www.elsevier.com / locate / bres
Research report
Aluminum-induced apoptosis in cultured astrocytes and its effect on
calcium homeostasis
a ,
*
bGui-Wen Guo
, You-Xin Liang
a
2nd Department of Pharmacology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 294 Taiyuan Road, Shanghai 200031, PR China
b
Department of Occupational Health, Shanghai Medical University, Shanghai 200032, PR China
Accepted 3 October 2000
Abstract
Aluminum exposure and apoptotic cell death has been implicated in several neurodegenerative conditions including Alzheimer’s disease. In this study, we use cultured astrocytes to investigate the ability of aluminum to induce the apoptosis of astrocytes. The proportion of apoptotic cells and cell cycle distribution were determined by flow cytometric analysis. Our results showed that exposure to aluminum at low levels (100 and 200mM) for up to 6 days did not result in the apoptosis of astrocytes, and a dramatic blockage of apoptotic cells was found at 200mM aluminum. However, at 400mM, aluminum markedly induced the apoptosis of astrocytes, which was associated with a significant change in cell cycle distribution characterized by increase of G2 / M phase cells (128%). Measurements
21
of intracellular Ca concentration using the fluorescent calcium indicator dye Fluo-3 demonstrated a significant increase in the levels of intracellular calcium after aluminum treatment. However, no differences were observed among aluminum-treated groups. These findings suggest that aluminum induce and block selectively the apoptosis of astrocytes, which depend upon the concentrations of aluminum.
21
Increased intracellular Ca may not be the primary mechanism of aluminum-mediated apoptotic cell death. 2001 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neurotoxicology
Keywords: Aluminum; Apoptosis; Calcium; Astrocytes; Cell cycle
1. Introduction Apoptosis is a distinct form of cell death, in which damaged cells activate a genetic program that leads to the Aluminum is well known to be a neurotoxic agent, destruction of their DNA. Inappropriate cell death may although the molecular basis of its toxicity is far from contribute to neurodegenerative disorders [2,17]. Apoptosis clear. The excessive accumulation in the CNS from of astrocytes has been shown in several experimental endogenous sources or environmental exposure has been [11,16,29] and clinical neuropathological conditions in-implicated in a variety of neurological disorders including cluding AD [20,28] and Huntington’s disease [33]. It has Alzheimer’s disease (AD) [6], dialysis encephalopathy been previously reported that aluminum can induce the [15] and the amyotryophic lateral sclerosis parkinsonism– apoptosis of astrocytes, however, some data suggested that dementia complex of Guam [24]. Neurodegenerative when astrocytes were treated with aluminum, their mor-changes in CNS neurons induced by exposure to aluminum phology or viability were not significantly affected [26]. salts in vivo and vitro have been analyzed extensively [30]. The difference may attributed to different experimental The existence of a causal relationship between aluminum conditions, such as aluminum concentration and exposure and neurodegenerative disorders, such as AD, remains time. In most cell lines, apoptosis is induced by increased
21
unclear. intracellular Ca [21]. Aluminum was shown as having
21
the capacity to interact with Ca binding sites [27] and
21
disrupt intracellular Ca homeostasis [10,13]. The majori-*Corresponding author. Fax:186-21-6437-0269.
E-mail address: [email protected] (G.-W. Guo). ty of work on the neurotoxic effects of aluminum has
involved an examination of the direct effects of aluminum routinely screened via immunocytochemistry technique for on neuronal cells, and very little work has been done to the expression of GFAP (GFAP position cells.95%). The study the effects of aluminum on astrocytes. Though nerve media was changed approximately every 3 days.
cells are the eventual functional target of aluminum toxicity, the extracellular environment as well as metabolic
2.3. Flow cytometric analyses of the cell cycle status of neurons are very dependent upon astrocytes
distribution and apoptosis [7,35], which have been reported to protect cortical
neurons from glutamate-induced toxicity [22,23], and this
For the flow cytometric analyses of DNA content, cells ability was found to be impaired by aluminum [26].
were harvested after aluminum exposure using trypsin, Furthermore, there is accumulated evidence that implicate
washed with PBS, fixed with 2.5 ml ice-cold 70% ethanol astrocytes as having a role in a number of
neurodegenera-at 48C for at least 30 min, and rinsed with PBS. The cell tive diseases [1,7]. It has been suggested that
aluminum-pellets were suspended in a cold solution containing 0.1% induced neurotoxicity is an indirect effect mediated by
Triton x-100, 0.08 N HCl, and 0.146 M NaCl, followed by astrocytes rather than a direct effect on neuronal cells [31].
