Summary We developed a protocol for the production of shoots from bud explants from 1- to 7-year-old trees of western white pine (Pinus monticola Dougl.). The best explant was a 2-mm-thick cross-sectional slice of the early winter bud. Geno-type of the donor tree was a significant factor affecting shoot production, but more than 80% of the genotypes tested produced shoots. Of the media tested, bud slices from 1- to 3-year-old trees grew best in Litvay’s medium containing N6 -benzyladenine in the range of 1 to 30 µM, whereas bud slices from older trees grew best in Gupta and Durzan’s DCR medium with zeatin riboside. Up to 400 shoots more than 3 mm in height were obtained from 100 bud-slice explants taken from 7-year-old western white pine trees.
Keywords: clonal propagation, N6-benzyladenine, organo-genesis, shoot induction, zeatin riboside.
Introduction
In western Canada and the Pacific northwest region of the United States, western white pine (Pinus monticola Dougl.) is a desirable species because it grows rapidly and produces high value wood (Govindaraju 1988, Stiff et al. 1989). However, the species has not been used for reforestation (Bingham 1983) because it is highly susceptible to white pine blister rust (Cronartium ribicola J. C. Fisch. ex Rabenh.). Several breed-ing programs for rust resistance in western white pine were initiated during the 1980s (Bingham 1983, Hagle et al. 1989). It takes only seven years to complete the assessment of western white pine for blister-rust resistance (Hunt 1988), which means that resistant strains may soon be identified and made available for use in plantation forestry. However, western white pine produces only a limited amount of seed (Fins 1987), which often germinates poorly (Dumroese and Wenny 1987). A clonal system for propagating what limited supply of rust-re-sistant plants becomes available during the next few years could, therefore, be of great value.
Induction of adventitious shoots from mature embryos of western white pine has been reported (Mott and Amerson 1981, Lapp et al. 1995), and the rooting response of these shoots has been investigated (Amerson and Mott 1982, McAfee et al. 1993a, 1993b). There have been several attempts to propagate western white pine vegetatively by rooting needle
fascicles (Hoff and McDonald 1968, McDonald and Hoff 1969, McDonald and Hoff 1970, Andrews 1980, Stiff et al. 1989). However, success has generally been limited to material from young trees and dependent on the genotype of the shoot; although Williams (1987) reported a very low rate of root induction in cuttings from 25- to 29-year-old trees, with suc-cess being dependent on genotype. Here we report a protocol that allows production of shoots from bud explants from most of the 1- to 7-year-old western white pine trees investigated.
Materials and methods
Explant source
Buds were collected from trees supplied by the Canadian Forestry Service, Pacific Forestry Centre, Victoria, British Columbia as one-year-old seedlings. The trees were main-tained in a greenhouse during the spring and summer and during the fall and winter they were placed in a growth cham-ber and maintained in a 16-h photoperiod at 3--4 °C.
Explant preparation and sterilization
Early winter buds were excised from trees and sterilized for 1 min with 70% ethanol, then rinsed with sterile double-dis-tilled water (sddw) before removal of the bud-scales. The buds were placed in 30% bleach (30 ml of 5% sodium hypochlorite plus 70 ml of sddw) containing 2--3 drops of Tween 80 and agitated for 1 h. The buds were rinsed three times with sddw. At no time were the buds allowed to dry out. For the first experiment, the buds were cut into a series of 2-mm thick cross-sectional slices, longitudinal half sections, or longitudi-nal quarter sections. Dissected out fascicle meristems, were also used as explants. For all other experiments, 2-mm thick cross-sectional slices were the only explants used. The number of slices obtained from one bud ranged from two to nine.
Media preparation
The media used were: AE (von Arnold and Eriksson 1981), DCR (Gupta and Durzan 1985), GD (GD1) (Gresshoff and Doy 1972), LV (Litvay et al. 1981), MCM (Bornman 1983), QP (Quoirin and Lepoivre 1977) and SH (Schenk and Hilde-brandt 1972). Two media, AGn and AGo, are modifications of
Microculture of western white pine (
Pinus monticola
) by induction of
shoots on bud explants from 1- to 7-year-old trees
MARTIN S. LAPP, JANA MALINEK and MAXINE COFFEY
Plant Biotechnology Institute, National Research Council, Saskatoon, Saskatchewan, Canada, S7N OW9
Received June 21, 1995
GD and differ only in that AGo has twice the amount of chelated iron as AGn. Hormones were added to each medium before adjusting the pH to 5.6. BiTek Agar (Difco Laborato-ries, Detroit, MI) at 8 g l−1 was added to all media before autoclaving at 121 °C for 15 min. The media were dispensed in 25 ml aliquots into 100 × 15 mm petri dishes.
