e106 Amb4391 S-adenosylhomocysteine hydrolase 0
Amb4440 Molecular chaperone 0 GroEL35*
Amb4486 Inorganic pyrophosphatase 1e62
&&&&&indicate oxidation/reduction proteins.
maintaining the balance of each process as well as protein or gene expression. The molecular mechanisms of each stage and the linkage of steps are expected to follow. Knowledge from whole genome sequence and gene reper-tories reveal organismal metabolism and insightful physiology.
The entire sequence of theMagnetospirillumsp. AMB-1
genome was determined to learn the mechanism of fine and nano-sized magnet formation, which we have investigated as novel material applicable for
recombina-tion. The genes were annotated with anEvalue 1 ·101
however, almost half of the 4559 ORFs were still
unknown and useless for functional prediction.
Therefore, Tn mutagenesis and magnetosome proteomics were performed to find several candidates for magneto-some formation among 2269 ORFs, and the resulting seven and six genes identified through Tn mutagenesis and magnetosome proteomics, respectively. Moreover, both analyses revealed several genes that were categor-ized into a signal transduction class. Remarkable numbers
of sensor and response domains were found in
Magneto-spirillum sp. AMB-1 in this study, and 65 chemotaxis
transducers were also reported in M. magnetotacticum
MS-1.31 The magnetosome synthesis pathway in
AMB-1 competes with oxygen respiration and couples with
res-piratory nitrate reduction whereas M. magnetotacticum
MS-1 magnetosome synthesis is coupled with oxygen respiration. Therefore, each species has its own signal transduction gene sets that respond to different environ-mental stimuli. Although gene predictions were confined within annotated genes, the predictions were also observed in other bacteria. Perhaps, machinery to pro-vide iron or others for magnetosome formation might be simple, but their controls must be complex and strict. Interestingly, magnetosome-related genes identified by Tn mutagenesis and proteome analysis are scattered throughout the genome, and similar genes have also been found in other bacteria. Therefore, magnetosome synthesis requires some genes encoded in the 100-kb region as well as other housekeeping genes. This genome analysis also suggests that the 100-kb region is a necessary element that is necessary but not sufficient for magneto-some formation. Magnetic bacteria are distributed over a heterogeneous group of Gram-negative bacteria with diverse morphologies and habitats. The wide diversity of these organisms suggests that their magnetic properties have no taxonomic significance. Comparative genomic approaches will reveal common factors for magnetosome formation or magnetotaxis. Unfortunately, the genome
sequencing of microaerobe M. magnetotacticum MS-1
or Magnetococcus sp. MC-1 (JGI Microbial Genomics,
http://genome.jgi-psf.org/microbial/) has not been com-pleted, but the draft sequences are comparable. The sequencing data provided lays the foundation for future studies to clarify magnetosome synthesis.
Acknowledgements: This work was funded in part by Grant-in-Aid for Specially Promoted Research, no. 13002005 from the Ministry of Education, Science, Sports and Culture of Japan. TC-DB version 2.0 was kindly provided by Dr Can Tran, University of California at San Diego.
Table 4.Regulatory and signaling proteins in Magnetospirillum sp.
AMB-1.
Protein Number Regulatory protein
Bacterial regulatory protein, LuxR family 10 Bacterial regulatory protein, LysR family 11 Bacterial regulatory protein, MarR family 9 Bacterial regulatory protein, ArsR family 4 Bacterial regulatory protein, AsnC family 2 Bacterial regulatory protein, Crp family 7 Bacterial regulatory protein, GntR family 6 Bacterial regulatory protein, MerR family 2 Bacterial regulatory protein, TetR family 8 Transcriptional regulatory protein 13
HTH Fis type 7
HTH CopG family 1
RpoD (Sigma 70/Sigma 32 ) 2
RpoN (Sigma 54 ) 1
Rpo32 (Sigma 32 ) 1
RpoE (Sigma 24) 3
Nitrogen regulatory protein PII 2 Signaling protein
Signal transduction histidine kinase
HATPase domain containing 98 HisKA domain containing 83 Methyl-accepting chemotaxis protein 44 Bacterial chemotaxis sensory transducer 26
CheA 1
CheB methylesterase 7
CheR 7
Chew 3
CheY 20
Response regulator receiver domain (CheY-like receiver) 45 Domain
SupplementaryMaterial:Supplementary material is available online at www.dnaresearch.oxfordjournals.org.
