Summary Relative absorptance of intact branches measured with an integrating sphere was compared to leaf area estimated by conventional methods (volume displacement and scanning area meter) for three conifer species: Picea mariana (Mill.) BSP, Pinus banksiana (Lamb.) and Pseudotsuga menziesii (Mirb.) Franco. A consistent relationship between relative ab-sorptance and surface area emerged for the three species. The ability to predict leaf area from absorptance was further ex-plored by measuring branches of Pseudotsuga menziesii grown in varying light and nutrient regimes. When a single equation was used to predict leaf area under all growth conditions, errors were as large as 40% primarily because of variation in leaf absorptivity, with the largest errors associated with extremely nutrient-deficient foliage. When separate empirical equations were developed for each growth treatment, predicted leaf sur-face area agreed to within 5% of the area determined by the volume displacement method. Leaf surface area estimated from theoretical principles was also in good agreement with total surface area estimated independently by conventional methods. With proper accounting for needle absorptivity, which varied with growth conditions, leaf area estimates ob-tained by the integrating sphere method were of similar accu-racy to those obtained by conventional methods, with the added advantage that the method allowed intact foliage to be sampled nondestructively in the field. Because the integrating sphere method preserves branch structure during measurement, it could provide a useful measure of needle area for photosyn-thetic or developmental studies requiring repeated sampling of the same branch.
Keywords: absorptance, leaf area determination, light absorp-tion, Picea mariana, Pinus banksiana, Pseudotsuga menziesii.
Introduction
Leaf area is a key parameter for gas exchange measurements and is often determined by methods that are both destructive and time-consuming. Leaf area measurement of needle-leaved species is notoriously difficult and may be confounded by differences between surface area and projected area. Most ‘‘leaf area meters’’ involve estimates of projected surface area, which may be a poor representation of actual surface area, particularly in sclerophyllous, needle-shaped leaves (e.g., most
conifers and many chaparral shrubs). For such cases, the vol-ume displacement method (Johnson 1984) is often employed to provide a more direct estimation of total surface area. However, use of either a scanning leaf area meter or, depending on how it is employed, the volume displacement method, typically involves destruction of the sample, which precludes repeated measurements of growing tissue.
Because light absorption scales with leaf area (Oquist et al. 1978), optically based absorption techniques present a possi-ble alternative to conventional methods of leaf area determina-tion. Integrating spheres provide a readily standardized method of determining light absorption (Idle and Proctor 1983). When a leaf or branch tip is enclosed in an integrating sphere, the decrease in internal light flux relates directly to the radiation absorbed by the enclosed sample, which in turn can be related to the surface area of the sample (Oquist et al. 1978). However, absorption depends not only on sample size or area but also on other factors, including leaf structure and pigmen-tation (Gates et al. 1965, Gausman 1985), and degree of mutual shading. We have used integrating spheres to explore the rela-tionship between relative absorptance and leaf area for three needle-leaved species grown under a variety of conditions. The objective of the study was to develop a fast, reliable, and nondestructive method for leaf area determination.
Materials and methods
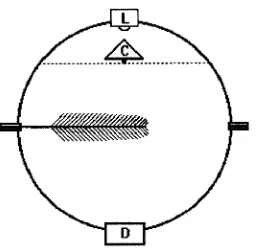
Two integrating spheres measuring 10.2 and 12.7 cm in diame-ter, respectively, were constructed from copper spheres, avail-able commercially as floats for water tanks. Each sphere was cut in half, a copper gasket was soldered to the rim of each hemisphere, and the gasket surfaces were padded with white foam (Li-Cor spare part 6000-10, Li-Cor, Inc., Lincoln, NE), providing a light-tight surface for complete enclosure of a branch tip (Figure 1). The inside of each sphere was first sealed with a high temperature paint (Colorworks, Krylon, Solon, OH) followed by several layers of barium sulfate suspension (white reflectance coating #6080, Eastman Kodak Co., Roch-ester, NY). Illumination was provided with a 1.2 A halogen lamp (L1030, Gilway Technical Lamp, Woburn, MA) located in a port at the top of the sphere. Quantum flux density was sampled with a Li-Cor quantum sensor (Model LI-189) placed
Estimation of leaf area with an integrating sphere
LYDIA SERRANO,
1J. A. GAMON
1,2and J. BERRY
3 1Department of Biology and Microbiology, California State University, Los Angeles, 5151 State University Drive, Los Angeles, CA 90032, USA
2 Author to whom correspondence should be addressed
3
Carnegie Institution of Washington, Department of Plant Biology, 290 Panama Street, Stanford, CA 94305, USA
Received June 25, 1996
in a port at the bottom of each sphere. To distribute the light homogeneously and to avoid direct illumination of the quan-tum sensor by the lamp, a small cone was mounted directly underneath the light bulb. The cone and the corresponding support were also painted with white reflectance coating.
