Summary We investigated the effects of simulated acid rain and elevated ozone on tissue water relations of mature clones of a fast-growing genotype of ponderosa pine (Pinus ponderosa Dougl. ex Laws.) and their half-sib seedlings. Whole seedlings and branches of mature trees were exposed to acid rain (pH 5.1 and 3.0) and ozone (ambient and twice-ambient) treatments in open-bottomed chambers. The acid rain treatment was applied to foliage weekly from January to April 1992. The ozone treatment was applied daily from September 1991 to November 1992. The treatments had little effect on the water relations of branches of mature trees. In contrast, water relations of seed-lings were affected by the treatments, particularly by the twice-ambient ozone treatment, and in many instances, there was a significant acid rain × ozone interaction. A combination of twice-ambient ozone and pH 3.0 rain lowered seedling solute potential, turgor loss point and cell-wall elasticity but increased pressure potential and symplastic water content, whereas total water content was unchanged. We conclude that twice-ambient ozone caused osmotic adjustment in seedlings, and the re-sponse was magnified by pH 3.0 rain. The long-term conse-quences of this response are unclear because although osmotic adjustment and increased pressure potential might be advanta-geous to seedlings in resisting drought and maintaining growth, the concurrent decrease in cell-wall elasticity might induce adverse effects. We note that the effects of elevated ozone on water relations of ponderosa pine seedlings were similar to the effects of drought on water relations of many species.
Keywords: branches, cell wall elasticity, drought, pH, ponder-osa pine, pressure potential, solute potential, symplastic water content, turgor loss point.
Introduction
Elevated tropospheric ozone and acid rain are among the air pollutants that can cause severe damage to terrestrial and aquatic ecosystems (Miller etal. 1977, Hileman 1984, Chap-pelka et al. 1985, Reich et al. 1986, Pye et al. 1988, USNAPAP 1991). It has been hypothesized that acid rain enters plant cells
through the cuticle of the leaf surface, alters the permeability of cell membranes, and produces osmotically active anions leading to decreased cell solute potential (Heath 1980). Ozone has been reported to cause a decrease in relative water content (Evans and Ting 1974), no significant changes in total water potential, solute potential and pressure potential of beech seed-lings (Taylor and Dobson 1989), a temporary increase in cell solute potential of water-stressed Norway spruce (Picea abies (L.) Karst.) seedlings (Dobsonet al.1990), an increase in total water potential of cotton (Temple 1990), and a decrease in total water potential of orange trees (Olszyk et al.1991). The con-flicting results obtained from studies of the effects of ozone on plant water relations may be related to the limited number of water relations components examined or to the use of short-term exposure to ozone under laboratory conditions, or both.
We investigated the long-term interactive effects of simu-lated acid rain and twice-ambient ozone on total water poten-tial (Ψw), solute potential (Ψs), pressure potential (Ψp), turgor
loss point (TLP), bulk modulus of elasticity (ε), and total water content (Vt) and its osmotically active portion, symplastic
water content (Vs), of foliage of mature clones of ponderosa
pine (Pinus ponderosa Dougl. ex Laws.) and their half-sib seedlings growing under field conditions.
Material and methods
Site
Field studies were conducted at the USDA Forest Service Chico Tree Improvement Center (CTIC) in Chico, CA. The site is located at the lower elevation of the natural range of ponder-osa pine. The climate is Mediterranean with hot dry summers and mild wet winters.
Plant material
Plants consisted of field-grown, grafted mature trees and cor-responding half-sib seedlings of ponderosa pine, Clone 3087. This clone was selected by the USDA Forest Service in the mid-1970s to produce seed for future plantations in California.
Osmotic adjustment induced by elevated ozone: interactive effects of
acid rain and ozone on water relations of field-grown seedlings and
mature trees of
Pinus ponderosa
B. MOMEN
1,2and J. A. HELMS
1 1Department of Environmental Science, Policy, and Management, University of California, Berkeley, CA 94720, USA
2
Present address: MRC, Biology Department, Rensselaer Polytechnic Institute, Troy, New York 12180, USA
Received January 3, 1995
In the mid-1970s, scions were collected from a 70-year-old tree growing at about 1200 m elevation on the El Dorado National Forest in the central Sierra Nevada (USDA-FS Seed Zone 526). Scions were grafted onto 3-year-old root stocks of unknown genetic origin and transplanted to a seed orchard in 1980. In 1990, platforms were constructed around the grafted trees (6- to 7-m tall) to facilitate access. In 1992, at the time of experimental measurements, the grafted trees possessed mor-phological characteristics of 80-year-old mature trees as indi-cated by branch diameter, branch angle, needle length, bark color and cone production.
