There is a renewal of interest in endosperm development. Recent studies are leading the way to a better
understanding of fundamental processes such as cell cycle control and the mechanisms of imprinting. A more global view of interactions between the endosperm and the embryo is emerging and will initiate an integrated approach to the study of seed development.
Addresses
RDP, UMR 9938, 46 allée d’Italie, 69364 Lyon cedex 07, France; e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:28–32 http://biomednet.com/elecref/1369526600200028 © Elsevier Science Ltd ISSN 1369-5266
Introduction
Double fertilisation is a characteristic unique to higher plants [1]. The pollen tube delivers two haploid male gametes to the embryo sac. One male gamete fuses with the oosphere to generate the embryo of the diploid daugh-ter plant. The other male gamete fuses with the central cell. The central cell occupies most of the volume of the embryo sac and contains two haploid nuclei. The triploid product of the second fertilisation develops into a peculiar organism, the endosperm. In cereals, the endosperm stores carbohydrates and proteins during seed maturation. These storage products represent a major source of food for mankind and, as a result, storage of reserves in endosperm has been the focus of research for many years [2].
Endosperm development is characterised by four phases: syncytial, cellularisation, differentiation and death (Figure 1). The duration and overlap of each of those phases varies from species to species [3]. Research in endosperm development is currently focused on two models: cereals, such as maize, rice and barley, and the dicot model species Arabidopsis. Endosperm develop-ment in these models is initiated by successive divisions of the triploid zygotic nucleus without cytokinesis. Hence, during initial stages of development, the endosperm is a syncytium, which is an unusual structure in higher plants. Later, each nucleus becomes isolated by the development of cell walls. This process, referred to as cellularisation, is gradual and does not affect all nuclei in species such as Arabidopsis. Cellularisation mechanisms appear to be independent of cytokinesis [4]. Cellularisation is followed by a differentiation of func-tional tissues. Eventually, most endosperm cells die during seed maturation.
Both zygotic products of double fertilisation inherit the same set of genes from their parents, as all nuclei involved in the process are products of mitoses. So, why is the
endosperm so different from the embryo? Double fertilisa-tion also occurs in Gnetales, the origin of which pre-dates the evolution of Angiosperms [5,6]. In these plants, two embryos develop and compete for resources from the nucellar tissues. It is thus possible that the endosperm evolved from a second embryo present in the ancestor of Angiosperms; however, as in vivo, isolated embryos and endosperms obtained through in vitro fertilisation follow completely different developmental paths [7••]. Thus, the differential expression of genes resulting in such dramatic differences in developmental features probably originates from an ensemble of genetic controls provided by cell determinants specific to the egg cell or the central cell. Recently, epigenetic controls of endosperm development have been identified and the importance of the mater-nal:paternal ratio has been re-examined in Arabidopsis[8•].
The role played by the endosperm during seed develop-ment has been a subject of debate [2]. In cereals, for example, the endosperm is a major site of reserve storage and is persistent in the mature dried seed. In these cases the endosperm provides nutrients and hormones to the germinating seed. In contrast, in many dicot seeds reserves are stored in the embryo and not in the endosperm — the role of which is thus not completely clear. Recent studies, however, have shown that in Arabidopsis seeds, the endosperm plays a crucial role in the control of nutrient delivery to the embryo [9•]. Other investigations in carrot seed strongly suggest that the endosperm could be a source of signals involved in embryogenesis [10•].