During measurements, voltage was set to 3.3 V to provide a quantum flux of approximately 150 µmol m−2 s−1 within the empty sphere. Absorptance was calculated as:
Absorptance =(Isphere −Ileaf)/Isphere, (1)
where Isphere is the irradiance at the bottom of the empty sphere, and Ileaf is the irradiance with the branch in the sphere. An absorptance determination for a single branch normally re-quired about 30--60 s to complete. To assess sphere radial symmetry, repeated measurements were taken by changing the position of the branch inside the sphere or by rotating the upper hemisphere. Standard errors associated with changes in either twig or lid position were, on average, smaller than 0.6% of the average relative absorptance value.
Measured absorptance can be affected by the interaction between sphere size and sample area (Idle and Proctor 1983), and possibly by geometric and optical variations between spheres. Preliminary measurements performed on the same set of twigs using both spheres showed that the measured absorp-tance values were slightly higher for the smaller, 10.2-cm-di-ameter sphere (data not shown). For these reasons, we apply the term ‘‘apparent’’ or ‘‘relative’’ absorptance to our results. Unless otherwise noted, the results reported are those meas-ured with the larger, 12.7-cm-diameter sphere.
We tested the absorptance--area relationship for three need-le-leaved species: Picea mariana (Mill.) BSP (black spruce), Pinus banksiana (Lamb.) (jack pine), and Pseudotsuga menzi-esii (Mirb.) Franco (Douglas-fir). The Picea and Pinus branches were collected near Candle Lake, Saskatchewan, Canada in the summer of 1994, as part of the BOREAS study (Sellers et al. 1995). Pseudotsuga menziesii branches were sampled in 1995 and 1996 in Los Angeles, CA, from potted, nursery-grown seedlings (California Department of Forestry, Ben Lomand Nursery, Santa Cruz, CA). For at least a year before measurement, the Pseudotsuga menziesii seedlings
were exposed to one of two growth irradiance regimes (full sun and shade) and the sun-exposed seedlings were grown in one of two nutrient regimes (high and low), determined by the addition of a slow-release fertilizer (Osmocote 17,7,12, Grace Sierra Co., Milpitas, CA) to the potting mix. The low-nutrient treatment had no supplemental fertilizer, resulting in visibly chlorotic needles (chlorophyll contents, as determined by HPLC, ranged from 114 to 372 µmol m−2, expressed on a projected area basis). Shade treatments were provided by a grove of Pinus canariensis Sweet ex K. Spreng. trees, which provided a monthly average integrated daily PPFD that ranged from 2.73 to 14.1 mol m−2, whereas sun treatments ranged from 9.49 to 38.9 mol m−2. The irradiance treatments had marked effects on branch tip morphology; the needles of sun branches were more erect than the relatively horizontal needles of shade leaves. Measurements were also conducted on two needle-age classes (current-year and 1-year-old foliage) that differed slightly in cross-sectional shape (mature needles had a larger area to volume ratio than current-year needles; data not shown).
Projected leaf area was determined with a flatbed scanner (Scanmaker IIXF, Microtek Laboratories Inc., Torrance, CA) attached to a Macintosh Centris 650. Images were digitized using Adobe Photoshop (Adobe Systems Inc., Mountain View, CA) and analyzed for area using IPLab Spectrum (Signal Analytics Corp., Vienna, VI). Surface area (SA) was also estimated from volume displacement (Johnson 1984) using the following formula:
SA=F√VnL, (2)
where L is the average length in cm from a subsample of 10 to 12 needles, n is the number of needles, V is the branch tip volume in cm3, and F is an empirical shape factor usually considered constant for a given species. (For this study, we used F values of 4.00 for Picea mariana, 4.10 for Pinus banksiana, and 4.17 for Pseudotsuga menziesii, as reported in Sellers et al. 1994.) In some cases, results were expressed as ‘‘hemi-surface area’’ (half of the surface area) to allow closer comparison with projected area measurements.
