L
Journal of Experimental Marine Biology and Ecology 248 (2000) 177–190
www.elsevier.nl / locate / jembe
Surface composition and orientation interact to affect subtidal
epibiota
* T.M. Glasby
Centre for Research on Ecological Impacts of Coastal Cities, Marine Ecology Laboratories, A11,
University of Sydney, Sydney, NSW 2006, Australia
Received 14 September 1999; received in revised form 12 January 2000; accepted 16 January 2000
Abstract
Settlement panels were used to evaluate the effects of composition of the substratum (sandstone, concrete, wood) and orientation (vertical, horizontal undersides) on subtidal epibiota. It was predicted that both factors would influence the development of epibiotic assemblages, but that differences due to composition would be less marked on horizontal undersides compared to vertical panels. Differences in assemblages among sandstone, concrete and wooden panels orientated vertically were predicted to be similar to those described previously among vertical surfaces of sandstone rocky reefs and concrete and wooden urban structures (pilings and pontoons). Multivariate analyses indicated that assemblages were influenced greatly by orientation, whereas the effects of surface composition differed for the two orientations and among sites. Assemblages on wood were always significantly different from those on sandstone or concrete — patterns between the latter two surfaces depended on the orientation of the panels. The taxa that dominated these surfaces were not similar in identity nor abundance to those on urban structures of the same composition. The covers of most taxa were influenced by orientation alone or by surface composition for just one orientation. This study demonstrates the need for caution in generalizing about effects of orientation and surface composition because they may interact with each other and / or with other factors and they are certainly quite different for different taxa and among sites. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Estuary; Fouling; Hard substrata; Recruitment; Sessile organisms
1. Introduction
Fouling organisms have been studied for many decades and some of the earliest
*Tel.: 161-2-9351-4282; fax: 161-2-9351-6713.
E-mail address: [email protected] (T.M. Glasby)
178 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190
research assessed the influences of surface characteristics on the settlement and growth of these sessile epibiotic species. Various surface characteristics have been found, at least in some experiments, to influence the numbers and types of organisms that settle on artificial surfaces, e.g. texture (Pomerat and Weiss, 1946; Crisp and Ryland, 1960; Harlin and Lindbergh, 1977), complexity (Hixon and Brostoff, 1985; Bourget et al., 1994; Lemire and Bourget, 1996; Walters and Wethey, 1996), size (Jackson, 1977; Keough, 1984a; Butler, 1991), composition (Caffey, 1982; Raimondi, 1988; McGuin-ness, 1989; Anderson and Underwood, 1994) and colour (Pomerat and Reiner, 1942; Wisely, 1959; James and Underwood, 1994) of the substratum. Artificial surfaces are continuously being added to waterways all over the world due to the rapid urbanization of coastal regions. Epibiota on pilings have been studied in detail (e.g. Karlson, 1978; Kay and Keough, 1981; Kay and Butler, 1983; Butler, 1986; Butler and Connolly, 1996, 1999), but far less information is available about epibiota on other urban structures. Recent work indicates that assemblages on vertical surfaces of pilings, pontoons and retaining walls (made of wood, concrete and sandstone) are quite different from those on nearby natural rocky reefs (Connell and Glasby, 1999; Glasby, 1999a).
Some of these differences in assemblages are attributable to differences in shading and proximity to the seafloor (Glasby, 1999b,c). It is not known to what extent surface composition may be responsible for differences in assemblages among natural and artificial structures. Although numerous studies have compared fouling assemblages on settlement panels made of different materials, few have compared materials that are common in urbanized waterways (but see McGuinness, 1989). Not only do urban structures provide surfaces of different compositions, they may also provide surfaces of various orientations. Most notably, floating pontoons provide large shaded undersurfaces in addition to smaller vertical sides. Very different types of epibiotic assemblages have been shown to occur on surfaces of different orientations (e.g. upper vs. lower surfaces: Fuller, 1946; Withers and Thorp, 1977; Todd and Turner, 1986; vertical vs. horizontal surfaces: Harris and Irons, 1982; Wendt et al., 1989; Hurlbut, 1991a; Baynes, 1999; Connell, 1999). It is likely, therefore, that the combined effects of surface composition and orientation may greatly influence the development of epibiotic assemblages.
In order to begin to understand the reasons for differences between assemblages on urban structures and natural substrata, it will almost certainly be necessary to design multifactorial experiments which can test for interactive effects (Underwood, 1981; Harris and Irons, 1982; Glasby, 1999c). Moreover, without an understanding of interactive effects, it may often be assumed incorrectly that the effects of various factors are consistent across a range of situations. This may explain, in part, some of the conflicting results from studies of the effects of surface characteristics on epibiota (Harris and Irons, 1982). Studies that compared similar factors were rarely done in the same manner by different researchers and, amongst other differences, settlement panels were often orientated differently in experiments.