Control of nuclear and cell division during
endosperm development
Endosperm development is characterised by the occur-rence of DNA replication which, if not followed by cytokinesis leads to the formation of syncytial structures and if not followed by nuclei division leads to endoredu-plication. In Arabidopsis [11] and maize [12], the endosperm initially develops as a syncytium and then undergoes cellularisation [2,4]. It has been shown that in the cytokinesis-deficient Arabidopsis mutant knolle, embryonic cells are syncytia containing large nuclei of heterogeneous sizes [13•]. This nuclear heterogeneity is not observed in wild-type endosperm, despite the absence of cellularisation. Synchronous divisions of endosperm nuclei have been observed in both wild-type maize [7••] and Arabidopsis(F Berger, unpublished data), implying the existence of mechanisms that prevent the merging of mitotic spindles and chromosome sets. Two recent studies report new phenotypes for mutant endosperms characterised by enlarged nuclei. A knockout for a newly identified small GTPase ERG isolated in
Antirhinum displays an anomalous syncytial endosperm filled with large nuclei of heterogeneous size [14]. ERG
Endosperm development
shows strong homology with the prokaryotic protein ERA which, in bacteria, is involved in vital processes such as cell division. The second report concerns the characterisa-tion of three mutants grouped under the titan(ttn) family [15•]. These mutants all exhibit enlarged endosperm nuclei. The mutation ttn3can be distinguished from ttn1
and ttn2because it affects neither the development of the embryo, nor endosperm cellularisation and mitosis. Unlike ttn2, which shows early embryo abortion, ttn1 is characterised by an embryo composed of a few enlarged cells with giant nuclei. The phenotype of the mutant
titan1 closely resembles phenotypes of the recently described class of mutants pilz (see note added in proof). Mutations have been isolated in four different genes which cause defects in cytokinesis and mitosis. In each mutant, the expression of cell cycle- and cytokinesis-relat-ed proteins is not impaircytokinesis-relat-ed. The common defect observcytokinesis-relat-ed in all four mutants is an absence of mitotic spindles, phragmoplasts and cortical arrays. This is correlated with a global absence of organised microtubules. It is likely that the titan family will be further sub-divided into vari-ous classes of mutants specifically impaired for essential components of the cell such as the cytoskeleton or ele-ments of nuclear structure.
Endosperm differentiation
Cytological studies clearly show that the cellularised endosperm both in cereals and Arabidopsiscontains a vari-ety of tissues [2,11,12]. This observation is supported by analysis of a number of mutants in which specific areas of the endosperm do not develop properly [16]. Endosperm differentiation may originate from a partitioning of mRNA within the syncytium, as suggested by the isola-tion of a molecular marker in barley [17]. In cereals,
concentric layers of cells define a pattern: the outermost aleurone layer, the subaleurone and the central starchy endosperm where enzymes involved in starch synthesis are located. The aleurone layer is the only layer of living cells in the mature kernel. During germination, the aleu-rone is responsible of the synthesis of enzymes which mobilise reserves from internal layers. It is also the site of anthocyanin synthesis [2]. In the maize mutant, crinkly4, in which epidermal specification is affected throughout the plant, the aleurone layer does not differentiate cor-rectly [18]. CRINKLY4 encodes a ser/thr kinase which could be involved in differentiation of the aleurone from the subaleurone layer [19]. Another indication of the epi-dermal nature of the aleurone is the fact that the homeobox containing gene ATML1, a very early epider-mal marker in Arabidopsis, is also expressed in the outer layer of endosperm cells [20].
In addition to the radial symmetry, a polar axis is set up between the pole where the embryo is located (the micropylar pole) and the chalazal region where vascular elements end. In maize, as in Arabidopsis, the area which surrounds the embryo at the micropylar pole is composed of small cells with dense cytoplasm associated normally with intense cytoplasmic activity [11,21]. The high meta-bolic activity of these cells is further supported by the high density of endoplasmic reticulum observed in this area before cellularisation in Arabidopsis [11]. Members of the
Esrgene family have been isolated in maize using a differ-ential display procedure [22•]. The expression pattern of Esr genes is endosperm-specific and is restricted to the small cytoplasmic cells around the base of the embryo. At least three Esrgenes with high homologies are present in the maize genome. These genes code for proteins of Figure 1