As a further test of sphere behavior, we compared empiri-cally derived absorptance measurements (using the methods described above) with leaf area estimates determined with the two spheres using theoretical principles. According to Idle and Proctor (1983), the radiant flux (Q) entering a sphere can be related to absorbing area (A), absorptivity (α), and flux density within the sphere (Φ1) as follows:
Q=Φ1(Asαs). (3)
Adding a branch segment of area AB and absorptivity αB reduces the flux density to Φ2, allowing Equation 3 to be modified as follows:
Q=Φ2(Asαs+ ABαB). (4)
Branch tip area can be estimated by combining Equations 3 and 4 and solving for AB:
AB=(Asαs/αB) ((Φ1/Φ2)− 1). (5)
Absorptivity of each sphere was estimated according to Idle and Proctor (1983) and Equations 3--5 by inserting pieces of black paper with known area and absorptivity. Paper absorp-tivity was determined with a separate integrating sphere (LI-1800, Li-Cor, Lincoln NE). Absorptivity of the small and large sphere was determined to be 0.049 and 0.060, respectively. Internal surface area was estimated to be 372.3 and 547.6 cm2 for the small and large sphere, respectively. Branch tip absorp-tivity (absorptance) was determined according to Equation 6 by measuring bidirectional reflectance with a fiber optic probe attached to a leaf reflectometer (Gamon et al. 1993) and as-suming negligible transmittance for these thick needles and twigs.
Absorptance = 1−(reflectance +transmittance). (6)
Branch tip absorptivity estimated in this way was approxi-mately 0.875, which is midway between the measured values of 0.85 (stems, assuming no transmittance) and 0.90 (needles, assuming no transmittance).
Hemi-surface and projected area data were log-transformed before statistical analysis (Statview 4.5, Abacus Concepts, Berkeley, CA). Analysis of covariance was used to study the effect of species and treatments on the absorptance--area rela-tionship and simple linear regressions were used to establish the relationship between ln(area) and absorptance.
Results and discussion
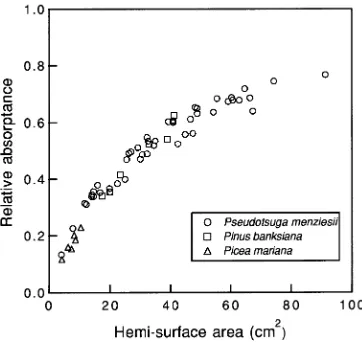
The relationship between branch relative absorptance and leaf area was nonlinear, and could be roughly described by a single relationship for all three species (Figure 2). This nonlinearity is an inherent function of canopy light absorption; the fraction of photosynthetically active radiation absorbed in canopies is an exponential function of leaf area (Monteith 1973).
When leaf area was determined by the volume displacement method, the absorptance--area relationship of Pseudotsuga menziesii was noticeably affected by the treatments (Fig-ure 3A). Even though the general relationship for all Pseudot-suga menziesii treatments combined was highly significant and the standard error of the estimate was small, analysis of covariance yielded significant differences between treatments (results not shown). Consequently, further analysis of the ab-sorptance--hemi-surface area relationship was performed with a separate linear regression for each treatment (Table 1). Un-like the results from the volume displacement method, the comparison of leaf area, estimated by the projected area method, with relative absorptance yielded very little scatter (Figure 3B). Because no significant treatment differences were found (data not shown) for this comparison, only one linear regression was calculated (Table 1).
Because the sphere provided diffuse illumination during area estimation, we expected absorptance to scale more closely with surface area than with projected area. It is likely that the better agreement with projected area than with surface area was due to errors inherent in applying a single species ‘‘shape factor’’ (F = 4.17) when using the volume displacement method. In reality, needle shape varied slightly with growth stage and treatment, as illustrated by a comparison of areas determined by the other methods (Figure 4). Although not a direct measure of F, variation in the ratio of areas determined by these two methods indicated variation in needle surface to volume ratio. Among treatments, the biggest variation in nee-dle shape was associated with neenee-dle age (Figure 4). This finding is consistent with the pattern reported in Figure 3A, indicating the highest absorptance--area values were for the mature, older foliage. These observations suggest that greatest accuracy with the volume displacement method could be ob-tained by empirically determining separate shape factors (F values) for each growth stage and condition encountered. However, calculation of multiple shape factors would signifi-cantly lengthen the time it takes to obtain an area estimate, reducing the simplicity of this method. Because the sphere method samples the effective light absorption of intact branches, it presumably minimizes potential errors associated with varying needle anatomy and branch morphology.