1.0 ml RNase A (10 mg / ml) at 378C for 10 min. In the For this purpose, we used primary cultures of astrocytes to
absence of light, the cells were stained with 1.0 ml PI observe the effect of aluminum on the apoptosis of
31 solution (50mg / ml PI, 1% Triton x-100, 0.9% NaCl), by astrocytes. Since the presence of free Al is probably low
incubation at room temperature for 30 min. Before analy-in the braanaly-in, development of alumanaly-inum-analy-induced
neuro-sis, cell suspensions were filtered through a stainless steel toxicity would be a long-term process as observed in the
filter to eliminated aggregates of cells. PI-stained cells brain degenerative changes of AD. One crucial question in
were analyzed using a FACS-CALIBUR Flow Cytometer aluminum toxicity is whether long-term, low-level
expo-(Becton-Dickinson, MountainView, CA). Cell fluorescence sure to aluminum may induce the apoptosis of astrocytes.
was measured by using an argon ion laser (488 nm) at a flow rate of 1000–1200 cells / s. The data obtained were analyzed by Cellfit and Lysis II software (Becton-Dickin-2. Materials and methods
son). 2.1. Materials
21 2.4. Fluorescent measurements of intracellular Ca Postnatal day 1–3 Sprague–Dawley rats were obtained
21 21
from Center of Lab Animals, Shanghai Medical University Intracellular Ca ([Ca ] ) values were obtained from
i
21
(Shanghai, China). DMEM was purchased from GIBICO single cell using dye Fluo-3, the fluorescent Ca sensitive RBL. Fifty-mm polystyrene culture dishes were obtained indicator. Cells were incubated with 4-mM Fluo-3 /AM at from Corning Glass Works, (Corning, NY). Trypsin and 378C for 30 min, and washed with PBS. After addition of Fluo-3 were obtained from Sigma Chemical (St. Louis, the culture medium, the temperature was maintained at USA). Other chemicals used were available locally and of 378C for 10 min and then the cells were placed on an analytical grade. Aluminum chloride (100, 200, 400 mM) inverted microscope (TMD-300, Nikon). Cells were in phosphate-buffered saline (PBS, pH7.0) was added to viewed using confocal laser scanning microscope (Leica, the culture medium from the 11th (or 15th) to the 16th day. Germany). Fluo-3 loaded cells were illuminated with the After the exposure, the cells were used for the following 488-nm line of an argon laser and emitted fluorescence
experiments. (526 nm) was collected through a 350 water-immersion
objective (NCF Fluor N.A. 1.15) and setting the confocal 2.2. Cell culture and treatment pinhole at 2 mM. The fluorescent intensity was detected
with one of two photomultipliers. To obtain good spatial Primary astrocycte cultures were prepared by the meth- image, four successive frames were collected for each cell.
21 od previously described [19]. Cerebral cortices were Data were analyzed by Exfoc software. [Ca ] was
i
dissected from newborn rats (postnatal days 1–3). The evaluated as fluorescent intensity. All experiments were meningeals and white matter was removed, and the performed at 24–268C.
remaining tissue was treated with 0.1% trypsin for 30 min at 378C. Dissociated neocortical cells were plated into a
5
culture dish (50 mm in diameter) at a density of 1310 2.5. Statistical analysis cells / ml of suspension composed of high glucose
3. Results
Astrocytes cultures were exposed to aluminum at vary-ing concentrations (100–400mM) in the growth medium for 1–6 days. No visible effects in the appearance and number of astrocytes was found compared to untreated cultures when viewed with phase contrast optics and transmission electron microscope (data not shown). We conclude that treatment of astrocytes with aluminum chloride does not dramatically affect cellular structure and their viability.