T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 179
naturally occurring rocky overhangs were not made because of the rarity and small size of overhangs in local estuaries. Nevertheless, assemblages on the undersides of pontoons appear to be similar to those under rocky overhangs and not as different as assemblages on vertical surfaces of the two structures (personal observation). It was proposed, therefore, that factors associated only with undersides of surfaces (perhaps increased shading, different patterns of water flow, etc.) can over-ride (at least partially) or obscure any differences that occur between assemblages on vertical surfaces of natural and artificial structures. Thus, different assemblages were predicted to develop on vertical surfaces of different compositions, but not on horizontal undersides. The differences among vertical sandstone, concrete and wooden surfaces were predicted to be similar to those described previously (Connell and Glasby, 1999) among sandstone reef and wooden and concrete urban structures.
2. Materials and methods
Settlement panels were deployed at three sites, each |200 m apart, in Middle
Harbour (338489 S, 1518149 E), the northern part of Sydney Harbour, in June (winter)
1998. Middle Harbour is a sheltered waterway (|5 km from the open ocean) and a
residential area. At each site, the panels were on reef adjacent to pontoons and jetties,
but not directly shaded by them. Settlement panels were 15315 cm and constructed of
either sandstone, concrete or marine plywood. These materials were chosen to represent the more common hard substrata in Sydney waterways — reefs and retaining walls are primarily sandstone, pilings are constructed of wood or concrete and many pontoons are concrete. Pilings are constructed of a variety of different timbers (mainly hardwood), some of which are treated with antifouling paints. The marine plywood used in the present study was not treated with antifouling paint and may have had different surface characteristics from many wooden pilings.
Aluminium beams (32332 mm aluminium 908 angle) were drilled onto concrete
blocks on the rocky reef at each site at a depth of 1.5 m below mean low water springs. PVC brackets were glued on to the backs of panels and these were bolted to the beams (as in Glasby, 1998). Three of the beams at each site supported vertical panels, three supported horizontal panels (facing downwards). The six beams were arranged in three pairs, such that vertical and horizontal panels were within 50 cm of each other. There were five panels on a beam and either one or two replicates of each surface were
attached to a beam. Replicates on the same beam were |1 m apart and replicates on
different beams were |5 m apart. There were five replicates of each surface, of each
orientation per site. All panels were 10–15 cm from the substratum, but were still accessible to benthic grazers such as urchins and gastropods.
Panels were collected in summer after 7 months, suspended and supported in tubs of seawater for transport back to the laboratory where they were transferred into filtered (10
mm filter) seawater and refrigerated at 58C until sorted (within 6 days of collection)
under a dissecting microscope. Primary cover (organisms attached directly to the panel) and secondary cover (organisms attached to primary cover) were estimated for sessile
180 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190
(i.e. a 1 cm border around each panel was not sampled to avoid ‘edge effects’). Taxa on the fronts of panels, but not under a point were assigned a cover of 0.5%. Species of filamentous algae were lumped together because often many species were present under a point and it was impossible to allocate the point to just one species.
Data for primary and secondary covers of each taxon were combined and expressed as a percentage for univariate (ANOVA) and non-parametric multivariate (PRIMER package) analyses. Variances were heterogeneous for many analyses (Cochran’s C-test,
P.0.05), despite transformation, so untransformed data were analysed. For balanced
designs, ANOVA is relatively robust to heterogeneous variances (Box, 1953), but
significant results at the level of P,0.05 should perhaps be interpreted with some
caution. Multivariate data were fourth root transformed and Bray–Curtis similarity matrices calculated. Data were presented graphically using non-metric multi-dimensional scaling (nMDS) ordinations. One-way analyses of similarities (ANOSIM; Clarke and Green, 1988) and multiple pairwise comparisons tested for differences in assemblages among treatments. The significance level for pairwise tests was reduced from 0.05 to 0.01 to adjust for multiple comparisons.
Some panels were lost from Site 1 (one sandstone, two concrete and two wood) and to maintain balanced univariate analyses, these values were replaced by the mean of the remaining replicates at that site and the residual degrees of freedom, mean square estimates and F-ratios adjusted accordingly for each taxon (Underwood, 1981).