Seed was collected at the same time as scions from the same parent tree, stored until April 1989, and then grown in contain-ers in a greenhouse. In February 1990, seedlings were trans-planted to a site adjacent to the grafted mature trees.
After transplanting, both mature trees and seedlings were watered every other week from May to October of both 1991 and 1992, and competing herbaceous species were removed regularly.
Acid rain and ozone treatments
Air pollution treatments were applied in branch exposure chambers (Houpis et al. 1991), which were installed in Sep-tember 1991. A single chamber covered 3 years of annual shoot growth of an individual branch of a mature tree, whereas each seedling was entirely covered by a single chamber. Cham-bers were open-bottomed, 1.5 m long × 0.7 m diameter, and were made of 5-mm Teflon sheeting supported by an alumi-num frame covered by nonreactive Teflon tape. Teflon was used because it is transparent to longwave heat radiation, does not alter light quality, and is nonreactive to ozone. The cham-ber included a mixing region at the top, a main exposure region in the middle, and an exhaust frustum at the bottom. A fan connected to the mixing region at the top of the chamber circulated ambient or ozonated air downward and prevented variations in chamber temperature.
Treatments consisted of four combinations of two ozone (ambient and twice-ambient) and two rain (pH 5.1 and 3.0) regimes: (1) pH 5.1 rain and ambient ozone (pH5.1 + Oamb ), (2)
pH 5.1 rain and twice-ambient ozone (pH5.1 + Otwice ), (3) pH
3.0 rain and ambient ozone (pH3.0 + Oamb ), and (4) pH 3.0 rain
and twice-ambient ozone (pH3.0 + Otwice ). Because the pH of
natural rain in Sierra Nevada is 5.1 to 5.6, the pH5.1 + Oamb
treatment was considered the control chamber treatment. Simulated rain regimes were applied weekly during the normal winter precipitation period from January 17 until April 30, 1992, based on records of Leonard et al. (1981) and on 25-year precipitation records from the University of California Blodgett Forest Research Station near Georgetown, CA. A total of 17 rain events, with a deposition of 5 cm per event, resulted in a total deposition of 85 cm. The rain solutions were based on a 2/3 (equivalent basis) mix of H2SO4 and HNO3 plus
the kinds and amounts of ions needed to simulate the chemical composition of normal and acidic rains in northern California (McColl and Johnson 1983). Rain droplets with diameters of 0.1 to 1.0 mm were produced with a calibrated nozzle mounted at the top of each branch exposure chamber. The solution
throughfall was collected in containers at the base of each chamber.
Twice-ambient ozone was applied daily (0600 to 2000 h in spring and summer, 0800 to 1700 h in fall and winter) from September 1991 to November 1992. Ozone fumigation was interrupted for 3 weeks in mid-December 1991 for system maintenance and calibration, and again for 3 weeks in late June 1992 because of the break-down of the ozone generator. Twice-ambient ozone was generated from compressed Twice-ambient air by electrical discharge with an ozone generator (PCI Ozone and Control Systems Inc., West Caldwell, NJ).
Ambient ozone concentration was monitored every 15 min and logged by a data acquisition system that calculated the twice-ambient value and transmitted signals to the ozone gen-erator to produce twice-ambient ozone concentration within the designated chambers. All fumigation and sample gases were passed through Teflon tubing. All sampling instruments were calibrated and serviced according to standard operating procedures (Houpis et al. 1988). Ozone analyzers (Model 1003, Dasibi Environmental Corp., Glendale, CA) were cali-brated against a California Air Resources Board certified trans-fer standard. Midday ambient ozone concentration increased from 0.038 ppm in February to 0.058 ppm in June and August, and then decreased to 0.020 ppm in November 1992. Midday elevated ozone concentration inside the chambers increased from 0.075 ppm in February to 0.115 ppm in June and August, and then decreased to 0.044 ppm in November 1992.