3.1. Effects of aluminum on the apoptosis and cell cycle distribution of astrocytes
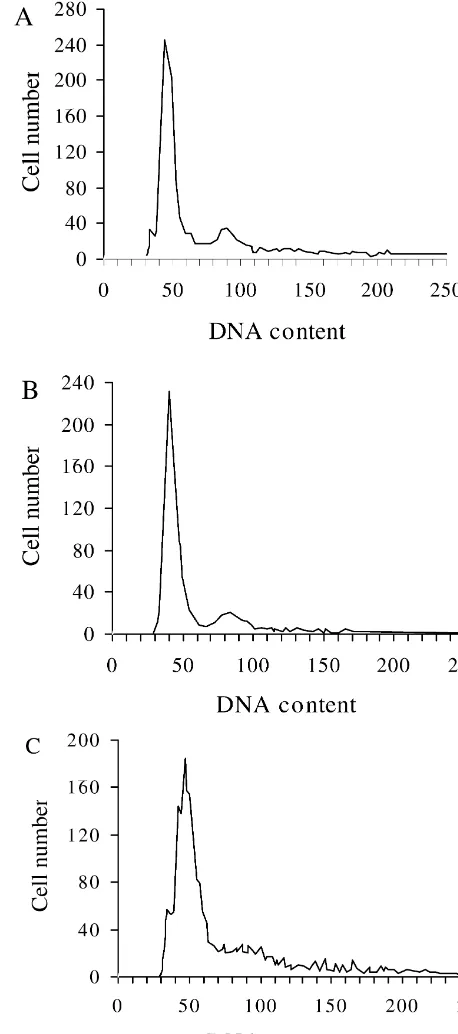
Many cell types have been shown to die by an active process known as apoptosis or programmed cell death, its deregulation may have important implications for tissue disorders. To investigate the effects of aluminum on apoptosis of astrocytes, we prepared the astrocyte cultures from newborn rat cortex, in which astrocytes accounted for approximately 95% of the overall number of cells in cultures as determined by GFAP immunocytochemistry. To quantify the aluminum-induced apoptotic activity of astrocytes, the number of apoptotic astrocytes was de-termined by measuring the percentages of hypodiploid nuclei using PI staining and flow cytometric methods as shown in Figs. 1 and 2. The DNA histogram of primary astrocytes, following 6 days of incubation with aluminum at various doses, revealed one DNA peak of high, narrow diploid form (relative nuclei number: G0 / G1.76%), no typical hypodiploid apoptotic peak was observed. Analysis of DNA content of astrocytes incubated with aluminum showed that the percentage of cells in the S phase significantly increased, and the percentage of cells in the G0 / G1 phase decreased after exposure to 100 mM aluminum chloride. We did not observe any apoptotic cells at 200 mM aluminum concentration, which did not result in a significant change in the cell cycle distribution of these cells. A dramatically increased number of apoptotic cells occurred under the experimental condition of aluminum concentration up to 400 mM, in which the percentage of cells in G2 / M phase markedly increased and the percentage of cells in the S phase decreased.