3. Results
Fifty taxa were identified on the panels (most to genus or species) and used (in addition to bare space) in multivariate analyses. These included species of spirorbid and serpulid polychaetes, encrusting and arborescent bryozoans, barnacles, sponges, solitary and colonial ascidians, oysters, mussels, scleractinian coral, algae, cyanobacteria, diatoms and the wood-boring bivalve Bankia australis (Calman). At each site, there
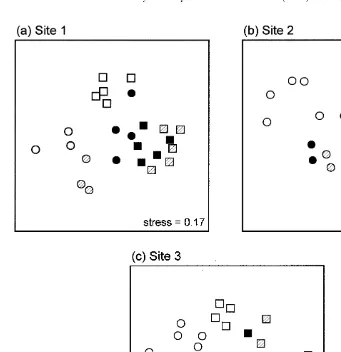
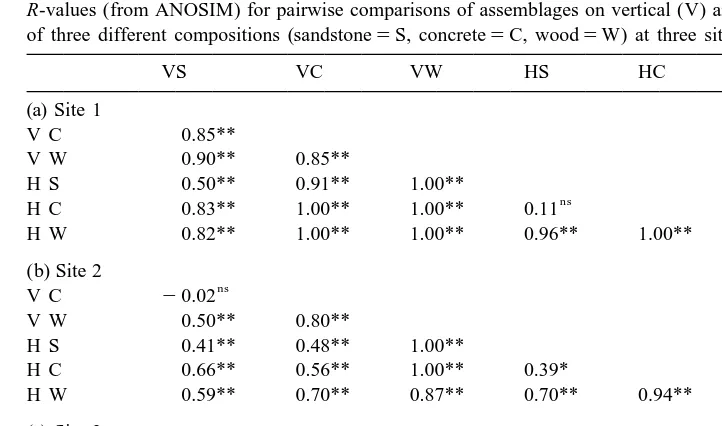
were significant differences among treatments (R50.87, R50.62, R50.58; P,0.001
for each), with assemblages on wooden panels being most distinct (Fig. 1).
Pairwise comparisons indicated that patterns among surfaces differed depending on the orientation of the panels. At sites 1 and 3, assemblages on vertical panels of each composition were all significantly different, whereas assemblages on horizontal under-sides of sandstone and concrete were similar to each other and different from wooden surfaces (Table 1a, c). The opposite pattern occurred at site 2 where assemblages on the three types of horizontal panels were all significantly different, whereas on vertical panels, sandstone and concrete panels were similar to each other and different from wood (Table 1b). The uniqueness of assemblages on wooden panels was not due simply to the numerical abundance of the wood-borer B. australis; when this species was removed from the analyses the same patterns were apparent. It is not known whether the presence of this species had any other influences (direct or indirect) on assemblages on wooden panels.
T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 181
Fig. 1. Non-metric multidimensional scaling ordinations of assemblages on sandstone (black), concrete (hatched) and wood (white) panels positioned vertically (circles) and horizontally (squares) at three sites. n55 except for site 1 where one vertical sandstone panel and two vertical concrete and wooden panels were lost. Note that stress values are large.
182 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 Table 1
R-values (from ANOSIM) for pairwise comparisons of assemblages on vertical (V) and horizontal (H) panels a
of three different compositions (sandstone5S, concrete5C, wood5W) at three sites
VS VC VW HS HC
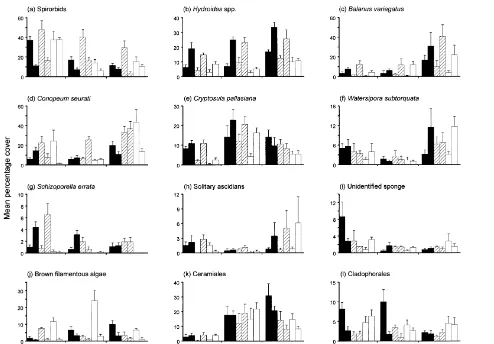
on serpulids were obvious for sandstone and concrete panels (vertical,horizontal), but
not for wooden panels (Fig. 2b; Table 3).
No significant differences were detected in the cover of the barnacle Balanus variegatus Darwin (Table 2), but it tended to be more abundant on horizontal undersides
Table 2
Summary of results from three-factor ANOVAs comparing percentage covers of taxa on three surfaces (Su) (fixed: sandstone, concrete, wood) of two orientations (Or) (fixed: vertical, horizontal undersides) at three sites
a (Si) (random) (n55)
Taxon Surface Orientation Su3Or Su3Site Or3Site Su3Or3Si
Spirorbids ns * ns *** ns ns
Hydroides spp. * ** ** ns ns ns
Balanus variegatus ns ns ns ns ns ns
Conopeum seurati ns ns ns ns ** *
Cryptosula pallasiana *** ns ns ns ** ns
Watersipora subtorquata ns ns ns ns ** ns
Schizoporella errata * ns ns ns *** ***
Solitary ascidians ns ns ns ns ns ns
Unidentified sponge ns ns ns *** ns **
Brown filamentous algae ns * ns *** ns **
Ceramiales ns ns ns ns * ns
Cladophorales ns ns ns ns * ns
a ns
T
.M
.