Sampling and measurement techniques
In April, July and August 1992, fully expanded fascicles were harvested 2 and 3 days after scheduled irrigation for seedlings and mature trees, respectively. In April and July, 1-year-old fascicles, and in November, current-year fascicles were exam-ined. Fascicles were collected from the upper part of the growing segments and were pulled so that a short strand remained extended from the base. When pressurizing fascicles, the extended strand prevented resin from obstructing the flow of sap from the xylem. Samples were harvested at predawn to prevent confounding effects of diurnal transpiration and kept on ice in the dark until water relations measurements were made.
Individual fascicles, each with three needles, were placed inside two serially connected pressure chambers (Soil Mois-ture Equipment Corp., Santa Barbara, CA), and pressurized with nitrogen gas. Bench-drying pressure-volume (P-V) tech-niques were used to construct P-V curves (Tyree and Hammel 1972, Cheung et al. 1976, Koide et al. 1989). The P-V meas-urements of all treatment combinations for seedlings or mature trees were made within 8 h.
Data analysis
For seedlings, a 22 factorial experiment within a completely randomized design was used with three replicates per treat-ment combination. To test for chamber effects, three replicates of nonchambered nontreated seedlings were compared with seedlings in the control (pH5.1 + Oamb) treatment. For mature
was a 22 factorial experiment within a split-plot, randomized complete block setting. Four mature trees were assigned ran-domly to either of the two rain regimes. On each tree, three similar branches were selected; two branches were assigned randomly to either of the two ozone regimes, and the third branch was used as a nonchambered notreated replicate (total of four) to test for chamber effects.
The GLM (General Linear Model) procedure with SS III option of the SAS System (SAS/STAT, SAS Institute Inc., Cary, NC) was used to analyze the data. When rain × ozone interaction was not significant, the main-effect means were compared. When rain × ozone interaction was significant, ANOVA was performed on four simple-effect means, and Student-Newman-Keuls (SNK) test was used for mutiple mean comparison (Steel and Torrie 1980). Main- or simple-effect means were declared significant at P < 0.05.
Results
There were no significant differences between the control (pH5.1 + Oamb ) and nonchambered nontreated treatments for
any water relations components of either seedlings or mature branches at any measurement date, indicating that the results were not confounded by chamber effects.
Seedlings
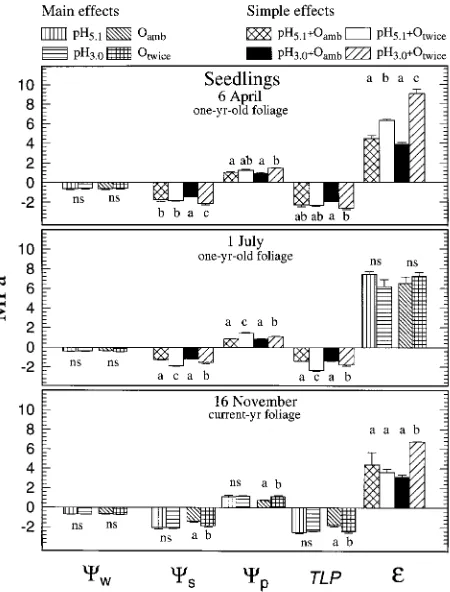
Exposure of seedlings to acid rain and twice-ambient ozone alone or in combination had no significant effect on predawn Ψw in April, July or November 1992. Water potential values of
seedlings in all treatments were near maximum at about −0.5 MPa on all measurement dates (Figure 1).
Interactions between rain and ozone effects on Ψs of
seed-lings were significant in April (P = 0.02) and July (P = 0.006) (Figure 1). In April, the simple-effect means of Ψs in the pH5.1
+ Oamb and pH5.1 + Otwice treatments were not significantly
different but they both differed (P < 0.05) from the simple-ef-fect means of Ψs in the pH3.0 + Oamb and pH3.0 + Otwice
treat-ments (Figure 1). Seedlings in the pH3.0 + Otwice treatment had
the lowest Ψs, whereas seedlings in the pH3.0 + Oamb treatment
had the highest Ψs. In July, simple-effect means of Ψs in the
pH5.1 + Oamb and pH3.0 + Oamb treatments did not differ
signifi-cantly but they both differed (P < 0.05) from the simple-effect means of Ψs in the pH5.1 + Otwice and pH3.0 + Otwice treatments.