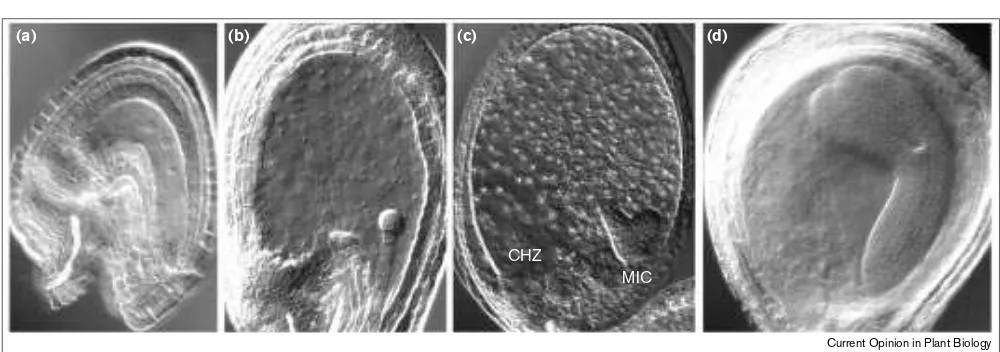
Major steps of endosperm development in Arabidopsis. Endosperm development is observed on whole-mount cleared seeds. (a)The nucleus of the central cell divides without cytokinesis prior to the first zygotic division. (b)The seed enlarges rapidly while further nuclear divisions take place and the endosperm forms a syncytium. (c)When the embryo reaches the heart stage most of the endosperm is cellularised with the exception of the nuclei at the chalazal pole (CHZ). In this region, nuclei are grouped in cytoplasmic pockets. At this stage endosperm differentiation occurs with production of a well-defined outer layer and a central vacuolar endosperm which is not visible on this picture. Micropylar, Mic. (d)After
cellularisation cell divisions are rare in the endosperm whereas embryonic cells divide rapidly and the embryo eventually occupies most of the seed. The endosperm dies during this stage with the exception of the outer cell layer.
(a) (b) (c) (d)
Current Opinion in Plant Biology
CHZ
unknown function but which could be involved in either nutrient transfer to the embryo or in signalling processes.
At the chalazal end, the endosperm usually presents a peculiar organisation. Haustoria develop at this pole in some species [3]. In Arabidopsis, cellularisation is extreme-ly delayed and large nuclei are present in pockets of cytoplasm [11]. In maize, the region adjacent to the pedi-cel is characterised by the presence of transfer pedi-cells. These cells are probably involved in the transport of nutrients delivered by the phloem to the endosperm. The gene
BET1is expressed in this region [23] and encodes a small 7Kda cell wall localised polypeptide, the role of which remains to be determined.
Imprinting and gene dosage
Imprinting refers to epigenetic modifications which alter gene expression depending on parental origin. The
Arabidopsis mutant medea is female gametophytic and is characterised by a general delay in the development of the seed [24••]. This results in the development of a giant tor-pedo embryo surrounded by an endosperm with fewer nuclei than are found in wild-type at the corresponding embryo-mature stage. Mutant seeds subsequently
col-lapse. The gene MEDEA encodes a protein that
resembles enhancer of Zestof the polycomb family of pro-teins. It is expressed in the mature female gametophyte and during seed development until seed maturation. The contribution via the pollen of a wild-type copy of MEDEA
cannot restore MEDEA function in the mutant and it is hypothesised that the paternal copy of the gene is silenced via a mechanism typical of imprinting.
Imprinting could explain the importance of the mater-nal:paternal ratio in endosperm development. In maize, any deviation from the normal 2:1 ratio results in endosperm developmental failure and seed collapse [25]. This problem has recently been thoroughly re-examined in Arabidopsis[8•]. Whereas selfing of tetraploid and hexa-ploid plants results in a normal endosperm of higher ploidy, any deviation in the maternal:paternal ratio results in endosperm development abnormalities. Increasing the maternal contribution to 6:1 results in premature cellulari-sation of an endosperm containing fewer nuclei than normal and leads ultimately to seed abortion. Extreme imbalance of the maternal:paternal ratio causes arrest of embryogenesis at the heart stage, probably as a conse-quence of endosperm developmental arrest. Conversely, decreasing the maternal contribution to 2:3 causes a delay in endosperm cellularisation. In the latter case, endosperm nuclei proliferate thus underlining the proposed link between cellularisation and mitotic control.
Endosperm: the cross roads in the
developing seed
The endosperm occupies a central position in the seed. It separates the embryo from the source of nutrients close to the chalazal pole and from the integuments. Thus,
irre-spective of its persistence in the mature seed, the endosperm is likely to assume a pivotal role during seed development. Analysis of rice mutants with altered embryo size has shown that a number of mutations specif-ically affect the endosperm, which indicates that the endosperm regulates embryo development [26]. Some mutants present larger embryos than normal with reduced endosperm, whereas in others an enlarged endosperm occupies the space left by an embryo of reduced size. Thus, the endosperm might exert positive and negative interactions with embryo growth. Recent evidence from
Arabidopsis suggests that the amino acid nutrition of the embryo is dependent on the endosperm [9•]. The amino acid transporter AAP1 appears to be specifically expressed in the endosperm during early seed development although its function remains to be assessed by a knockout strategy.
