e*g#.."
d
EdDt.iIEl-
?
C.
l
tyL
iI.tGiIl
IE53
I.,l-.tl ICESS
KE
Yl
ICT'E
PTJRE
A3$M
AFtrh}EM
Cffi
INTERNATx*}TTA.T
C&NFHRENCE
KffiS
SUSTAINABKH
MHVHTSP}WENT
TN
CHHMISTRY
BA$EN
QhT
INMIGHNSUS
K}$SWIHMGE
Ier*qJARY 14.$S- kS(}$
AN uNIvER.
]TK S*xxrysamq:a*K
T]*ffrffiqx>
o\
a
U)
bt
()
q
0)
a
OEJ
"E
aa
0ro
b0
(,) rJ1
0)
(,
Q
bI
(J
A
'cc k d 'c
CN J
o
.o
a_)
4)
o p
o
o o
() ()
()
U
a a () a
a a ()
bI
a a
.A)
.o
G
F
(H
o
d)
o
o
a
o o
U
qo.l
9O ! !
otr
a= L". A-v
hI
>rd
-N
^!
=
9a
otr
OG !!
(c
xa
r€
L.
oe
\2
6A,()
'=OFI E a '*.
50ts c6 c!
v7
o-o:
O::
.Ao
()=j
()-96
aa
a a
a
ro
a
I
(,) 'l
>,
C)
a tO rg
trB
.9<
cE(a OE Lq)
.'" bJ
QnA
=r'
S
crD6J
!> aQ)
=>
a-oo)
2a
bTB
VN
3z
.=v
o0
C.)
a a
.o
U
a aG)
I
a a ,o)
>,
a
O
C)
o,
a.)
>. a !
()
D
()
li
Z
{.) (.)
()
o
a +
o
o
fJr
o
0.) >,
-o
tr
o -o
Q
o
(-)
! i) b0
F-a
k
o
il
t-..1
tr
o
a
,9 o
! +i
o o o .d o
!
U
a aO
0 a ()
bo
4)
a
O
.a)
()
6)
,]
o
()
9 q,
a
() ()
I
at
N
bI
a
OI
()
o
bI
a
(,
a a c) I
a
U)
()
AT
tt
a a
a
(,
U)
ZZ
Je
() I
a
I
a
a
L9
r-'-Eoo
trs
Err
e. ;a
0!
a
z
o
U U
o
o
o
L
'!l
tr
63
J
50
o o
.q
o
a) F
o
F
()
)
63
50 6
(d
'a
'o
E
!
o
!
a
tr5{)
o
p
L
a)
=
o
L
z
+ro
tr
o
()
!
o
l-.1
'tr
i.t !-ocd
cE
92 t
oO (a^
cux
*a
=x
VF *a---cocc,
>-6
trrLo 6()o'
F'o
xc
EEI tuy@ i ., 6- x<v
e
=-i9
=CD TA 6
CJtrXX : iq il
f9.!
cs
E
rl
O:F
,
.a-r]
O* -.'1 tat CN+ CO
oo
:^'
vl .1--
OO rl= OOO-++
,_,a
--i=
t
6l
-lr
t-Z
nof
ttee
iarm roud Pure
nt
in tlaryr ists the
)of
I
all10rn 10te
14ry rif-ic
io4
the
nts"
nce
1
ng
I
a
J _
=-
==aa-== = ja
.=---.)
=
=/-=,=
==, *a?
-=
- = a = -.=
=-;---, =' = - r
=- ,'-
=:,
a.
t:
l-l
=Z Z=
Z
-:-, =-
-
-==t-=
,a-=
==
=!
=-Z
Z
z
,r,r
:
,l- lr.
r
F
-N
co
aa
oo
aaa ()
a
z
() ()
c,)
O
a
(,
tr
(,
Z
=
J
a
fr
a
.a
o
a
o
b!
bn
o
a
ocoo
CCCC qa6a@
!n
t- J
)z
=
z=a
ooo
O=Caaa&
o
= a
">
Y:z
car 4
.i!