The simple-effect mean of Ψs in the pH5.1 + Otwice treatment
was also significantly (P < 0.05) lower than the simple-effect mean of Ψs in the pH3.0 + Otwice treatment. Because the acid
rain by ozone interaction was not significant in November, we compared main-effect means for this date. The comparison showed that Ψs was significantly (P = 0.014) decreased in
seedlings in the twice-ambient ozone treatment.
Acid rain and ozone effects on Ψp of seedlings were of
similar magnitude to those on Ψs but the direction of the
changes was reversed (Figure 1).
Interactions between rain and ozone effects on TLP of seed-lings were significant in April (P = 0.026) and July (P = 0.009). In April, the only significant difference was a lower TLP in seedlings in the pH3.0 + Otwice treatment compared with
seed-lings in the pH3.0 + Oamb treatment (Figure 1). In July, acid rain
had no significant effect on TLP in seedlings exposed to ambient ozone, whereas the twice-ambient ozone treatment decreased (P < 0.05) TLP, and the decrease was greater (P < 0.05) in the presence of pH 5.1 rain than in the presence of pH 3.0 rain. In November, we compared main-effect means, be-cause the acid rain × ozone interaction was not significant, and found that the twice-ambient ozone treatment caused a signifi-cant decrease (P = 0.009) in TLP.
In April and November, signifiacnt interactions between rain and ozone effects were detected on ε (P = 0.001 and 0.007, respectively). The twice-ambient ozone treatment caused ε to increase, particularly in seedlings exposed to pH 3.0 rain (Figure 1). In July, ε was not significantly affected by acid rain or twice-ambient ozone either alone or in combination.
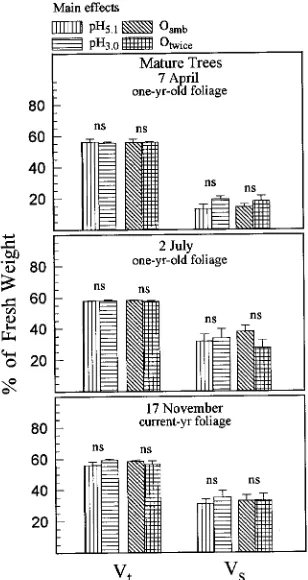
Acid rain, ozone and a combination of the two treatments had no significant effects on Vt in April, July or November
(Figure 2). Because there were no significant acid rain × ozone interactions on Vs, we compared the main-effect means
(Fig-ure 2). There was no significant effect of acid rain or twice-am-bient ozone on Vs in April. In July, Vs increased (P = 0.004) in
seedlings exposed to twice-ambient ozone, whereas in Novem-ber, Vs increased in seedlings in both the pH 3.0 rain (P =
0.047) and twice-ambient ozone (P = 0.002) treatments.
Figure 1. Effects of acid rain and O3 on total water potential (Ψw),
solute potential (Ψs), pressure potential (Ψp), turgor loss point (TLP)
and bulk modulus of elasticity (ε) of ponderosa pine seedlings. Main-effect means are shown when rain × O3 interaction was not significant.
Simple-effect means are shown when rain × O3 interaction was
Mature trees
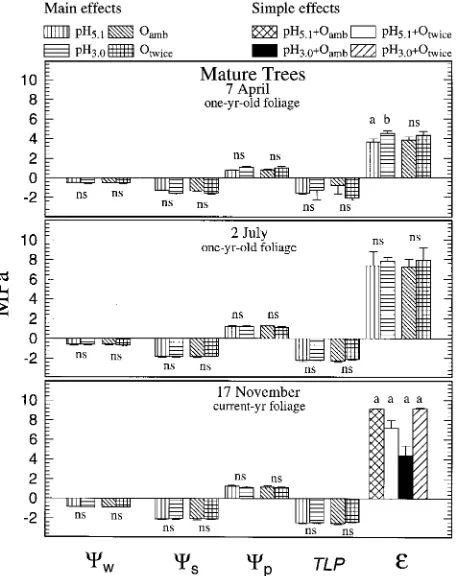
The only significant effects of acid rain and twice-ambient ozone on tissue water relations of mature trees were (1) an increase (P = 0.028) in ε in April caused by the main-effect of pH 3.0 rain, and (2) rain × ozone interaction (P = 0.038) on ε in November. There were no significant simple-effect mean differences in November (Figures 3 and 4).