Fig. 1. DNA fluorescence histogram of hypodiploid nuclei in PI-stained astrocytes, which was analyzed by Flow Cytometer. (A): controls; (B): 3.2. Effects of aluminum on intracellular calcium levels treated with 200mM AlCl for 6 days; (C): treated with 400mM AlCl
3 3
for 6 days. Aluminum was previously shown to promote the influx
21
and intracellular accumulation of Ca in neurons and astrocytes. In this study, we adopted confocal laser
micro-scopy to detect the fluorescence signal arising from the chloride (100, 200 and 400 mM), aluminum caused a
21
individual cells. Astrocytes treated with aluminum for 1 significant increase of intracellular [Ca ] over 50% asi
and 6 days exhibited a strong fluorescent intensity in compared to the control astrocytes. No significant change
21
nuclear staining with Fluo-3 (Fig. 3). [Ca ] was quan-i was found among aluminum-treated astrocytes at various
21
tified by fluorescence ratio imaging of Ca indicator dye doses. After exposure to aluminum for 6 days, intracellular
21
Fig. 2. Effects of aluminum on the cell cycle distribution and apoptosis in astrocyte. The cells with pretreatment of 100, 200 and 400mM AlCl for3
6 days were stained with PI. On day 7 after aluminum treatment, the cells were harvested and the percentage of apoptotic cells (A) and cell cycle distribution (B) were determined as described under Materials and methods. Each point represents a mean6S.E.M. (n55 cultures per group). *P,0.05, **P,0.01 compared with control group (one-way ANOVA followed by the Fisher’s LSD test).
that of controls, and there is a slight decrease compared with astrocytes exposed to aluminum for 1 day (Fig. 4).
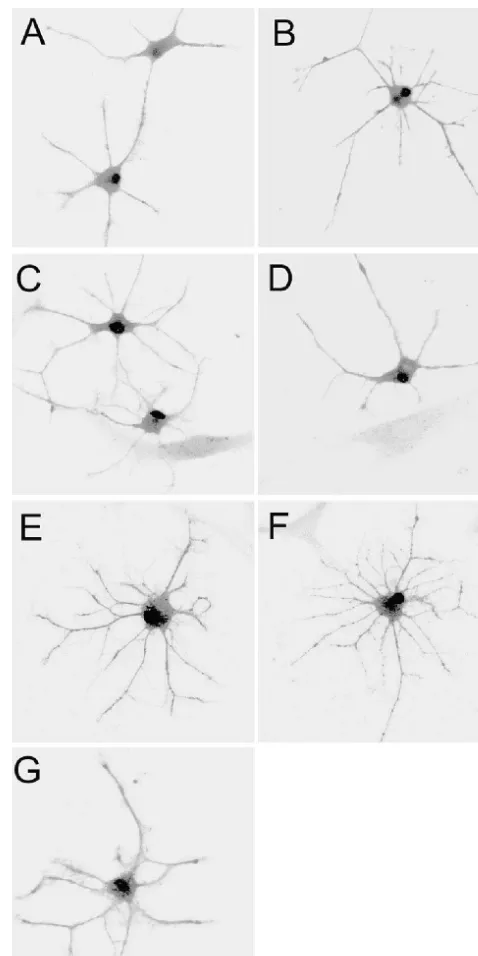
Fig. 3. Confocal images of astrocyte stained with Fluo-3, which were
4. Discussion
average of 32 frames collected through a 350 water-immersion. (A) Control cells; (B–D) treated cells with 100, 200 and 400 mM AlCl ,3 The present results demonstrate that aluminum selective- respectively, for 1 day ; (E–G) treated cells with 100, 200, 400 mM ly modulates astrocyte apoptosis. At higher concentrations, AlCl , respectively, for 6 days. Astrocytes treated with aluminum3
exhibited a high fluorescent intensity at various doses and exposure time aluminum induced astrocyte apoptosis, on the contrary, at
under confocal laser scanning microscope. Results are representative of lower concentrations, aluminum blocks the apoptotic cell
five similar experiments. death. Due to the lack of typical morphological changes of
apoptosis and relatively low apoptotic rate, no visible effects in the appearance and number of astrocytes were
21
intracellular Ca are important in regulation of astrocyte function as they are in neurons [5,8], either an elevation or a marked reduction in calcium can result in degeneration of astrocytes [3,14]. In astrocytes, aluminum has been
21 shown to stimulate an increase in intracellular Ca concentration [25], but the relationship of intracellular
21
Ca levels and aluminum-induced apoptosis of astrocytes has not been extensively studied. In the present study, we
21 wish to know whether changes of intracellular Ca concentrations regulate aluminum-mediated pro- and anti-apoptosis of astrocytes. Our results demonstrate that
21
aluminum may elevate the intracellular Ca of astrocytes
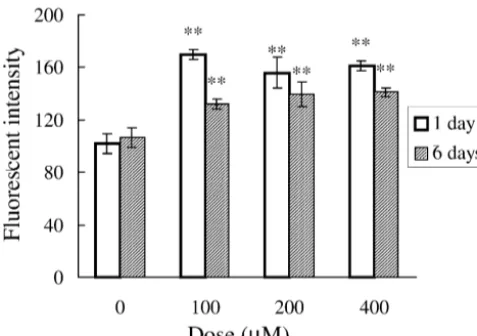
21 cultured with aluminum for 1 day. Intracellular Ca remains at a higher level after exposure to aluminum for 6 days, but there is a slight decrease compared with as-Fig. 4. Effects of aluminum on intracellular calcium. Astrocytes were trocytes exposed to aluminum for 1 day. In addition, no treated with AlCl at various doses for 1 day and 6 days respectively.3 21
correlation was found between the intracellular Ca level Data were expressed as mean6S.E.M. (n56). **P,0.01 compared to
and apoptosis of astrocytes. Our results suggested that in control group (one-way ANOVA followed by the Fisher’s LSD test).