>a
9r€5-+-+-d **J**
---F-rrrr a6aaa
! !N
--!->.Y 2
-- = !
:r.:t
d&d)
^-^=
NNr-l av6';
-.il
ta
*a
Ea=E
EZ
2z
-on
Oc
-a ! o3,r_
ez
z;
6
-No+'-, nJ**p
OOOOO rrrrr aaaaa
-r= '-. > '.E )Z F ir ?
=al-*N-t'a) ***Jp
rrrrr
a)A)AA)A
=_
d> i:
2d = '=a\
+dr,a'., Jda*alcl
QQQqq
ddNNN
AAqAq
jjtr 5!
= >,r, N E L'= ?
A c'= t.= .: EF:{ i-] !,
L
2- a
J7 t
!d = '=Qa
csrrl
**)*l NdNd^,,
a aaaA
'-Qf+d ***)J
---€€€oc 6AAAA
E e* q )z=a .ra a= i
c. t;
oooc
AA)qA>.:
=-:}\a'
a1';.iFZ
A
*EF€.=
J-r^ -!
co v'; s v
-N-+d U*J**
ooooo
-r) -99 qA)AAoa
-a- A
e >.'- -. =.2 Z.z z: j I
UU
,
)Z a
Ara
';
u)it
&.4 XYY_l_t€g ,
aa aa
.,=
=-=
!i=
-'<>+-e
)z!a EaZ a
,5
= >.c
i e.V
oooo
aa6a=?'
d z'e
L rd - E
'i-, :: \a== rzli
E^>n
\.?q=
6 AA;
!
'1 >=
>a
de&d.
oooo
9949 a9
aa
rxo.-dd&) aa aA
=
'.
=
'_i,
-=
=
=
,
=:
= =
-)
=
t
-||l
ttt
ttt
!t
II
tl
ttl
]Il
ttt
rttt
t.ttt
i=l I
Il.'l I I
ia,t
tt
lrl ll
:l I
I
_!t I I
-ttt
it lt
:t I tu zl I l;'=l
I
l=al I l:
'!l llF =l I
l-'lll€
=l I l.
:l I I'
2l I l:
, .. tl I lJ :: =l I lo
)-=l I
li.
.:,-l I
l=- ^ al I I u , -
-l I l-t,
.=
alll l;
- = - - -l ,l --t )t t:lil
- : -l-l l-- - =t I I a)
- = 1l tl l;i
< - )l-l I
:'
=1.-l ,la'=::lrlilt
- - =t =t ll 9
- :l=l+1., ' 1-.1 .1.?l . - - :lvlel =
:
-.- zl
1.9
!= nl I!
- - -) =t I ; -'
=j.l t!
= , =lal l:l : a =t-t I o . - , =lo , l!
-t-- I rl - :l-l l-= - )l)1 l! - ' ^l.l I =
- - ' : t =1.,1 | =
19 l=
i !-'a--l)
--z
:
= i'!
- - - , Ic - - - -Il I :
: _ - a) tE - = - It
1:=- lv
, : i z l--I :.
: , t
= la
=_::=221 il
:-tr
-:
ti-_tl' - : :l
I
:- ---l l.vl
: : .
f l i:
; : - ' I l- l
lrl
a F o o C a t a o I o tr C (, a = a a N E 1 a =';
1 a .t. .c /\ a a = L = .2 vc
€ c o =-
= Lil';
= /. E ! E ao = O p a 5 l-E a = o-o & a ,. ,= ! E ? .,2 a o = (, c.