Discussion
Total water potentials of seedlings were near maximum (−0.5 MPa) throughout the study and, contrary to reports by Taylor and Dobson (1989), they were not affected by the pollutant treatments. Similar Ψw values indicate that the potential effects
of water deficit and transpiration were minimized as a result of regular irrigation and the use of predawn measurements, re-spectively, and that confounding effects of differences in Ψw
on other water relations components were prevented. Solute potential of seedlings was decreased by twice-ambi-ent ozone, particularly in the presence of pH 3.0 rain. This finding is contrary to previous reports (Taylor and Dobson 1989, Dobsonet al. 1990) and differs from the expectation that elevated ozone increases osmotic potential as a result of a reduction in photosynthesis and, hence, cellular concentration.
It may be argued that Ψs decreased as a result of the movement
of N ions from the acid rain to leaf cells through the cuticle, or that Ψs changed in response to the formation of N2O5 and
HNO3 during generation of ozone from ambient air via electric
discharge (Brown and Roberts 1988). However, we have pre-viously found that foliar N concentration of seedlings and mature trees either decreased or did not change in response to elevated ozone or acid rain, respectively (Momen and Helms 1995).
Decreased Ψs might be caused by an increased
concentra-tion of osmotically active metabolites in response to an ozone-induced increase in allocation of photosynthate to foliage at the expense of decreased root growth. Alteration of carbon partitioning by elevated ozone has been attributed to disturbed phloem loading or increased accumulation of starch and sugar in foliage (Hanson and Stewart 1970, McLaughlin et al. 1982, Okano et al. 1984, Cooley and Manning 1987, Küppers and Klumpp 1988, Gorissen and Van Veen 1988, Edwards 1991, Gorissen et al. 1991, Olszyk et al. 1991, Smeulders et al. 1995). However, in this study, the decreased Ψs is unlikely to
be the result of an ozone-induced increase in the allocation of photosynthate to foliage because only very small changes in foliar C concentration occurred in response to elevated ozone (Momen and Helms 1995). We hypothesize that Ψs was
de-Figure 2. Effects of acid rain and O3 on total water content (Vt) and
symplastic water (Vs) of ponderosa pine seedlings. Main-effect means
are shown because rain × O3 interaction was not significant. Different
letters over bars show significant treatment effects (ns = nonsignificant effects); bars = 1 SE.
Figure 3. Effects of acid rain and O3 on total water potential (Ψw),
solute potential (Ψs), pressure potential (Ψp), turgor loss point (TLP),
and bulk modulus of elasticity (ε) of mature ponderosa pine trees. Main-effect means are shown when rain × O3 interaction was not
significant. Simple-effect means are shown when rain × O3 interaction
creased by elevated ozone as a result of osmotic adjustment (Morgan 1984, Salisbury and Ross 1985). Concurrent in-creases in sugar concentrations and dein-creases in starch concen-trations in response to elevated ozone have been observed in many studies (Barnes 1972, Koziol 1984, Ito et al. 1985, Cooley and Manning 1987, Friend and Tomlinson 1992, Kelly et al. 1993, Smeulders et al. 1995). In our study, osmotic adjustment in response to elevated ozone is indicated by de-creases in Ψs and TLP, and concurrent increases in Ψp and Vs
but unchanged Vt. Because osmotic adjustment facilitates
water extraction and turgor maintenance during drought (Hsiao et al. 1976, Tyree et al. 1978, Turner and Jones 1980, Tyree and Jarvis 1982, Morgan 1984, Turner 1986, Momen et al. 1992, Momen et al. 1994), our findings support the hy-pothesis that elevated ozone enhances drought resistance of plants (Temple 1990).
Ozone-induced osmotic adjustment and increases in Ψp
should enhance plant growth; however, many reports suggest that plant growth is inhibited by elevated ozone. Growth or plastic (irreversible) cell expansion depends partly on the rate at which Ψp exceeds a threshold value that controls cell-wall
extensibility (Salisbury and Ross 1985). Our finding that ele-vated ozone caused an increase in ε may indicate an increase in the threshold pressure required to permit cell expansion and hence a decrease in growth rate. Decreased elasticity of guard
cell walls may also affect plant growth through changes in stomatal conductance (Runeckles and Rosen 1977) and water use efficiency. Therefore, the positive effects of osmotic ad-justment and increased Ψp on plant growth might be offset by
the negative effect of decreased cell wall elasticity.