Plant growth regulators
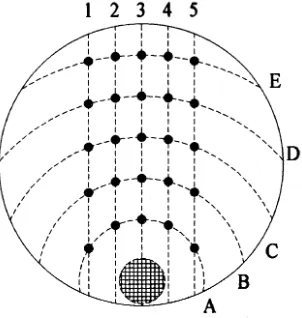
For bud slices from 1- to 5-year-old trees, 1 to 100 µM BA (N6-benzyladenine) was used. For bud slices from 6- to 7-year-old trees a range of cytokinins was used in a hormone-gradient system developed by Anderson and Camper (1987). To prepare the system, 100 ml of autoclaved medium was dispensed per 150 × 15 mm petri dish. A 2.1-cm diameter filter disk of glass microfibre (Whatman No. 934-AH, Whatman International Ltd., Maidstone, England) was placed on top of the medium and loaded with 200 µl of filter-sterilized 5 mM cytokinin solution. A hormone gradient was allowed to establish for 24 h after which the bud slices were placed on the plates in the radial pattern shown in Figure 1. This arrangement was used to ensure that each row of five slices was supplied with the same concentration of hormone.
Culture environment and protocol
Cultures were kept in growth chambers at 25 °C in a 16-h photoperiod provided by both incandescent and 40-W cool-white fluorescent lights which gave illumination of 90 µmol m−2 s−1. We used a modification of the culture regime de-scribed by Patel and Thorpe (1984). On Day 0, prepared explants were placed flat on the hormone-containing medium (the cross-sectional slices were oriented apical side up) in 100
× 15 mm petri dishes. On Day 21, explants were moved to hormone-free medium in 100 × 15 mm petri dishes. On Day 28, explants were transferred to hormone-free medium con-taining 0.1% charcoal in 100 × 25 mm petri dishes. On Day 42, explants were transferred to fresh medium containing 0.1% charcoal in 500-g specimen jars. Finally, on Day 77, shoots
taller than 3 mm were harvested and the remaining explants discarded.
Measurements and statistical analysis
We recorded the number of test explants (the initial number less any explants lost to contamination) and the number of shoots obtained per explant. These data were summed for each initial plate to give one treatment-replicate. The number of shoots produced per test explant, i.e., the number of shoots divided by the number of original explants was determined. The data were subjected to either analysis of variance or contingency Chi-square using NWA Statpak Version 4.1 (Northwest Analytical, Inc., Portland, Oregon, USA). The ANOVA tables and their interpretations were made according to Mize and Chun (1988). All of the formulations and concen-trations reported have been tested at least three times.
Results and discussion
Explant
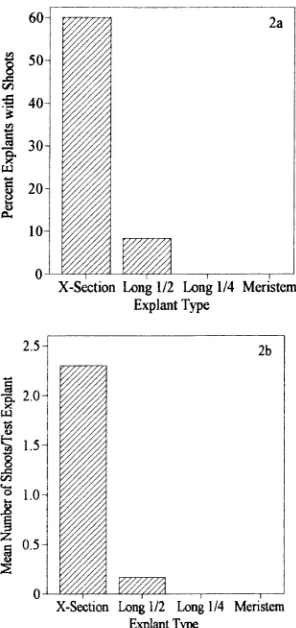
Selection of the explant is probably the most important factor for successful micropropagation of conifers (Thorpe et al. 1991). Four different portions or parts of the western white pine buds were tested. The most productive explants in terms of percent of explants forming shoots and the mean number of shoots per test explant were the 2-mm thick cross-sectional bud slices. Bud explants comprising longitudinal half sections, longitudinal quarter sections or individual meristems produced very few shoots (Figures 2a and 2b). Whole buds were also tried but did not give rise to any shoots (data not shown). Bud slices from lateral buds were as productive in forming shoots as bud slices from terminal buds (16.4 versus 15.2%, contin-gency Chi-square P = 0.8244). A similar result was found with buds of Picea glauca (Moench) Voss (Dunstan et al. 1987). There was no significant difference in the number of shoots formed relative to the location of the slice within the source bud (Table 1).