the presence of aluminum, it is difficult for astrocytes to
21
remain calcium homeostasis. Ca transients is not in-voked as a trigger for the apoptotic effects, given that even this finding yet, it was consistent with proliferation and in cells that were seemingly protected from apoptosis (200
21 activation of astrocytes induced by aluminum in vivo. This mM Al). So, simple elevation of intracellular Ca is finding may have clues for the effects of aluminum in vivo, insufficient to explain the action of aluminum. The process but careful correlation needs to be drawn between the in of astrocytic cell death induced by aluminum is likely to be situ situation and the primary cultures, which only unpre- extremely complex, we cannot rule out the possibility, for dictably reflect in vivo conditions. The mechanism by example, that aluminum mediates the survival of astrocytes
21
which aluminum induces and blocks selectively apoptosis through elevating the intracellular Ca concentrations.
21
in cultured astrocytes remains unclear. Recent extensive However, a rise of intracellular Ca in glia cells may play studies suggested that the mechanism by which cells a role in developing aluminum-induced neuropathological committed to undergo apoptosis is associated with cell events.
cycle control [18,36], and most studies on apoptosis also In summary, this study demonstrates that aluminum involve in the cell cycle. Sensitivity of proliferating cells selectively mediated the apoptosis of astrocytes, and an to some death stimuli is frequently dependent on the phase elevated concentration of aluminum is necessary to induce of the cell cycle [12]. Analysis of cell kinetics indicated the apoptosis. However, low concentration of aluminum that aluminum influences the cell cycle process of as- may induce neurotoxicity, which might not be acted via trocytes. The mechanism by which aluminum-induce pro- apoptosis of astrocytes. Since the concentration of liferation and apoptosis of astrocytes is associated with the aluminum in the human brain is usually very low, the high cell cycle distribution. High concentrations of aluminum concentration of aluminum associated to cultured cells may induce apoptotic cell death by inducing transition from S be rather unlikely to occur in vivo. Proliferation of to G2 / M phase of the cell cycle. It was speculated that astrocytes, not cell death is a primary consequence as aluminum-induced apoptosis of astrocytes could occur at observed in AD and neurotoxicity of aluminum. The dual G2 / M phase or in the process of transition from S to role aluminum-induced and blocked apoptosis of astrocytes
G2 / M phase. was also observed in modulation of GABAA
receptor-Further experiments are necessary to elucidate the mediated currents [34]. These findings suggest a clue for specific mechanisms by which aluminum causes prolifer- the elucidation of mechanism of aluminum neurotoxicity. ation, degeneration or death of astrocytes. Most studies, to
21 date, have investigated the increase of intracellular Ca as an early event in the activation of cell death in vivo and in
vitro models of pathological conditions [3,4]. Induction of Acknowledgements
21
apoptosis by Ca ionophores has been demonstrated in
primary neuronal cell, the increase of intracellular calcium The authors would like to thank Ms. Yaling Huang, Ms. is thought to play a primary role in starting up the Kangmei Huang and Ms. Leiming Huang for their excel-apoptotic pathway, probably through the activation of lent technical assistance. This work was supported by the
21 21
[19] R.S. Morrison, J. deVellis, Growth of purified astrocytes in a
References
chemically defined medium, Proc. Natl. Acad. Sci. USA 78 (1981) 7205–7209.