In addition, evidence supports roles other than embryo nutri-tion for the endosperm. The characterisanutri-tion of the early expression of specific genes in the area surrounding the embryo suggests the existence of interactions between the endosperm and the embryo [22•]. Support for this hypothe-sis has recently been provided by the characterisation of the expression of the gene EP3 encoding for a type IV endo-chitinase in carrot [10•]. Endochitinase activity was shown to rescue a mutant line deficient for somatic embryo produc-tion. In cell cultures, EP3 appears to be expressed in cells which do not develop into embryos. In the developing seed,
EP3 is strongly expressed initially in the seed integument and later in the endosperm. The protein was immunolo-calised, however, in the endosperm at the time of expression in the integuments. The chitinase EP3 may act on substrates containing chitin motifs such as arabinogalactan proteins and release oligosaccharides important for embryogenesis. In somatic embryogenic cultures, EP3-expressing cells would, therefore, assume an endosperm-like role.
Thus it seems likely that the endosperm has functions critical for the development of its neighbour embryo. It appears, however, that endosperm development itself may be relatively independent from interactions with the embryo. Recently, in vitrofertilisation of the central cell has been achieved and the fertilisation product was cul-tured in vitro [7••]. The in vitro fertilised endosperm underwent developmental steps similar to the in vivo
developmental sequence. Synchronous nuclear divisions led to the formation of a syncytium which later cellu-larised in a polar manner. In vitro culture, however, is achieved using media containing various nutrients and hormones and a role played by the embryo during endosperm development cannot be excluded. The
favor of a relative independence for endosperm develop-ment from interactions with the embryo.
Endosperm death
The endosperm reaches the end of its life at germina-tion. Recent studies in maize have indicated the occurrence of programmed cell death in the endosperm [27•]. Characteristic internucleosomal degradation of DNA and nuclease activity were detected. Moreover, programmed cell death appears to be linked to ethylene signalling. Cell death is coincident with an increase of ethylene production and the mutant shrunken2, which produces high levels of ethylene, is characterised by a precocious and more extensive cell death than wild-type. It is not clear in seeds with non-persistent endosperm whether endosperm death is autonomous, or linked to signals provided by other parts of the seed. It has been shown in Petunia that the seed integument might play a role in the maintenance of endosperm during late seed development [28•]. Seeds of plants where the expression of the MADS box genes Floral Binding Protein 7 (FBP7)
and FBP11 was cosuppressed are characterised by a degeneration of the inner layer of the integument. This causes the degeneration of the endosperm which sug-gests that endosperm death may be controlled by interactions with the seed coat.
Conclusions
There has been a strong renewal of interest in the devel-opment of endosperm during the past year. Despite the fact that cereals with persistent, economically-relevant endosperm have until now attracted most attention it is becoming clear that Arabidopsis with its minimal endosperm, easily amenable to cytology, leads the way as a model for characterisation of new mutant phenotypes and to a thorough understanding of the basis of endosperm development. The description of new mutant phenotypes has shown that the syncytial endosperm is a model of choice to study mitosis and other aspects of cell biology in plants. Mechanisms involved in imprinting may be studied using recently characterised endosperm mutants. Altogether, current data indicate that the endosperm and the embryo inter-act to some degree during their development and understanding the nature of those interactions is crucial for research in seed biology.
Note added in proof
A recent paper by Mayer et al. has provided further insights into genes involved in cytokinesis and mitosis [29•].
Acknowledgements
This work was supported by the INRA. The author thanks Ueli Grossniklaus and Chun-ming Liu for sharing results before publication. Gwyneth Ingram, Mark Cock, Corrine Boisnard-Lorig, Christian Dumas and Peter Rogowsky kindly contributed to corrections and discussions of the manuscript.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Chaudhury AM, Craig S, Dennis ES, Peacock WJ: Ovule and embryo development, apomixis and fertilization.Curr Opin Plant Biol 1998, 1:26-31.