To test the predictive ability of the sphere method, we applied the empirical equations shown in Table 1 to estimate leaf areas of an independent set of branches, and then com-pared these estimated areas with leaf areas determined by the volume displacement method (Figure 5). When a general equa-tion for all treatments together was used, leaf areas of high-nu-trient plants grown in either sun or shade were close to the expected values, but leaf areas of severely chlorotic branches
Figure 2. Relationship between relative absorptance and hemi-surface area estimated by the volume displacement method for twigs of
(chlorophyll content of 33 µmol m−2) were underestimated by as much as 40% (Figure 5A). Use of specific regressions for each treatment (sun, shade, and low nutrient; Table 1) reduced this error to approximately 5% (Figure 5B). Similar error estimates for projected area were even smaller (< 1%, data not shown). Clearly, to obtain accurate area estimates with the integrating sphere method, careful consideration must be given to growth conditions and to leaf pigmentation when develop-ing empirical absorptance--area calibrations, because these factors can influence leaf absorptivity and thus apparent leaf area.
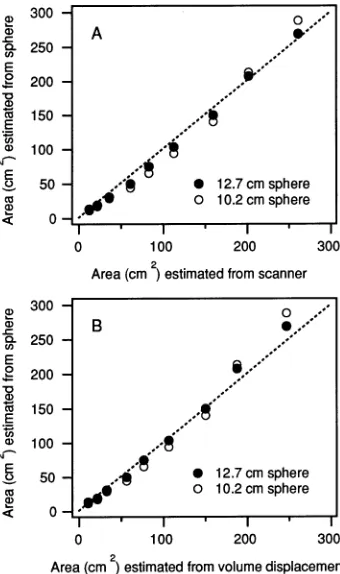
Needle areas estimated with the sphere from theoretical principles (Equation 5) were in good agreement with both twice the projected area measured by the scanner and total surface area estimated by volume displacement (Figure 6). Compared to the volume displacement method, the scanner yielded values that were in slightly closer agreement to the sphere-determined needle areas (Table 2). Compared to the smaller sphere, the larger sphere yielded values that were in slightly better agreement with areas determined by either the scanner or volume displacement method (Table 2). Because each method carries inherent errors, it is not possible to deter-mine which of these methods provides the most accurate
Figure 3. Panel A: Relationship between relative absorptance and hemi-surface area esti-mated by the volume displace-ment method for Pseudotsuga menziesii, where different sym-bols indicate different growth treatments. Treatments include high and low nutrient treat-ments in full sun, 1-year-old fo-liage (old) and current-year (young) foliage, and shade branches. Panel B: Relationship between relative absorptance and projected area for Pseudot-suga menziesii.
Table 1. Regression parameters for the linear relationship between ln(hemi-surface area) (HA) or ln(projected area) (PA) and absorptance of Picea mariana, Pinus banksiana or Pseudotsuga menziesii. For Pseudotsuga menziesii, the effects of several treatments on the relationship were considered: high nutrient (HN), low nutrient (LN), current-year twigs (Y), 1-year-old twigs (O) and shade plants (SH). The SEE indicates standard error of the estimates.
Species Area mea- Treatment x-Intercept Slope r2 P n SEE surement
method
Picea mariana HA −0.054 0.116 0.87 0.006 6 0.016
Pinus banksiana HA −0.556 0.308 0.96 0.001 6 0.026
Pseudotsuga menziesii HA HN-Y −0.450 0.272 0.99 < 0.0001 7 0.010
Pseudotsuga menziesii HA LN-Y −0.363 0.239 0.99 < 0.0001 7 0.008
Pseudotsuga menziesii HA HN-O −0.283 0.230 0.98 < 0.0001 7 0.022
Pseudotsuga menziesii HA LN-O −0.327 0.019 0.99 < 0.0001 7 0.007
Pseudotsuga menziesii HA SH −0.206 0.215 0.99 < 0.0001 11 0.023
Pseudotsuga menziesii PA −0.337 0.253 0.98 < 0.0001 27 0.018
measurements, and the choice of method is probably best determined by the particular experimental goal and tools at hand. The good agreement between sphere measurements based on theoretical principles and needle areas estimated by conventional empirical methods further confirms the ability of the integrating sphere to determine needle area accurately, even in the absence of the calibrations presented in Table 1. However, according to Equation 5, the accuracy of this theo-retical approach depends on accurate estimates of sphere area, sphere absorptivity, and needle absorptivity. In particular, nee-dle absorptivity can be difficult to measure, especially in the field. For this reason, empirical calibration of sphere-deter-mined needle areas with conventional methods would typi-cally be needed.