The interactive effects of acid rain and elevated ozone on ε and Ψs could support the hypothesis (Heath 1980) that elevated
ozone reacts with the plasmalemma and increases the perme-ability of membranes thereby enhancing the movement of S and N ions of acid rain into leaf cells leading to decreased Ψs.
However, analysis of foliar chemistry (Momen and Helms 1995) indicated no increase in foliar N concentration of pon-derosa pine seedlings or mature trees in response to increased rain acidity.
The effects of elevated ozone on water relations of ponder-osa pine seedlings were similar to the effects of drought in many species (cf. Chapin 1991). It seems unlikely that elevated ozone induced water stress in seedlings as a result of decreased root growth and water uptake, because Ψw of seedlings were
not affected by twice-ambient ozone and were near maximum. Interactive effects of acid rain and elevated ozone indicate that the effects of one pollutant depended on the level of the other, and thus no accurate conclusion can be drawn from single pollutant studies in regions exposed to multiple pollutants. The large responses of seedling water relations to pollutants con-trasted with those of mature trees and may indicate that the parent genotype had adapted to environmental stresses. It is possible that seedlings and mature trees differed in their re-sponses to the treatments because seedlings were entirely exposed to pollutants whereas mature trees were not, or be-cause the branches of mature trees were not entirely autono-mous with regard to plant water relations. However, cellular damage by elevated ozone is local, and branches of mature trees have been found autonomous with respect to carbon transport (Cregg 1990, Mattson et al. 1990, Sprugel et al. 1991) and water relations (Zimmermann 1983, Tyree 1988).
Acknowledgments
We thank P.D. Anderson and J.L.J. Houpis for their help throughout the study. We also thank F.S. Chapin and G. Biging for comments on an earlier version of this paper. This study was supported by the California Air Resources Board through Contract A132-101 and by the McIntire-Stennis project 4635-MS.
References
Barnes, R.L. 1972. Effects of chronic exposure to ozone on soluble sugar and ascorbic acid content of pine seedlings. Can. J. Bot. 110:435--441.
Brown, K.A. and T.M. Roberts. 1988. Effects of ozone on foliar leaching in Norway spruce (Picea abies (L.) Karst.): confounding factors due to NOx production during ozone generation. Environ. Pollut. 55:55--73.
Chapin, F.S., III. 1991. Integrated responses of plants to stress. Bio-Science 41:29--36.
Chappelka, A.H., B.I. Chevone and T.E. Burk. 1985. Growth re-sponses of yellow-poplar (Liriodendron tulipfera L.) seedlings to ozone, sulfur dioxide, and simulated acidic precipitation alone and in combination. Environ. Exp. Bot. 25:233--244.
Figure 4. Effects of acid rain and O3 on total water content (Vt) and
symplastic water (Vs) of mature ponderosa pine trees. Main-effect means are shown because rain × O3 interaction was not significant.
Cheung, Y.N.S., M.T. Tyree and J. Dainty. 1976. Some possible sources of errors in determining bulk elastic moduli and other parameters from pressure-volume curves of shoots and leaves. Can. J. Bot. 54:756--765.
Cooley, D.R. and W.J. Manning. 1987. The impact of ozone on assimilate partitioning in plants: a review. Environ. Pollut. 47:95--113.
Cregg, B.M. 1990. Net photosynthesis and carbon allocation of lob-lolly pine (Pinus taeda L.) branches in relation to three levels of shade. Ph.D. Diss. University of Georgia, Athens, GA, 169 p. Dobson, M.C., G. Taylor and P.H. Freer-Smith. 1990. The control of
ozone uptake by Picea abies (L.) Karst. and P. sitchensis (Bong.) Carr. during drought and interacting effects on shoot water rela-tions. New Phytol. 116:465--474.
Edwards, N.T. 1991. Root and soil respiration responses to ozone in
Pinus taeda L. seedlings. New Phytol. 118:315--321.
Evans, L.S. and I.P. Ting. 1974. Ozone sensitivity of leaves: relation-ship to leaf water potential, gas transfer resistance, and anatomical characteristic. Am. J. Bot. 61:592--597.
Friend, A.L. and P.T. Tomlinson. 1992. Mild ozone exposure alters 14C dynamics in foliage of Pinus taeda L. Tree Physiol. 11:214--227. Gorissen, A. and J.A. Van Veen. 1988. Temporary disturbance of
translocation of assimilates in Douglas-firs caused by low levels of ozone and sulfur dioxide. Plant Physiol. 88:559--563.