Glasby
/
J.
Exp
.
Mar
.
Biol
.
Ecol
.
248
(2000
)
177
–
190
183
184
T
.M
.
Glasby
/
J.
Exp
.
Mar
.
Biol
.
Ecol
.
248
(2000
)
177
–
190
Table 3
a Summary of SNK results for significant interaction terms in Table 2
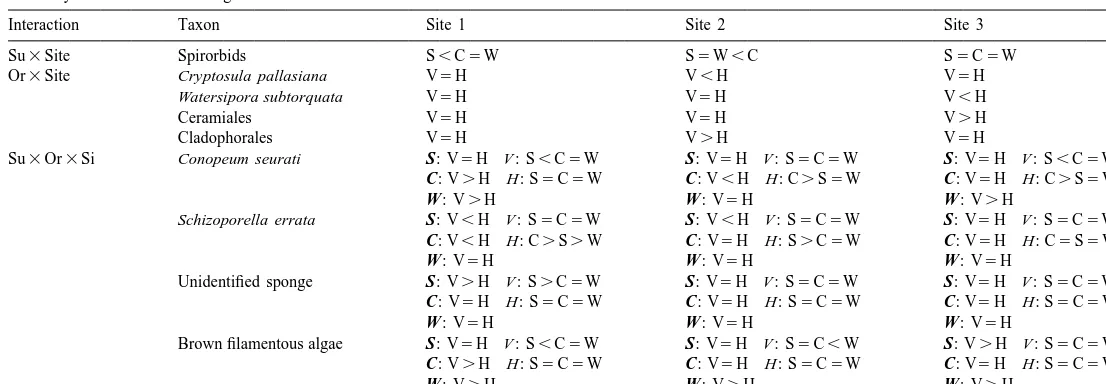
Interaction Taxon Site 1 Site 2 Site 3
Su3Site Spirorbids S,C5W S5W,C S5C5W
Or3Site Cryptosula pallasiana V5H V,H V5H
Watersipora subtorquata V5H V5H V,H
Ceramiales V5H V5H V.H
Cladophorales V5H V.H V5H
Su3Or3Si Conopeum seurati S: V5H V : S,C5W S: V5H V : S5C5W S: V5H V : S,C5W
C: V.H H: S5C5W C: V,H H: C.S5W C: V5H H: C.S5W
W : V.H W : V5H W : V.H
Schizoporella errata S: V,H V : S5C5W S: V,H V : S5C5W S: V5H V : S5C5W
C: V,H H: C.S.W C: V5H H: S.C5W C: V5H H: C5S5W
W : V5H W : V5H W : V5H
Unidentified sponge S: V.H V : S.C5W S: V5H V : S5C5W S: V5H V : S5C5W
C: V5H H: S5C5W C: V5H H: S5C5W C: V5H H: S5C5W
W : V5H W : V5H W : V5H
Brown filamentous algae S: V5H V : S,C5W S: V5H V : S5C,W S: V.H V : S5C5W
C: V.H H: S5C5W C: V5H H: S5C5W C: V5H H: S5C5W
W : V.H W : V.H W : V.H a
T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 185
than on vertical panels of each surface (Fig. 2c). Covers were very similar among the three surfaces. For the bryozoan Conopeum seurati (Canu), patterns among surfaces differed between vertical and horizontal panels and among sites (Tables 2 and 3; Fig. 2d). C. seurati was most abundant on vertical concrete and wooden panels at site 1, horizontal concrete panels at site 2 and vertical wooden panels at site 3 (Fig. 2d; Table 3).
The cover of the encrusting bryozoan Cryptosula pallasiana (Moll) was greatest on sandstone surfaces, less on concrete and least on wooden surfaces (Table 2; Fig. 2e). Its cover tended to be less on vertical panels than on horizontal undersides, but only at two sites (Fig. 2e; Table 3). Watersipora subtorquata (d’Orbigny) also tended to be less common on vertical panels than horizontal undersides, but the difference was significant only at the site where the bryozoan was most abundant (Fig. 2f; Tables 2 and 3). The same pattern occurred for Schizoporella errata (Waters) (Fig. 2g; Tables 2 and 3). Differences in the cover of this bryozoan also occurred among surfaces, but only for horizontal panels and, like C. pallasiana, it was generally least common on wooden surfaces (Fig. 2g; Table 3).
Although uncommon on panels, solitary ascidians tended to be most abundant on the horizontal undersides of each surface (Fig. 2h). Sponges were also rare. The cover of the most common, an unidentified species (Class Calcarea), was variable and the significant difference detected was due to the relatively large cover on vertical sandstone panels at one site (Fig. 2i; Tables 2 and 3).