The most meaningful expression of conifer leaf area is open to debate and a subject of continued research. Several recent studies have considered the ratio of shoot silhouette area to total needle surface area (‘‘STAR’’), which may be an impor-tant factor in the photosynthetic responses of conifer shoots (Smith et al. 1991, Oker-Blom et al. 1992, Hemmerlein and Smith 1994). Clearly, the interpretation of gas exchange re-sults, which are typically expressed on an area basis, and the comparison of results from different studies and species can be confounded by the lack of a single, standard method for sam-pling leaf area. The issue of ‘‘effective leaf area’’ versus meas-ured area is further complicated by the complex light fields
present in forest canopies, which can vary from largely direct to largely diffuse. Although the integrating sphere method presented here does not resolve these issues, it does present a simple, rapid, and field portable tool for assessing leaf area on intact shoots. Because it is nondestructive, the integrating sphere method could be especially useful in developmental or photosynthetic studies requiring repeated sampling of the same branch.
Table 2. Regression parameters for the relationships in Figure 6. Regressions were forced through the origin. The SEE indicates stand-ard error of the estimates.
Figure Sphere Slope r2 P SEE panel diameter
(cm)
A 12.7 0.995 0.997 < 0.0001 8.003 A 10.2 1.010 0.987 < 0.0001 16.379 B 12.7 1.061 0.997 < 0.0001 7.860 B 10.2 1.091 0.987 < 0.0001 14.954 Figure 5. Panel A: Relationship between actual and predicted
hemi-surface area for Pseudotsuga menziesii derived from a single equation for all treatments (Table 1). Panel B: Relationship between actual and predicted area derived from separate equations for each treatment.
Figure 6. Comparison between needle areas for Pseudotsuga menziesii
Acknowledgments
This work was supported by grants from NSF (# DEB-9220258 to J.G.) and NASA(# NAGW-3707 to J.G., and # NAG52239 to J.B.). Technical support was provided by R. Stuber, R. Johnson, D. Horvarth and B. Welsh. Additional thanks go to J. Collatz for the provision of one of the copper spheres and to A. Fredeen, B. Yoder, and D. Sprugel for comments on the manuscript.
References
Gamon, J.A., I. Filella and J. Peñuelas. 1993. The dynamic 531 nm ∆ reflectance signal: a survey of twenty angiosperm species. In Pho-tosynthetic responses to the environment. Eds. H.Y. Yamamoto and C.M. Smith. Am. Soc. Plant Physiologists, Rockville, MD, pp 172--177.
Gates, D.M., H.J. Keegan, J.C. Scheleterand and V.R. Weidner. 1965. Spectral properties of plants. Appl. Opt. 4:11--20.
Gausman, H.W. 1985. Plant leaf optical properties in visible and near-infrared light. Graduate Studies No. 29, Texas Technical Uni-versity, Texas Tech. Press, Lubbock, TX, 78 p.
Hemmerlein, M.T. and W.K. Smith. 1994. Structural scaling of light interception efficiency in Picea engelmannii and Abies lasiocarpa. Tree Physiol. 14:1139--1148.
Idle, D.B. and C.W. Proctor. 1983. An integrating sphere leaf chamber. Plant Cell Environ. 6:437--439.
Johnson, J.D. 1984. A rapid technique for estimating total surface area of pine needles. For. Sci. 30:913--921.
Monteith, J.L. 1973. Principles of environmental physics. Edward Arnold, London, 241 p.
Oker-Blom, P., T. Lahti and H. Smolander. 1992. Photosynthesis of a Scots pine shoot: a comparison of two models of shoot photosyn-thesis in direct and diffuse radiation fields. Tree Physiol. 10:111--125.
Oquist, G., J.E. Hallgren and L. Brunes. 1978. An apparatus for measuring photosynthetic quantum yields and quanta absorption spectra of intact plants. Plant Cell Environ. 1:21--27.
Sellers, P.J., F.G. Hall, D. Baldocchi, J. Cihlar, P. Crill, J.D. Hartog, B. Goodison, R.D. Kelly, D. Lettenmeier, H. Margolis, J. Ranson and M. Ryan. 1994. Appendix K. Measurement methodologies: experimental plan. Boreal Ecosystem--Atmosphere Study, Version 3.1. NASA Goddard Space Flight Center, Greenbelt, MD, pp K1--K3.
Sellers, P.J., F.G. Hall, H. Margolis, R.D. Kelly, D. Baldocchi, J.D. Hartog, J. Cihlar, M. Ryan, B. Goodison, P. Crill, J. Ranson, D. Lettenmeier and D.E. Wickland. 1995. The boreal ecosystem--atmosphere study (BOREAS): an overview and early results from the 1994 field year. Bull. Am. Meteorol. Soc. 76:1549--1577. Smith, W.K., A.W. Schoettle and M. Cui. 1991. Importance of the