Gorissen, A., N.N. Joosten and A.E. Jansen. 1991. Effects of ozone and ammonium sulfate on carbon partitioning to mycorrhizal roots of juvenile Douglas-fir. New Phytol. 119:243--250.
Hanson, G.P. and W.S. Stewart. 1970. Photochemical oxidants: effect on starch hydrolysis in leaves. Science 168:1223--1224.
Heath, R.L. 1980. Initial events in injury to plants by air pollutants. Annu. Rev. Plant Physiol. 31:394--431.
Hileman, B. 1984. Forest decline from air pollution. Environ. Sci. Technol. 18:8--10.
Houpis, J.L.J., K.A. Surano and S. Cowles. 1988. Research plan: comparison of the response of mature branches and seedlings of
Pinus ponderosa to atmospheric pollution. Lawrence Livermore National Lab, Report UCRL-21001-88-4, University of California, Livermore, CA, 155 p.
Houpis, J.L.J., M.P. Costella and S. Cowles. 1991. A branch exposure chamber for fumigating ponderosa pine to atmospheric pollution. J. Environ. Qual. 20:467--474.
Hsiao, T.C., E. Acevedo, E. Ferres, and D.W. Henderson. 1976. Water stress, growth and osmotic adjustment. Philos. Trans. R. Soc. Lond. B 273:479--500.
Ito, O, F. Mitsumori and T. Totsuka. 1985. Effects of NO2 and O3 alone
or in combination on kidney bean plants (Phaseolus vulgaris L.): products of 13CO2 assimilation detected by 13C nuclear magnetic
resonance. J. Exp. Bot. 36:281--289.
Kelly, J.M., G.E. Taylor, Jr., N.T. Edwards, M.B. Adams, G.S. Ed-wards and A.L. Friend. 1993. Growth, physiology, and nutrition of loblolly pine seedlings stressed by ozone and acidic precipitation: a summary of the ROPIS-south project. Water Air Soil Pollut. 69:363--391.
Koide, R.T., R.H. Robichaux, S.R. Morse, and C.M. Smith. 1989. Plant water status, hydraulic resistance and capacitance. In Plant Physiological Ecology: Field Methods and Instrumentation. Eds. R.W. Pearcy, J. Ehleringer, H.A. Mooney and P.W. Rundel. Chap-man and Hall, New York, NY, pp 161--183.
Koziol, M.J. 1984. Interactions of gaseous pollutants with carbohy-drate metabolism. In Gaseous Air Pollutants and Plant Metabolism. Eds. M.J. Koziol and F.R. Whatley. Butterworths, London, pp 251--273.
Küppers, K. and G. Klumpp. 1988. Effects of ozone, sulfur dioxide, and nitrogen dioxide on gas exchange and starch economy in Norway spruce (Picea abies (L.) Karst.). GeoJournal 17:271--275. Leonard, R.L., C.R. Goldman and G.E. Likens. 1981. Some measure-ments of the pH and chemistry of precipitation at Davis and Lake Tahoe, California. Water Air Soil Pollut. 15:153--167.
Mattson, K.G., L.Y. Amaut, G.A. Reams and S.O. Cline. 1990. Re-sponse of forest trees to sulfur, nitrogen, and associated pollutants. USEPA/USDA Forest Service Response Program, Major Program Output No. 4, p 108.
McColl, J.G. and R. Johnson. 1983. Effects of simulated acid rain on germination and early growth of Douglas-fir and ponderosa pine. Plant Soil 74:125--129.
McLaughlin, S.B., R.K. McConathy, D. Duvick and L.K. Mann. 1982. Effects of chronic air pollution stress on photosynthesis, carbon allocation and growth of white pine trees. For. Sci. 28:60--70. Miller, P.R., R.N. Kickert, O.C. Taylor, R.J. Arkley, F.M. Cobb, D.L.
Dahlsten, P.J. Gersper, R.F. Luck, J.R. McBride, J.R. Parameter, J.M. Wenz, M. White and W.W. Wilcox. 1977. Photochemical oxidant air pollutant effects on a mixed conifer forest ecosystem: a progress report. Corvallis Environ. Res. Lab., US EPA, Corvallis, OR, 339 p.