2. Lopes MA, Larkins BA: Endosperm origin, development and function. Plant Cell1993, 5:1383-1399.
3. Vijayaraghavan MR, Prabhakar K: The endosperm.In Embryology of Angiosperms.Edited by BM Johri. Berlin: Springer-Verlag;
1984:319-376.
4. Olsen OA, Brown RC, Lemmon BE:Pattern and process of wall formation in developing endosperm.Bioessay1995, 17:803-812.
5. Friedman WE: Evidence of a pre-angiosperm origin of endosperm: implications for the evolution of flowering plants.Science1992, 255:336-339.
6. Friedman WE: Organismal duplication, inclusive fitness theory and altruism: understanding the evolution of endosperm and the angiosperm reproductive syndrome.Proc Natl Acad Sci USA 1995, 92:3913-3917.
7. Kranz E, von Wiegen P, Quader H, Lörz H: Endosperm development •• after fusion of isolated single maize sperm and central cells in
vitro.Plant Cell1998, 10:511-524.
The first report of in vitrofertilisation of isolated central cells. This outlines the uniqueness of endosperm development and its independence from the development of the embryo.
8. Scott RJ; Spielman M, Bailey J, Dickinson HG:Parent-of-origin • effects on seed development in Arabidopsis thaliana.
Development 1998, 125:3329-3341.
Crosses between parents of different ploidy in Arabidopsis confirm the importance of the maternal:paternal ratio for endosperm development. Imbalance results in an abnormal timing of cellularisation and can be lethal to the embryo. These results could be related to the phenotype of the mutant tetraspore where aberrant endosperm has been observed.
9. Hirner B, Fischer WN, Rentsch D, Kwart M, Frommer WB: • Developmental control of H+/amino acid permease gene
expression during seed development of Arabidopsis.Plant J 1998, 14:535-544.
The expression of the amino acid transporters AAP1 and AAP2 is studied in Arabidopsis using GUS-promoter fusions. At the globular stage, AAP1 is localised in the endosperm surrounding the embryo and the embryosac wall as well as in the cotyledons of the embryo during late stages of develop-ment. Despite its non-persistent nature in Arabidopsis seed, the endosperm is most likely to play a role in embryo nutrition.
10. van Hengel AJ, Guzzo F, van Kammen A, de Vries SC: Expression • pattern of the carrot EP3 endochitinase genes in suspension
cultures and in developing seeds.Plant Physiol1998, 117:43-53.
The carrot chitinase EP3 was previously identified on the basis of its ability to rescue somatic embryogenesis in a defective cell line. Its expression pattern is dissected both in somatic embryo cultures and during zygotic embryogenesis. This shows that EP3 is expressed in non-embryogenic cells in cultures and in the integuments and endosperm of developing seeds. These results indicate interactions between the various parts of the seed to support embryogenesis.
11. Mansfield SG, Briarty LG: Endosperm development InArabidopsis, an Atlas of Morphology and Development. Edited by J Bowman. Berlin: Springer-Verlag; 1993:385-397.
12. Randolph LF: Developmental morphology of the caryopsis in maize.J Agr Res1936, 53:881-916.
13. Lauber MH, Waizenegger I, Steinman T, Schwarz H, Mayer U, • Hwang I, Lukowitz W, Jürgens G: The ArabidopsisKNOLLE protein
is a cytokinesis-specific syntaxin.J Cell Biol1997, 6:1485-1493. The characterisation of a key player in cytokinesis in plants. Immunolocalisation techniques show the localisation of the syntaxin KNOLLE to membranes at the plane of division during cytokinesis. Cytological analysis of the mutant defective for KNOLLE shows that vesi-cle fusion at the cell plate is affected. This syntaxin is involved in cytoki-nesis in all cell types with the exception of pollen second meiotic division.
15. Liu C, Meinke DW: The titanmutants of Arabidopsisare disrupted • in mitosis and cell cycle control during seed development.Plant J
1998, in press.
An interesting report of novel phenotypes affecting endosperm nuclei size. The mutants described probably represent the first example of a large and heterogeneous class of mutants defective in basic cell biologi-cal functions.