Brown filamentous algae (a mixture of various species, but mainly Feldmania sp. and Sphacelaria sp.) were generally more abundant on vertical surfaces than on horizontal undersides (Fig. 2j), although significant differences were detected only for certain surfaces at some sites (Tables 2 and 3). Effects of surface composition were evident only for vertical panels at two sites and the patterns were not consistent (Table 3; Fig. 2j). Patterns for red filamentous algae (grouped as Ceramiales) were quite variable among sites. There were no clear effects of surface or orientation (Fig. 2k) except at site 3 where ceramialean algae were more abundant on vertical surfaces than on horizontal undersides (Table 3). Green filamentous algae (grouped as Cladophorales) were significantly more abundant on vertical surfaces than horizontal undersides only at one site (Tables 2 and 3) and there was generally no evidence of an effect of orientation or surface at the other sites (Fig. 2l).
4. Discussion
186 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190
marine organisms and without more knowledge of these it would be unwise to generalize about the effects of surface composition or orientation on these organisms.
Although there were many significant differences detected in this study, the results were not as predicted. Effects of surface composition were not always apparent in that covers of various organisms were often similar on sandstone and concrete surfaces, which is consistent with Sousa (1979). Wooden surfaces did, however, support quite different assemblages, as also noted by Coe (1932). It was predicted that differences among surfaces would not occur for panels orientated horizontally. This was the case for some taxa, but certainly not for all. In fact, for certain taxa at some sites, effects of surface composition were evident for horizontal undersides and not for vertical panels. The large variability in patterns among sites separated by only 200 m is important to note. It is by no means a novel finding, but highlights the need for adequate spatial replication of experiments and suggests that the generality of results of experiments done at a single place is by no means assured.
Differences in biota among natural and urban structures made of sandstone, concrete and wood described previously in Middle Harbour, Sydney, were not apparent in the present study. Subtidal sandstone reefs in estuaries around Sydney tend to be dominated by spirorbid polychaetes and foliose and filamentous algae, whereas wooden pilings typically support large covers of serpulid polychaetes, sponges, solitary ascidians and encrusting bryozoans (Glasby, 1999a). These differences in assemblages between pilings (of various types) and rocks are quite consistent both spatially and temporally (Glasby, 1999a). Concrete pontoons and pilings are covered largely by spirorbids, serpulids, ceramialean algae and barnacles (Connell and Glasby, 1999). In the present study, the covers of some of these taxa were influenced by surface composition, but certainly not consistently, nor to the same degree that they differed among structures sampled previously.
Of course it is not known whether the patterns among surfaces may have been different if the experiment ran longer or began at a different time. The experiment was, however, almost certainly run for sufficient time to allow differences to develop among surfaces. Although the recruitment of most species is temporally variable and inverte-brates such as sponges may take a long time to become abundant on settlement panels (Osman, 1977; Kay and Keough, 1981; Russ, 1982), this probably did not influence the conclusions of this study. The species sampled on panels included those that are common on urban structures (Connell and Glasby, 1999) and their covers were generally similar, and in some cases far greater, in the present study. Although the surfaces used in this study were not exactly comparable to those of the pilings and pontoons sampled previously, these results and those of similar studies (Glasby, 1999a; Connell, 2000), indicate that differences in surface composition do not play a major role in explaining the large and consistent differences in biota on urban structures and rocky reef. Instead these differences in biota are likely to be due largely to differences in shading and proximity to the seafloor — experimental manipulations of these two factors produced comparable differences in assemblages over just 8 months (Glasby, 1999c).
T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 187
different compositions (e.g. Coe, 1932; Pomerat and Weiss, 1946; Caffey, 1982; McGuinness, 1989; Anderson and Underwood, 1994), but none has examined the interactive effects of the two factors. This study showed that the influence of surface composition on the covers of Hydroides spp., C. seurati, S. errata and an unidentified calcareous sponge can depend upon orientation. Pomerat and Reiner (1942) demon-strated a strong interactive effect of surface colour / illumination and orientation on the settlement of barnacles — far more settled on the horizontal undersides of black glass panels than on white or clear glass at various angles. In the present study, there was a tendency for the abundance of barnacles to be greatest on horizontal undersides, but no differences were apparent among the three surfaces which were all a similar light colour. These results suggest that it may be difficult to compare many studies investigating surface characteristics because different experiments done to test for effects of orientation often used surfaces of different compositions and vice versa. The interaction between these two factors may account for some of the variability in results of previous experiments. Of the eight studies investigating the effects of orientation cited above, at least five different surfaces were used, including glass, asbestos, naturally occurring rock, acrylic and fibreglass. Conversely, studies of the effects of surface composition on epibiota have used settlement panels orientated vertically or horizontally and some researchers have examined the undersides of panels while others have examined the tops. Interactive effects of surface composition and orientation on epibiota will confound any comparisons among these studies. Furthermore, the present study identified significant small-scale spatial variability in patterns of epibiota among surfaces of different compositions and orientations. This will of course also increase the likelihood that studies done in different places will produce different results, even if identical techniques are used.