Genotype
For a microculture technique to be useful it is important that most genotypes of the species respond to the protocol (Dun-stan et al. 1986). We tested 14 individual tree genotypes and found significant differences among the trees in the percent of bud-slice explants forming shoots (Figure 3). However, 12 of the 14 trees tested were induced to form shoots, which is encouraging given the high genetic variability of western white pine (Hunt et al. 1985).
Medium composition
We used bud slices from 2-year-old trees to test the effects of eight medium formulations on shoot production (Figure 4). The production of shoots was highest when the bud-slice explants were grown on LV or SH medium and lowest on MCM medium. However, with increasing age of the source trees and thus presumably the loss of juvenility, the rate of shoot production declined. A similar experiment with bud Figure 1. Layout of a 150 × 15 mm petri dish set up as a
slices from 4-year-old trees indicated that shoot production was higher on DCR medium than on LV, SH or GD medium (Figure 5). Therefore, we used DCR medium in all subsequent experiments with older trees.
Plant hormones
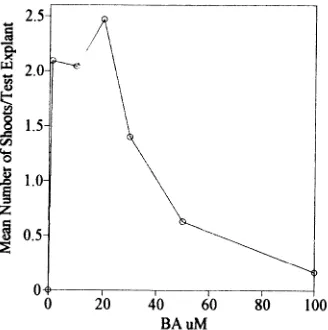
Both the amount and the type of cytokinin are important factors affecting the induction of shoots. Bud-slice explants from 2-year-old trees produced the highest number of shoots in the presence of BA in the range of 1 to 30 µM (Figure 6). Because the rate of shoot production declined with increasing age of the donor trees, we used bud-slice explants from 7-year-old trees to test the effects of six cytokinins on the induction of shoots. The hormone-gradient system allowed the testing of several concentrations of one cytokinin on one plate (Figure 7). Zeatin riboside followed by zeatin were significantly more effective in inducing shoots than the other cytokinins tested. Figure 2. Effect of explant type on shoot production of buds from
1-year-old western white pine trees. The cultures were grown on LV medium with 5 to 50 µM BA and 18 to 24 explants per treatment. (a) Percent of explants forming shoots by explant type. (b) Mean number of shoots per test explant by explant type. X-Section = 2-mm thick cross-sectional slice, Long 1/2 = longitudinal half section of the bud, Long 1/4 = longitudinal quarter section of the bud, Meristem = indi-vidual needle bundle meristem.
Table 1. Percentage of bud-slice explants from 2-year-old trees form-ing shoots versus the source location of the slice within the bud. The bud slices are numbered from the apex down. Bud slices numbered 5 to 9 are grouped together so that each category has sufficient slices to allow statistical treatment. There was no significant difference in shoot production among bud slices (contingency Chi-square P = 0.9296). The cultures were grown on LV medium with 10 µM BA.
Bud-slice no. Bud slices with shoots (%)
1 13.0
2 19.2
3 18.4
4 16.2
5--9 13.6
Figure 3. Percent of bud-slice explants from 7-year-old western white pine trees producing shoots in response to zeatin or zeatin riboside versus the individual tree genotype. There were significant differences among the trees (contingency Chi-square P < 0.001) but shoots were produced on 12 of the 14 trees. The cultures were grown on zeatin riboside or zeatin gradient plates with DCR medium. There were four to 20 explants per individual tree for a total of 152 explants.
Figure 4. Effects of eight medium formulations on the production of shoots from bud slices from 2-year-old western white pine trees. Explants cultured on the LV or SH medium produced significantly more shoots than explants cultures on the other media (ANOVA P =
The bud slices closest to the hormone source (the highest concentration) produced the most shoots. The protocol was also applied to bud-slice explants from cuttings of 7-year-old trees that had been tested for blister rust resistance and results similar to those presented in Figure 7 for zeatin riboside and zeatin were obtained. Thus, for the explants from cuttings, the difference between zeatin riboside and zeatin and the differ-ences between concentrations were significant (ANOVA P = 0.0401 and 0.0012, respectively).