References
1. Frankel, R. B., Blakemore, R. P., and Wolfe, R. S. 1979, Magnetite in freshwater magnetotactic bacteria, Science, 203, 1355–1356.
2. Heywood, B. R., Bazylinski, D. A., Garratt-Reed, A. J., Mann, S., and Frankel, R. B. 1990, Controlled biosynthesis of greigite (Fe3S4) in magnetotactic bacteria, Naturwiss.,
77, 536–538.
3. Mann, S. 1986, On the nature of boundary-organized bio-mineralization (BOB),J. Inorg. Biochem.,28, 363–371. 4. Towe, K. and Moench, T.T. 1981, Electron-optical
charac-terization of bacterial magnetite,Earth Planet. Sci. Lett., 52, 213–220.
5. Nakamura, N. and Matsunaga, T. 1993, Highly sensitive detection of allergen using bacterial magnetic particles, Anal. Chim. Acta,281, 585–589.
6. Matsunaga, T., Kawasaki, M., Yu, X., Tsujimura, N., and Nakamura, N. 1996, Chemiluminescence enzyme immunoas-say using bacterial magnetic particles, Anal. Chem., 68, 3551–3554.
7. Matsunaga, T., Sato, R., Kamiya, S., Tanaka, T., and Takeyama, H. 1999, Chemiluminescence enzyme immuno-assay using protein A-bacterial magnetite complex,J. Magn. Magn. Mater.,194, 126–131.
8. Tanaka, T. and Matsunaga, T. 2000, Fully automated chemiluminescence immunoassay of insulin using antibody-protein A-bacterial magnetic particle complexes, Anal. Chem.,72, 3518–3522.
9. Matsunaga, T., Ueki, F., Obata, K., et al. 2003, Fully automated immunoassay system of endocrine disrupting chemicals using monoclonal antibodies chemically conjugated to bacterial magnetic particles, Anal. Chim. Acta.,475, 75–83.
10. Sode, K., Kudo, S., Sakaguchi, T., Nakamura, N., and Matsunaga, T. 1993, Application of bacterial magnetic particles for highly selective mRNA recovery system, Biotechnol. Lett.,7, 688–694.
11. Yoza, B., Matsumoto, M., and Matsunaga, T. 2002, DNA extraction using modified bacterial magnetic particles in the presence of amino silane compound, J. Biotechnol., 94, 217–224.
12. Yoza, B., Arakaki, A., Maruyama, K., Takeyama, H., and Matsunaga, T. 2003, Fully automated DNA extraction from blood using magnetic particles modified with hyper-branched polyamidoamine dendrimer,J. Biosc. Bioeng.,95, 21–26.
13. Takeyama, H., Yamazawa, A., Nakamura, C., and Matsunaga, T. 1995, Application of bacterial magnetic par-ticles as novel DNA carriers for ballistic transformation of a marine cyanobacterium,Biotechnol. Lett.,9, 355–360. 14. Nakamura, C., Kikuchi, T., Burgess, J. G., and
Matsunaga, T. 1995, Iron regulated expression and mem-brane localization of the MagA protein inMagnetospirillum sp. strain AMB-1,J. Biochem.,118, 23–27.
15. Yoshino, T., Takahashi, M., Takeyama, H., Okamura, Y., Kato, F., and Matsunaga, T. 2004, Assembly of G
protein-coupled receptors onto nanosized bacterial magnetic parti-cles using Mms16 as an anchor molecule, Appl. Environ. Microbiol.,70, 2880–2885.
16. Matsunaga, T., Sakaguchi, T., and Tadokoro, F. 1991, Magnetite formation by a magnetic bacterium capable of growing aerobically, Appl. Microbiol. Biotechnol., 35, 651–655.