The reasons for the interactive effects of surface composition and orientation are no doubt complex, but I suggest that differential light intensity, predation and larval behaviour (and interactions among these factors) may play important roles. Light intensity would certainly have been less on horizontal undersides compared to vertical panels. Differences in light intensity can influence (directly or indirectly) the covers of various species, including algae, serpulid polychaetes, bryozoans and sponges (Miura and Kajihara, 1984; Reed and Foster, 1984; Kennelly, 1989; Duggins et al., 1990; Glasby, 1999b). Biofilms may be also be influenced by light intensity and these are known to strongly affect the settlement of various epifaunal species (Todd and Keough, 1994; Keough and Raimondi, 1996; Keough, 1998). Predators such as invertebrate grazers and fishes can also have large effects on abundances of epibiota (e.g. Breitburg, 1985; Sebens, 1985; Keough and Downes, 1986; Osman et al., 1992; Connell and Anderson, 1999), but this is certainly not always the case (e.g. Osman, 1977; Keough and Butler, 1979; Keough, 1984b).
188 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190
almost never involved complex interactions among species and physical factors which are known to influence settlement in the field. There is, however, evidence that the behaviour of larvae in the field can be extremely variable and may differ greatly among populations (Raimondi and Keough, 1990).
In conclusion, the results of this study and others suggest that effects of substratum may be relatively minor in explaining why different assemblages develop on natural and artificial substrata in estuaries around Sydney. Few, if any, of the differences docu-mented in the present study were comparable to those described between assemblages on artificial and natural surfaces. Perhaps most importantly, this study demonstrated that the effects of surface composition on epibiota can depend upon the orientation of the surface and may be variable over relatively small spatial scales. Thus, it is impossible to generalize about effects of these factors on most sessile species in newly developed assemblages. This situation may be redressed if future studies focus on the importance of interactive effects on the development of epibiotic assemblages. Such studies will need to be done at a variety of spatial and temporal scales.
Acknowledgements
This research was funded by the Centre for Research on Ecological Impacts of Coastal Cities (University of Sydney). I thank S. Heislers, G. Housefield, N. Knott and S. Connell for assistance in the field. D. Gordon kindly identified many of the bryozoans. This manuscript benefited from comments by P. Archambault, M.G. Chapman, S.D. Connell and two anonymous referees. [AU]
References
Anderson, M.J., Underwood, A.J., 1994. Effects of substratum on the recruitment and development of an intertidal estuarine fouling assemblage. J. Exp. Mar. Biol. Ecol. 184, 217–236.
Baynes, T.W., 1999. Factors structuring a subtidal encrusting community in the southern Gulf of California. Bull. Mar. Sci. 64, 419–450.
Bourget, E., DeGuise, J., Daigle, G., 1994. Scales of substratum heterogeneity, structural complexity, and the early establishment of a marine epibenthic community. J. Exp. Mar. Biol. Ecol. 181, 31–51.
Box, G.E.P., 1953. Non-normality and tests on variances. Biometrika 40, 318–335.
Breitburg, D.L., 1985. Development of a subtidal epibenthic community: factors affecting species composition and the mechanisms of succession. Oecologia 65, 173–184.
Buss, L.W., 1979. Habitat selection, directional growth and spatial refuges: why colonial animals have more hiding places. In: Larwood, G., Rosen, B.R. (Eds.), Biology and Systematics of Colonial Organisms, Academic Press, London, pp. 459–497.
Butler, A.J., 1986. Recruitment of sessile invertebrates at five sites in Gulf St. Vincent, South Australia. J. Exp. Mar. Biol. Ecol. 97, 13–36.
Butler, A.J., 1991. Effect of patch size on communities of sessile invertebrates in Gulf St. Vincent, South Australia. J. Exp. Mar. Biol. Ecol. 153, 255–280.
Butler, A.J., Connolly, R.M., 1996. Development and long term dynamics of a fouling assemblage of sessile marine invertebrates. Biofouling 9, 187–209.
Butler, A.J., Connolly, R.M., 1999. Assemblages of sessile marine invertebrates: still changing after all these years? Mar. Ecol. Prog. Ser. 182, 109–118.