= j ! e F':
,= = -l) = 1/
;-a =tl
.,1 -lOI =l ol ol ol =t -l ,'| =l;)
ol :l EI -.1 =l ^l;
": .:l '--t Ll tlrl
.=l Llrl
i E d -a U o O a a L c :I EI a c :l 5 o .,2 '6 4. .) O 6 ,ar
a .= = =a a a t a U O = -la 3 o a = -= c g o a 6 Ej a a a;
= = ?.:
':
-i E c' (, .2 p-I C = a Ez
t t).
r z a O 0 !. o o I a p o a \, t a /. o Ez
a a c L a s a oil
a l) a,
a -a at s E t) =€l .:l ,rl =l EI 3l =l =t;
=l =-l!t:l
.ul zl al 3l -t at;
-al =l at sl =l;l
EI dl -l -l >l O:
L)i
{
Z 1; ? t; ah _t *t zt:
-r = = a ! a a I U o = a a{
z
= o o € = c cc a o a = .= n '? a C o = 2a l I I I I I I lo l! l= l--lz t, 5 -iu t-IN l= .= = t-lc< la*lN
rl=
ol=:
t\-=-)
Z=
.ta
'1:=ln
oli -,-l;
-> l= I- a
-lv =l! <tl =
t --l=
.;l;
:'l u.ztz
olE -15 -t.,=
=o =ll?: :!2 C. <1v7l
-il<
rt -|-l= AP ! o -Z = a I U .J = aI .= q = a a t! .= .9 3
-I c o a = C = a = o = O a L a 7, 'i a O N -!, 9 ? c u Z .t a = .9 -a r ! o. C -s o 2 a d t
-)
a -a tr !:
E .= L .t O-z
-F a "1, o = o U a c c4 o = a -I-
= = ,2 = 5 c E = q E .: a = ! i-(
;
= 6i
= = = ,= =z
-cO i-= = a o a F)
;
c 5a t ! .o I ! g '5 = =a a = .2 a = 1l .= = a o d = al o € a E 2 A) ? .9 Y c = F a, e -a-
,
e .a! .= I I I I t-lL i _-lola
li
lc to i="o )= lp la 12l2
lr tq oz
i a -Z E:
=C q = E =a = 'il c =\
(. C = E \, a = 2\
I 2 s U;
2 6 a o a:-.,N L* ;i _-a a!E=
i= ci E,^ LJ v'-IZ t: t--3 t=
).
l; l=-l=l;]
2l l3lI t, I
LI l=l t'-t-, l+1 ZI lEl
i:l
l:l
tdtlll
l= 41 ?,1 zl;l
;l
,'l =l _t =l = ol il ':l -lalEl il -l =l j O I L -Z r a r a ,a n = P e I! -o a o L o c -O c U F Ir o a I F E. E E r r f F r o E o Eo I = EI ';, a N o = ! 6 = a = a -a E = -.2 .: c L = a o\
U bi = 51 .f; a a q t,
c t ,2 o E F ! slr
l-l+ IU 1,,t:
I t)
lz lo IE l= lo iLa z
-= -r n = aj
= = € U =:
a .= = o = c:
F E e L-a .i .: F l;l U 6 l-t',. t.-El =^l
--t>
-lL ,lr .'IL t; It I= l- t-elv arl .t ,a,l;l
=t =l =t +l = o E o = o s F -a co a\ i (, -a .o q o a $ -O a :l o = a 5-l .= a a a o O a a d = a -o o -c d t-EO !C ltt 9-:a=
6A o 9 ? c d 1 a O o ? 7 o n L = ,1 o az
I a an O (, a !J a J 5' -3/
LLJ a o .9 o c = C :E c a 'a a J E-i
cl cl g .l a = cl C C I I J)
c
"\l"i
-i = a cl C .., ac
C al o a. T Z T J v c4 a T) l liw.
rlo
;tj
I I + cL C f a' :l o a t' cl C I C, r t + V)-lv
=
t--< l-=
t-/. C a
-t + a = EIc
J C a a af c, a a q+cl
c
a a o a aili
,l
.-.i-1.