17. Sambrook, J. and Russell, D. W. 2001, Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
18. Fleischmann, R. D., Adams, M. D., White, O., et al. 1995, Whole genome random sequencing and assembly of Haemophilus influenzaeRd,Science,269, 496–512. 19. Saito, I. and Stark, G. R. 1986, Charomids: cosmid
vectors for efficient cloning and mapping of large or small restriction fragments, Proc. Natl. Acad. Sci. USA, 83, 8664–8668.
20. Wahyudi, A. T., Takeyama, H., and Matsunaga, T. 2001, Isolation of Magnetospirillum magneticum AMB-1 mutants defective in bacterial magnetic particle synthesis by trans-poson mutagenesis, Appl. Biochem. Biotechnol., 91-93, 147–154.
21. Arakaki, A., Webb, J., and Matsunaga, T. 2003, A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1, J. Biol. Chem.,278, 8745–8750.
22. Schubbe, S., Kube, M., Scheffel, A., et al. 2003, Characterization of a spontaneous nonmagnetic mutant of Magnetospirillum gryphiswaldense reveals a large deletion comprising a putative magnetosome island, J. Bacteriol., 185, 5779–5790.
23. Nakamura, C., Burgess, J. G., Sode, K., and Matsunaga, T. 1995, An iron-regulated gene, magA, encoding an iron trans-port protein ofMagnetospirillumsp. strain AMB-1,J. Biol. Chem.,270, 28392–28396.
24. Matsunaga, T., Tsujimura, N., Okamura, Y., and Takeyama, H. 2000, Cloning and characterization of a gene mpsA, encoding a protein associated with intracellular magnetic particles from Magnetospirillum sp. strain AMB-1, Biochem. Biophys. Res. Commun., 268, 932–937.
25. Matsunaga, T. and Okamura, Y. 2003, Genes and proteins involved in bacterial magnetic particle formation, Trends Microbiol.,11, 536–541.
26. Komeili, A., Vali, H., Beveridge, T. J., and Newman, D. K. 2004, Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation, Proc. Natl Acad. Sci. USA,101, 3839–3844.
27. Okamura, Y., Takeyama, H. and Matsunaga, T. 2001, A magnetosome-specific GTPase from the magnetic bacterium Magnetospirillum magneticumAMB-1,J. Biol. Chem.,276, 48183–48188.
28. Noguchi, Y., Fujiwara, T., Yoshimatsu, K., and Fukumori, Y. 1999, Iron reductase for magnetite synthesis in the magnetotactic bacteriumMagnetospirillum magneto-tacticum,J. Bacteriol.,181, 2142–2147.
29. Short, K. and Blakemore, R. P. 1986, Iron respiration-driven proton translocation in aerobic bacteria, J. Bacteriol.,167, 729–731.
capable of growing aerobically,Appl. Microbiol. Biotechnol., 39, 368–371.
31. Galperin, M. Y., Nikolskaya, A. N., and Koonin, E. V. 2001, Novel domains of the prokaryotic two-component signal transduction systems,FEMS Microbiol. Lett.,203, 11–21. 32. Alexandre, G., Greer-Phillips, S., and Zhulin, I. B. 2004, Ecological role of energy taxis in microorganisms, FEMS Microbiol. Rev.,28, 113–126.
33. Frankel, R. B., Bazylinski, D. A., Johnson, M. S., and Taylor, B. L. 1997, Magneto-aerotaxis in marine coccoid bacteria,Biophys. J.,73, 994–1000.
34. Okuda, Y., Denda, K. and Fukumori, Y. 1996, Cloning and sequencing of a gene encoding a new member of
the tetratricopeptide protein family from magneto-somes of Magnetospirillum magnetotacticum, Gene, 171, 99–102.
35. Grunberg, K., Muller, E-C., Otto, A., et al. 2004, Biochemical and proteomic analysis of the magnetosome membrane in Magnetospirillum gryphiswaldens, Appl. Environ. Microbiol.,70, 1040–1050.