T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190 189 Clarke, K.R., Green, R.H., 1988. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol.
Prog. Ser. 46, 213–226.
Coe, W.R., 1932. Season of attachment and rate of growth of sedentary marine organisms at the pier of the Scripps Institution of Oceanography, La Jolla, California. Bull. Scripps Inst. Oceanogr., Tech. Ser. 3, 37–86.
Connell, S.D., 1999. Effects of surface orientation on the cover of epibiota. Biofouling 14, 219–226. Connell, S. D., 2000. Floating pontoons create novel habitats for subtidal epibiota. J. Exp. Mar. Biol. Ecol. (in
press).
Connell, S.D., Anderson, M.J., 1999. Predation by fish on assemblages of intertidal epibiota: effects of predator size and patch size. J. Exp. Mar. Biol. Ecol. 241, 15–29.
Connell, S.D., Glasby, T.M., 1999. Do urban structures influence local abundance and diversity of subtidal epibiota? A case study from Sydney Harbour, Australia. Mar. Environ. Res. 47, 373–387.
Crisp, D.J., Ryland, J.S., 1960. Influence of filming and of surface texture on the settlement of marine organisms. Nature 185, 119.
Duggins, D.O., Eckman, J.E., Sewell, A.T., 1990. Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. J. Exp. Mar. Biol. Ecol. 143, 27–45.
Fuller, J.L., 1946. Season of attachment and growth of sedentary marine organisms at Lamoine, Maine. Ecology 27, 150–158.
Glasby, T.M., 1998. Estimating spatial variability in developing assemblages of epibiota on subtidal hard substrata. Mar. Freshwater Res. 49, 429–437.
Glasby, T.M., 1999a. Differences between subtidal epibiota on pier pilings and rocky reefs at marinas in Sydney, Australia. Estuar. Coast. Shelf Sci. 48, 281–290.
Glasby, T.M., 1999b. Effects of shading on subtidal epibiotic assemblages. J. Exp. Mar. Biol. Ecol. 234, 275–290.
Glasby, T.M., 1999c. Interactive effects of shading and proximity to the seafloor on the development of subtidal epibiotic assemblages. Mar. Ecol. Prog. Ser. 190, 113–124.
Harlin, M.M., Lindbergh, J.M., 1977. Selection of substrata by seaweeds: optimal surface relief. Mar. Biol. 40, 33–40.
Harris, L.G., Irons, K.P., 1982. Substrate angle and predation as determinants in fouling community succession. In: Cairns, J. (Ed.), Artificial Substrates, Ann Arbor Science, Michigan, pp. 131–174. Hixon, M.A., Brostoff, W.N., 1985. Substrate characteristics, fish grazing, and epibenthic reef assemblages off
Hawaii. Bull. Mar. Sci. 37, 200–213.
Hurlbut, C.J., 1991a. The effects of larval abundance, settlement and juvenile mortality on the depth distribution of a colonial ascidian. J. Exp. Mar. Biol. Ecol. 150, 183–202.
Hurlbut, C.J., 1991b. Larval substratum selection and postsettlement mortality as determinants of the distribution of two bryozoans. J. Exp. Mar. Biol. Ecol. 147, 103–119.
Jackson, J.B.C., 1977. Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am. Nat. 111, 743–767.
James, R.J., Underwood, A.J., 1994. Influence of colour of substratum on recruitment of spirorbid tubeworms to different types of intertidal boulders. J. Exp. Mar. Biol. Ecol. 181, 105–115.
Karlson, R., 1978. Predation and space utlization patterns in a marine epifaunal community. J. Exp. Mar. Biol. Ecol. 31, 225–239.
Kay, A.M., Butler, A.J., 1983. ‘Stability’ of the fouling communities on the pilings of two piers in South Australia. Oecologia 56, 70–78.
Kay, A.M., Keough, M.J., 1981. Occupation of patches in the epifaunal communities on pier pilings and the bivalve Pinna bicolour at Edithburgh, South Australia. Oecologia 48, 123–130.
Kennelly, S.J., 1989. Effects of kelp canopies on understorey species due to shade and scour. J. Exp. Mar. Biol. Ecol. 50, 215–224.
Keough, M.J., 1984a. Effects of patch size on the abundance of sessile marine invertebrates. Ecology 65, 423–437.
Keough, M.J., 1984b. Dynamics of the epifauna of the bivalve Pinna bicolor: interactions among recruitment, predation, and competition. Ecology 65, 677–688.
Keough, M.J., 1998. Responses of settling invertebrate larvae to the presence of established recruits. J. Exp. Mar. Biol. Ecol. 231, 1–19.