_ = _t..,1_- - - .1,.1,. -- -,
.
llli1
- - -l-l-- -l-l-- -l-l-- rl,r,l,r, ----t-i-€ 1 a € a EI L ra Ca ta.,
-t:
^i + -1 =l -l '-l -l 3 d +:
a (. a f + = a + 3 -1 (,. I a ll - 1,.,-,i-r -t--t_. I I I c.j -l -t
-l 1+
ll
ll
l:l
tLl
ta
I .r*l
lql
zlr
=l tli I :l I
EI: Y',1=
.1!
,ol: -t-=l' EI "_l !Fl rEl lzl l'=
l!l
t;l
l=1 lzl l= lEl l>t:
l= I:t;
t;
t', l= t. IJt.
t.. l= l= t^t:
l= t... li t'-lc l= t2 lal2
t;
t..-]Y l:"1 6l =t:l
;\
,-i\ t,l z,l ol tz IE lo IEl{
l;
!-tf t, IL ta to lo.I r,
l-ln l= to It t.=
l\
l= l= -l! :Ia=:
E= ts: =a -\= 'lj .\ -,] ilo=t;--l a
lll=
=l t
'tl
-=t,,
Jl a : I i-= )li-= : -l: , -lo . :Dt=
.:tr
--t'-i -l= : l -. -l -.-.
i
tla
. :lx
. .t=
, al=
. :tJ
THE STUDY OF CROSSED ALDOL CONDENSATION AT THE
SYNTHESIS OF ASYMMETRIC DIBENZALACETONE
Sri Handayani*, Indyah Sulistyo Arty and Retno Arianingrum
Department of Chemical Education, Faculty of Mathematics and Natural Sciences, State University of Yogyakarta, Karangmalang, Depok, Yogyakarta 55281, Indonesia
*Email: [email protected]
Abstract: The synthesis of asymmetric dibenzalacetone has been done by crossed aldol condensation. It can be made from 3,4-dimethoxybenzaldehyde, benzaldehyde and acetone as the starting materials. As a nucleophile, acetone, has α-hydrogens in two side. So, it can attack two kinds of aldehydes. The product will be characterized by 1H-NMR, 13C-NMR, HMQC and HMBC spectrometer. Therefore, it was identified as 1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one.
Introduction
Aldol Condensation is occured by a nucleophilic addition of the enolate ion to a carbonyl. Acetone also undergoes aldol condensation, but the equilibrium concentration of the product is generally small. Cross aldol condensation between p-annisaldehyde from fennel oil with acetophenone produce 2-hydroxy-4-methoxychalcone [1]. The influence of the base concentration and reaction time on the cross aldol condensation reaction also has been reported [2]. Alnustone or 4(E),6(E)-1,7-diphenyl-4,6-heptadiene-3-one is an asymmetric compound that isolated from
Curcuma xanthorrhiza (Zingiberaceae). This
compound was synthesized by Goksu, et al. using crossed aldol condensation between benzaldehyde and acetone, followed by reaction with cinnamaldehyde [3].
Handayani and Arty have synthesized 1.5-diphenyl-penta-1,4-diene-3-one and its derivatives known as symmetrical dibenzalacetone. It made by crossed aldol condensation between acetone : benzaldehyde by 1:2 mol ratio. It also tested as a radical hydroxyl scavengers [4]. Asymmetric crossed aldol condensation have been done with various catalyst [5,6,7]. Tutik D had synthesized of a symmetrical dibenzalacetone that have a similar structure with the cinnamic acid derivatives[8]. From its structure, it is estimated that benzalacetone and dibenzalacetone will absorb ultraviolet in the same
range
.
Thus, asymmetric dibenzalacetone will act as aradical scavenger and also a sun screen. In this research asymmetric dibenzalacetone, compound 5 namely
1(E),4(E)-1-phenyl-5-(3',4'-dimethoxyphenyl)-penta-1,4-diene-3-one will be synthesized. This
compound was made by crossed aldol condensation
between acetone with two aldehydes which are the
benzaldehyde and 3.4-dimethoxybenzaldehyde
.