Momen, B., J.W. Menke and J.M. Welker. 1992. Tissue water relations of Quercus wislizenii seedlings: drought resistance in a California evergreen oak. Acta Oecol. 13:127--136.
Momen, B., J.W. Menke, J.M. Welker, K.J. Rice and F.S. Chapin III. 1994. Blue-oak regeneration and seedling water relations in four sites within a California oak savanna. Int. J. Plant Sci. 155:744--749.
Momen, B. and J.A. Helms. 1995. Effects of acid rain and ozone on foliar chemistry of field-grown seedlings and mature trees of Pinus ponderosa. Environ. Pollut. In press.
Morgan, J.M. 1984. Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol. 35:299--319.
Okano, K., O. Ito, G. Takeba, A. Shimizu and T. Totsuka. 1984. Alteration of 13C-assimilate partitioning in plants of Phaseolus vulgaris exposed to ozone. New Phytol. 97:155--163.
Olszyk, D.M., B.K. Takemoto and M. Poe. 1991. Leaf photosynthetic and water relations responses for ‘‘Valencia’’ orange trees exposed to oxidant pollution. Environ. Exp. Bot. 31:427--436.
Pye, J.M., J.E. de Steiguer, and C. Love. 1988. Expert opinion survey on the impacts of air pollutants on forests of the USA. In Air Pollution and Forest Decline. Proc. 14th Int. Meeting for Specialists in Air Pollution Effects on Forest Ecosystems, Vol. 1. Eds. J.B. Bucher and I. Bucher-Wallin. IUFRO, Interlaken, Birmensdorf, Switzerland, pp 355--360.
Reich, P.B., A.W. Schoettle and R.G. Amundson. 1986. Effects of ozone and acidic rain on photosynthesis and growth in sugar maple and northern red oak seedlings. Environ. Pollut. 40:1--15. Runeckles, V.C. and P.M. Rosen. 1977. Effects of ambient ozone
pretreatment on transpiration and susceptibility to ozone injury. Can. J. Bot. 55:193--197.
Salisbury, F.B. and C.W. Ross. 1985. Plant physiology. Wadsworth Inc., Belmont, CA, 540 p.
Smeulders, S.M., A. Gorissen, N.N. Joosten and J.A. Van Veen. 1995. Effects of short-term ozone exposure on the carbon economy of mature and juvenile Douglas-firs (Pseudotsuga menziesii (Mirb.) Franco). New Phytol. 129:45--53.
Sprugel, D.G., T.M. Hinckley and W. Schaap. 1991. The theory and practice of branch autonomy. Annu. Rev. Ecol. Syst. 22:309--334. Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of
Taylor, G. and M.C. Dobson. 1989. Photosynthetic characteristics, stomatal responses and water relations of Fagus sylvatica: impact of air quality at a site in southern Britain. New Phytol. 113:265--273.
Temple, P.J. 1990. Water relations of differentially irrigated cotton exposed to ozone. Agron. J. 82:800--805.
Turner, N.C. and M.M. Jones. 1980. Turgor maintenance by osmotic adjustment: a review and evaluation. In Adaptation of Plants to Water and High Temperature Stress. Eds. N.C. Turner and P.J. Kramer. John Wiley and Sons, New York, NY, pp 89--103. Turner, N.C. 1986. Adaptation to water deficits: a change in
perspec-tive. Aust. J. Plant Physiol. 13:175--190.
Tyree, M.T. and H.T. Hammel. 1972. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J. Exp. Bot. 23:267--282.
Tyree, M.T., Y.N.S. Cheung, M.E. Macgregor and A.J.B. Talbot. 1978. The characteristics of seasonal and ontogenetic changes in the tissue water relations of Acer, Populus, Tsuga, and Picea. Can. J. Bot. 56:635--647.
Tyree, M.T. and P.G. Jarvis. 1982. Water in tissues and cells. In
Encyclopedia of Plant Physiology. Vol. 12B. New Series, Physi-ological Plant Ecology II. Eds. O.L. Lange, P.S. Nobel, C.B. Os-mond and H. Ziegler. Springer-Verlag, Berlin, pp 36--77. Tyree, M.T. 1988. A dynamic model for water flow in a single tree:
evidence that models must account for hydraulic architecture. Tree Physiol. 4:195--218.
USNAPAP. 1991. Acidic deposition: state of science and technology. Vol. I--IV. Ed. P. M. Irving. Government Printing Office, Washing-ton, DC.