190 T.M. Glasby / J. Exp. Mar. Biol. Ecol. 248 (2000) 177 –190
Keough, M.J., Downes, B.J., 1986. Effects of settlement and post-settlement mortality on the distribution of the ascidian Trididemnum opacum. Mar. Ecol. Prog. Ser. 33, 279–285.
Keough, M.J., Raimondi, P.T., 1996. Responses of settling invertebrate larvae to bioorganic films: effects of large-scale variation in films. J. Exp. Mar. Biol. Ecol. 207, 59–78.
Lemire, M., Bourget, E., 1996. Substratum heterogeneity and complexity influence micro-habitat selection of
Balanus sp. and Tubularia crocea larvae. Mar. Ecol. Prog. Ser. 135, 77–87.
Lilly, S.J., Sloane, J.F., Bassindale, R., Ebling, F.J., Kitching, J.A., 1953. The ecology of the Lough Ine rapids with special reference to water currents. IV. The sedentary fauna of sublittoral boulders. J. Anim. Ecol. 22, 87–96.
McGuinness, K.A., 1989. Effects of some natural and artificial substrata on sessile marine organisms at Goleta Reef, Panama. Mar. Ecol. Prog. Ser. 52, 201–208.
Meadows, P.S., Campbell, J.I., 1972. Habitat selection by aquatic invertebrates. Adv. Mar. Biol. 10, 271–382. Miura, T., Kajihara, T., 1984. An ecological study of the life histories of two Japanese serpulid worms,
Hydroides ezoensis and Pomatoleios kraussii. In: Hutchings, P.A. (Ed.), Proceedings of the First
International Polychaete Conference, Sydney, The Linnean Society of New South Wales, Sydney, pp. 338–354.
Osman, R.W., 1977. The establishment and development of a marine epifaunal community. Ecol. Monogr. 47, 37–63.
Osman, R.W., Whitlatch, R.B., Malatesta, R.J., 1992. Potential role of micro-predators in determining recruitment into a marine benthic community. Mar. Ecol. Prog. Ser. 83, 35–43.
Pomerat, C.M., Reiner, E.R., 1942. The influence of surface angle and of light on the attachment of barnacles and other sedentary organisms. Biol. Bull. Mar. Biol. Lab., Woods Hole 82, 14–25.
Pomerat, C.M., Weiss, C.M., 1946. The influence of texture and composition of surface on the attachment of sedentary marine organisms. Biol. Bull. Mar. Biol. Lab., Woods Hole 91, 57–65.
Raimondi, P.T., 1988. Rock type affects settlement, recruitment, and zonation of the barnacle Chthamalus
anisopoma Pilsbury. J. Exp. Mar. Biol. Ecol. 123, 253–267.
Raimondi, P.T., Keough, M.J., 1990. Behavioural variability in marine larvae. Aust. J. Ecol. 15, 427–437. Reed, D.C., Foster, M.S., 1984. The effects of canopy shading on algal recruitment and growth in a giant kelp
forest. Ecology 65, 937–948.
Russ, G.R., 1982. Overgrowth in a marine epifaunal community: competitive hierarchies and competitive networks. Oecologia 53, 12–19.
Sebens, K.P., 1985. Community ecology of vertical rock walls in the Gulf of Maine, USA: Small-scale processes and alternative community states. In: Moore, P.G., Seed, R. (Eds.), The Ecology of Rocky Coasts, Hodder & Stoughton, London, pp. 346–371.
Sousa, W.P., 1979. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol. Monogr. 49, 227–254.
Todd, C.D., Keough, M.J., 1994. Larval settlement in hard substratum epifaunal assemblages: a manipulative field study of the effects of substratum filming and the presence of incumbents. J. Exp. Mar. Biol. Ecol. 181, 159–187.
Todd, C.D., Turner, S.J., 1986. Ecology of intertidal and sublittoral cryptic epifaunal assemblages. I. Experimental rationale and the analysis of larval settlement. J. Exp. Mar. Biol. Ecol. 99, 199–231. Underwood, A.J., 1981. Techniques of analysis of variance in experimental marine biology and ecology. Annu.
Rev. Oceanogr. Mar. Biol. 19, 513–605.
Walters, L.J., Wethey, D.S., 1996. Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: the importance of refuge dimensions and adult morphology. Mar. Ecol. Prog. Ser. 137, 161–171.
Wendt, P.H., Knott, D.M., Van Dolah, R.F., 1989. Community structure of the sessile biota on five artificial reefs of different ages. Bull. Mar. Sci. 44, 1106–1122.
Wisely, B., 1959. Factors influencing the settling of the principal marine fouling organisms in Sydney Harbour. Aust. J. Mar. Freshwater Res. 10, 30–44.