Materials and Methods
General . All materials were from Merck, among
other acetone, benzaldehyde,
3.4-dimethoxybenzaldehyde, ethanol, chloroform, hexane, and ethyl acetate. TLC was carried out using 0.25-mm
plate Silica gel Merck 60 F254, column
chromatography were performed by Silica gel 60
(230-400 mesh). The 1H-NMR, 13C-NMR, HMQC and
HMBC spectra were recorded on 500 MHz Jeol instrument. IR spectra were conducted using a Shimadzu 8300 FTIR spectrometer.
1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one (5). Into a solution of NaOH
(0.025 mol, 1g) in aqueous ethanol (1:1) that was prepared at ambient temperature, benzaldehyde (0.01 mol, 1.06 g), acetone (0.01 mol, 0.58 g) and 3,4-dimethoxybenzaldehyde (0.01 mol, 1.66 g) were added drop wise alternately. After additional stirring for 60 minutes, water (20 ml) was added to the reaction mixture which was then filtered. The extract was washed with water (20 ml x 3) and separated by
column chromatography (d 2.5 cm, h 50 cm), with
silica gel 60 (230-400 mesh) as the stationary phase and ethylacetate-hexane by 1:9 as the eluent. Four
fractions were obtained from the column
chromatography. The target compound was identified using thin layer chromatography with chloroform-hexane 4: 6.
Results and Discussion
Improved Synthesis of 1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one (5).
The preparation of compound 5 was initiated by the mixing of 1, 2 and 4 to give 5 ((Figure 1). The product of crossed aldol condensation between benzaldehyde, 3,4-dimethoxybenzaldehyde and acetone is a mixture of 4 compounds. There was separated by Column Chromatography (EtOAc-hexane, 1:9) to provide the asymmetric dibenzalacetone 5 (15.53%) as pale yellow oil.
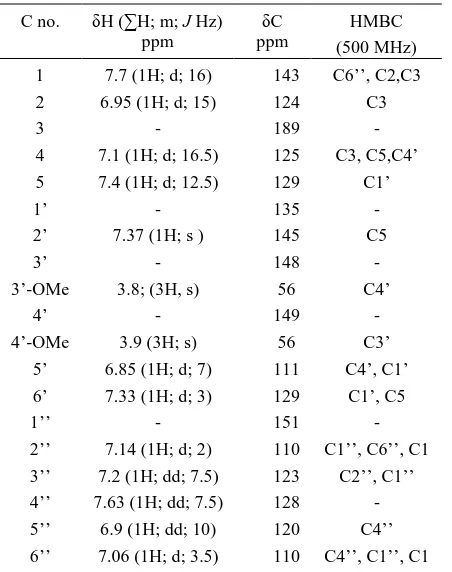
The multiple bond correlation of HMBC supported
the structure (Table 1, Figure 2). In the 1H-NMR
spectrum (500 MHz, CDCl3), three protons singlet and
three protons double dublet were observed. The singlet
at δ = 7.37 was assignable to H2’, 3.8 to H3’ and 3.9
to H4’. The double dublet at δ = 7.2; 7.63; and 6.9 was
388
PACCON2009 (Pure and Applied Chemistry International Conference)
Edited with the trial version of Foxit Advanced PDF Editor
To remove this notice, visit:
6" 5" 4"
3" 2"
1"
6' 5'
4' 3' 2'
1'
5
4 3 2 1
O
H
H
H
H
H
H
H H
H
H
H
OMe
OMe
assignable to H3”, H4” and H5” respectively. Two
equivalence methoxy signals at δ 3.8 and 3.9 were
assigned to C3’ and C4’. Support spectra data provided by the IR (KBr), which indicates the
existence of C=O (1645cm-1), aromatic C=C
(1514-1417 cm-1) and CO ether (1255-1139 cm-1). Therefore,
the structure of 5 was 1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one.