16. Bosnes M, Weideman F, Olsen O-A: Endosperm differentiation in barley wild-type and sex mutants.Plant J1992, 2:661-674.
17. Doan DNP, Linnestad C, Olsen O-A:Isolation of molecular markers from the barley endosperm coenocyte and the surrounding nucellus cell layers.Plant Mol Biol1996, 31:877-886.
18. Becraft PW, Stinard PS, McCarty DR: CRINKLY 4: a TNFR-like receptor kinase involved in maize epidermal differentiation. Science1996, 273:1406-1408.
19. Olsen OA, Brown RC, Lemmon BE:A model for aleurone development.Trends Plant Sci1998, 3:168-169.
20. Lu P, Porat R, Nadeau JA, O’Neill SD: Identification of a meristem L1 layer-specific gene in Arabidopsisthat is expressed during embryonic pattern formation and defines a new class of homeobox genes.Plant Cell1996: 8:2155-2168.
21. Schel JHN, Kieft H, van Lammeren AAM: Interactions between embryo and endosperm during early developmental stages of maize caryopses (Zea mays).Can J Bot1984, 62:2842-2853.
22. Opsahl-Ferstad H-G, Le Deunff E, Dumas C, Rogowsky PM: ZmEsr, a • novel endosperm-specific gene expressed in a restricted region
around the maize embryo.Plant J1997, 12:235-246.
This work describes a family of novel genes expressed specifically in the endosperm located around the embryo. No sequence homology has been found but the localised expression of these genes suggests a role in inter-actions between the embryo and the endosperm.
23. Hueros G, Varotto S, Salamini F Thompson RD: Molecular characterisation of BET1, a gene expressed in the endosperm transfer cells of maize.Plant Cell1995, 7:747-757.
24. Grossniklaus U, Vielle-Calzada J-P, Hoeppner MA, Gagliano WB: •• Maternal control of embryogenesis by MEDEAa Polycombgroup
gene in Arabidopsis.Science1998, 280:446-448.
MEDEA encodes a potential regulator of transcriptional activators. The female gametophytic nature of the mutation and its associated phenotype
are rather complex and intriguing. This might be a door opened towards the understanding of imprinting.
25. Lin B-Y: Ploidy barrier to endosperm development in maize. Genetics1984, 107:103-115.
26. Hong SK, Kitano H, Satoh H, Nagato Y: How is embryo size genetically regulated in rice?Development1996, 122:2051-2058.
27. Young TE, Gallie DR, DeMason DA: Ethylene-mediated • programmed cell death during maize endosperm development of
wild type and shrunken2genotypes.Plant Physiol1997, 115:737-751. A thorough study of cell death associated with endosperm maturation in maize. Nuclease activity is detected in the endosperm of developing kernels. The activity is high before reserve storage and, later, a second smaller peak of activity occurs during seed maturation. An increase in the production of ethylene and of its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) takes place in the endosperm at the time of the onset of cell death. Ethylene treatments result in more rapid and extensive cell death. The mutant shrunk-en 2characterised by more extensive and earlier cell death produces high-er levels of ethylene and ACC. Finally, treatment with an ethylene synthesis inhibitor reduces the abnormally large extent of DNA fragmentation in sh2.
28. Columbo L, Franken J, Van der Krol AR, Wittich PE, Dons HJM,
· Angenent GC: Downregulation of ovule-specific MADS box genes from Petunia results in maternally controlled defects in seed development. Plant Cell 1997, 9:703-715.
Downregulation of the Petunia MADS box genes FBP7 and FBP11 using cosuppression results in abnormal seed development under a genetic mater-nal control. The cosuppression appears to result in the degeneration of the innermost cell layer of the seed-coat, the endothelium. In parallel, the endosperm developing at proximity of degenerating endothelium cells shows some abnormalities, which indicate interactions between these two compo-nents of the seed. These interactions may involve the supply of nutrients as well as developmental signals.
29. Mayer U, Herzog U, Berger F, Inzé D, Jürgens G: Mutations in the
· PILZ group genes disrupt the microtubule cytoskeleton and uncouple cell cycle progression from cell division in Arabidopsis embryo and endosperm.Eur J Cell Biol 1999, in press.