[1] A. Aschner, H.K. Kimelberg, The use of astrocytes in cultures as [20] T. Nishimura, H. Akiyama, S. Yonehara, H. Kondo, K. Ikeda, M. model systems for evaluating neurotoxic-induced injury, Neuro- Kato, E. Iseki, K. Kosaka, Fas antigen expression in brains of Toxicology 12 (1991) 505–518. patients with Alzheimer-type dementia, Brain Res. 695 (1995) [2] M. Barinaga, Death gives birth to the nervous system. But how?, 137–145.
Science 259 (1993) 762–763. [21] T. Pozzan, R. Rizzuto, P. Volpe, J. Meldolesi, Molecular and cellular [3] R. Chiesa, N. Angeretti, R.D. Bo, E. Lucca, E. Munna, G. Forloni, physiology of intracellular calcium stores, Physiol. Rev. 74 (1994)
Extracellular calcium deprivation in astrocytes: regulation of mRNA 595–636.
expression and apoptosis, J. Neurochem. 70 (1998) 1474–1483. [22] P.A. Rosenberg, Accumulation of extracellular glutamate and
neuro-21
[4] K. Dunlap, J.I. Luebke, T.J. Turner, Exocytotic Ca channels in nal death in astrocyte-poor cortical cultures exposed to glutamine, mammalian central neurons, Trends Neurosci. 18 (1995) 89–98. Glia 4 (1991) 91–100.
[5] S.R. Glaum, J.A. Holzwarth, R.J. Miller, Glutamate receptors [23] P.A. Rosenberg, E. Aizenman, Hundred-fold increase in neuronal
21 21
activate Ca mobilization and Ca influx into astrocytes, Proc. vulnerability to glutamate toxicity in astrocyte-poor cultures of rat Natl. Acad. Sci. USA. 87 (1990) 3454–3458. cerebral cortex, Neurosci. Lett. 103 (1989) 162–168.
[6] P.F. Good, D.P. Perl, L.M. Bierer, J. Schmeidler, Selective accumu- [24] L.P. Rowland, Motor neuron diseases and amyotrophic lateral lation of aluminum and iron in the neurofibrillary tangles of sclerosis: research progress, Trends Neurosci. 10 (1987) 393–398.
Alzheimer’s disease: a laser microprobe (LAMMA) study, Ann. 21
[25] M.W. Salter, J.L. Hicks, ATP causes release of intracellular Ca via Neurol. 31 (1992) 286–292. the phospholipase C beta / IP3 pathway in astrocytes from the dorsal [7] L. Hertz, Neuronal-astrocytic interactions in brain development, spinal cord, J. Neurosci. 15 (1995) 2961–2971.
brain function and brain disease, in: P.S. Timeras, A. Vernadakis, J. [26] J.B. Sass, L.-C. Ang, B.H.J. Juurlink, Aluminum pretreatment Lauder, A. Private (Eds.), Brain Differentiation in Aging, Plenum, impairs the ability of astrocytes to protect neurons from glutamate New York, 1991, pp. 143–159. mediated toxicity, Brain Res. 621 (1993) 207–214.
[8] J.A. Holzwarth, S.T. Gibbons, J.R. Brorson, L.H. Philipson, R.J. [27] N. Siegel, A. Haug, Aluminum interaction with calmodulin: evi-Miller, Glutamate receptor agonist stimulates diverse calcium re- dence for altered structure and function from optical and enzymatic sponses in different cell types of cultured rat cortical glial cells, J. studies, Biochem. Biophys. Acta 744 (1983) 36–45.