CHO CH3CCH3
O
O CH3
1
2
3 OMe
OMe
OHC 4
5
OMe
OMe
O
Figure 1. Synthesis of 1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one.
Table 1. 1H and 13C-NMR data of compound 5
(CDCl3)
C no. δH (∑H; m; J Hz) ppm
δC ppm
HMBC (500 MHz) 1
2 3 4 5 1’ 2’ 3’ 3’-OMe
4’ 4’-OMe
5’ 6’ 1’’ 2’’ 3’’ 4’’ 5’’ 6’’
7.7 (1H; d; 16) 6.95 (1H; d; 15)
- 7.1 (1H; d; 16.5) 7.4 (1H; d; 12.5)
- 7.37 (1H; s )
- 3.8; (3H, s)
- 3.9 (3H; s) 6.85 (1H; d; 7) 7.33 (1H; d; 3)
- 7.14 (1H; d; 2) 7.2 (1H; dd; 7.5) 7.63 (1H; dd; 7.5)
6.9 (1H; dd; 10) 7.06 (1H; d; 3.5)
143 124 189 125 129 135 145 148 56 149
56 111 129 151 110 123 128 120 110
C6’’, C2,C3 C3
- C3, C5,C4’
C1’ - C5
- C4’
- C3’ C4’, C1’
C1’, C5 - C1’’, C6’’, C1
[image:8.595.328.525.82.211.2]C2’’, C1’’ - C4’’ C4’’, C1’’, C1
Figure 2. The HMBC of compound 5
Conclusions
1(E),4(E)-1-phenyl-5-(3’,4’-dimethoxyphenyl)-penta-1,4-diene-3-one can be made from acetone,
benzaldehyde and 3,4-dimethoxybenzaldehyde by crossed aldol condensation.
Acknowledgement
This experiment was sponsored by Hibah Bersaing Project, Ditjen Dikti RI.
References
[1]Handayani, S., Sintesis 4’-metoksiflavanon Menggunakan o-hidroksiasetofenon dan p-anisaldehida dari minyak Adas, Master's Thesis, Gadjah Mada University, 2000.
[2] Handayani, S., Sunarto and Kristianingrum, S., Optimization of time reaction and hydroxide ion concentration on flavonoid synthesis from benzaldehyde and its derivatives, Indonesian journal of Chemistry Vol 5 No.2 Juli 2005 Indonesian Journal of Chemistry, 5, No.2 (2005), pp 88-93.
[3] Goksu, S., Celik, H. and Secen, H., Turk. J. Chem. 27 (2003), pp 31-34.
[4] Handayani, S. and Arty, I.S., Synthesis Of Hydroxil Radical Scavenger From Benzalacetone And Its Derivatives, 2nd USM Penang International Postgraduate Convention, Proc. Penang, Malaysia, 2008
[5] Yamano, Y., Fujita, Y., Mizuguchi, Y., Nakagawa, K., Okano, T., Ito, M. And Wada, A., Chem. Pharm. Bull.,
55(9) (2007), pp 1365-1370.
[6] Mlynarski, J., European Journal of Organic Chemistry,
21 (2006), pp 4779-4786.
[7] M.Manuel B. Marques, Asymmetric Cross-Aldol Reaction of Aldehides : A Formidable Synthetic Chalengge, Requimte-FCT-New University of Lisbon (2005).
[8] Tutik, D., Oksidasi Anetol dan Kajian Pengaruh Gugus Metoksi Turunan benzaldehide Terhadap Reaksi Kondensasi Benzoin dan Aldol Silang, Master Thesis, Gadjah Mada University, 1996.
389
PACCON2009 (Pure and Applied Chemistry International Conference)
Edited with the trial version of Foxit Advanced PDF Editor
To remove this notice, visit:
[image:8.595.86.281.182.404.2] [image:8.595.72.298.491.777.2]