Neurosci. 14 (1994) 1879–1891. [28] G. Smale, N.R. Nichols, D.R. Brady, C.E. Finch, W.E. Horton Jr., [9] R. Joseph, E. Han, Neuronal death, cytoplasmatic calcium and Evidence for apoptotic cell death in Alzheimer’s disease, Exp.
internucleosomal DNA fragmentation: evidence for DNA fragments Neurol. 133 (1995) 225–230.
being released from cells, Mol. Brain Res. 17 (1993) 70–76. [29] E. Sochocka, B.H.J. Juurlink, W.E. Code, V. Hertz, L. Peng, L. Hertz, [10] D. Julka, K.D. Gill, Altered calcium homeostasis: a possible Cell death in primary cultures of mouse neurons and astrocytes mechanism of aluminum-induced neurotoxicity, Biochim. Biophys. during exposure to and ‘recovery’ from hypoxia, substrate depriva-Acta 1315 (1996) 47–54. tion and simulated ischemia, Brain Res. 638 (1994) 21–28. [11] B.H.J. Juurlink, L. Hertz, Ischemia-induced death of astrocytes and [30] J.A. Sturman, H.M. Wisniewski, Aluminum, in: S.C. Bondy, L.L.
neurons in primary culture: pitfalls in quantifying neuronal cell Prasad (Eds.), Metal Neurotoxicity, CRC Press, Boca Raton, death, Dev. Brain Res. 71 (1993) 239–246. Florida, 1988, pp. 61–85.
[12] K.L. King, J.A. Cidlowski, Cell cycle and apoptosis: common [31] M.B. Suarez-Fernandez, A.B. Soldado, A. Sanz-Medel, J.-A. Vega,´ ´ pathways to life and death, J. Cell Biochem. 58 (1995) 175–180. A. Novelli, M.T. Fernandez-Sanchez, Aluminum-induced degenera-´ ´ [13] M.L. Koening, R.-S. Jope, Aluminum inhibits the fast phase of tion of astrocytes occurs via apoptosis and results in neuronal death,
voltage-dependent calcium inflex into synaptosomes, J. Neurochem. Brain Res. 835 (1999) 125–136.
49 (1987) 316–320. 21
[32] N. Takei, Y. Endo, Ca ionophore-induced apoptosis on cultured
21
[14] T. Koike, D. Martin, E.M. Johnson, Role of Ca channels in the embryonic rat cortical neurons, Brain Res. 652 (1994) 65–70. ability of membrane depolarization to prevent neuronal death [33] L.B. Thomas, D.J. Gates, E.K. Richfield, T.F. O’Brien, J.B. induced by trophic factor deprivaiton: evidence that levels of Schweitzer, D.A. Steindler, DNA and labeling (TUNEL) in Huntin-internal calcium determine growth factor dependence of sympathetic gton’s disease and other neuropathological conditions, Exp. Neurol. ganglion cells, Proc. Natl. Acad. Sci. USA 86 (1989) 6421–6425. 133 (1995) 265–272.
[15] L.T. Kurland, Amyotrophic lateral sclerosis and Parkinson’s disease [34] P.Q. Trombley, Selective modulation of GABA receptors by
A
complex on Guam linked to an environmental neurotoxin, Trends aluminum, J. Neurophysiol. 80 (1998) 755–761.
Neurosci. 11 (1988) 51–58. [35] W. Waltz, Role of glial cells in the regulation of the brain ion [16] F.M. LaFerla, B.T. Tinkle, C.J. Bierberich, C.C. Haudenschild, G. environment, Prog. Neurobiol. 33 (1989) 309–333.
Jay, The Alzheimer’s Ab peptide induces neurodegeneration and [36] L. Zhu, C. Anasetti, Cell cycle control of apoptosis in human apoptotic cell death in transgenic mice, Nat. Genet. 9 (1995) 21–30. leukemic T cells, J. Immunol. 154 (1995) 192–200.
[17] J. Marx, Cell death studies yield cancer clues, Science 259 (1993) 760–761.