Conifers become increasingly difficult to micropropagate as they mature. However, the greatest gains from clonal propaga-tion will come from the reproducpropaga-tion phenotypically superior
trees that are old enough to be identified as such (Dunstan 1988). With increasing maturity of the explant source, tech-niques that work well with bud explants from 1- to 3-year-old trees gave poor results with bud explants from 4- and 5-year-old trees. However, we were able to develop an effective protocol for the production of shoots from most 7-year-old trees. The protocol was successfully applied to bud-slice ex-plants from cuttings of 7-year-old trees that had been tested for blister rust resistance. Roots have now been induced on these shoots using a treatment with either ectomycorrhizal fungi or Agrobacterium rhizogenes (McAfee et al. 1993a, McAfee et al. 1993b). A number of these plantlets were established in the greenhouse and grew normally. Also published as NRCC Pub-lication No. 38935.
Acknowledgments
The authors thank Drs. E. White and R. Hunt of the Pacific Forest Centre, Canadian Forestry Service, Victoria, British Columbia for supplying the trees and cuttings used in this work. The authors would also like to express their appreciation to Dr. D. Dunstan for his encouragement and advice. This is NRCC Publication No. 38935.
References
Amerson, H.V. and R.L. Mott. 1982. Improved rooting of western white pine shoots from tissue culture. For. Sci. 28:822--825. Anderson, D.R. and N.D. Camper. 1987. Application of the disk
method: response to growth regulators. J. Plant Growth Regul. 6:57--65.
Figure 5. Effects of four medium formulations on the production of shoots from bud slices from 4-year-old western white pine trees. Explants cultured on DCR medium produced significantly more shoots than explants cultured on the other media (ANOVA P =
0.0343). All of the media contained 30 µM BA. There were five replicates per treatment with 10 bud slices per plate, considered one treatment-replicate, for a total of 200 explants.
Figure 6. Effect of BA concentration on the production of shoots from bud slices from 2-year-old western white pine trees. The 1 to 30 µM concentration range significantly enhanced shoot induction (ANOVA
P = 0.0006). The cultures were grown on LV medium with five
replicates per treatment and five bud slices per plate, considered one treatment-replicate, for a total of 175 explants.
Figure 7. Effects of six cytokinins on the production of shoots from bud slices from 7-year-old western white pine trees. The x axis shows
the distance in mm from the cytokinin source and thus reflects a decreasing cytokinin concentration with increasing distance. The dif-ferences between cytokinins are significant (ANOVA P = 0.0160) as
Andrews, D.S. 1980. Rooting western white pine, Pinus monticola
Dougl., needle fascicles and branch cuttings. USDA Forest Service, Intermountain Forest and Range Expt. Stn., Res. Note INT-291, 11 p.
Bingham, R.T. 1983. Blister rust resistant western white pine for the inland empire: the story of the first 25 years of the research and development program. USDA Forest Service, Intermountain Forest and Range Expt. Stn., General Tech. Rep. INT-146, 45 p. Bornman, C.H. 1983. Possibilities and constraints in the regeneration
of trees from cotyledonary needles of Picea abies in vitro. Physiol. Plant. 57:5--16.
Dumroese, R.K. and D.L. Wenny. 1987. Sowing sized seed of western white pine in a containerized nursery. West. J. Appl. For. 2:128--130.
Dunstan, D.I. 1988. Prospects and progress in conifer biotechnology. Can. J. For. Res. 18:1497--1506.
Dunstan, D.I., G.H. Mohammed, and T.A. Thorpe. 1986. Shoot pro-duction and elongation on explants from vegetative buds excised from 17- to 20-year-old Pseudotsuga menziesii. N.Z. J. For. Sci.
16:269--282
Dunstan, D.I., G.H. Mohammed, and T.A. Thorpe. 1987. Morphoge-netic response of vegetative bud explants of adolescent and mature
Picea glauca Moench Voss in vitro. New Phytol. 106:225--236. Fins, L. 1987. The Inland Empire Tree Improvement Cooperative
Eleventh Progress Report. Idaho Forest, Wildlife and Range Experi-ment Station, College of Forest, Wildlife and Range Sciences, Univ. Idaho, Moscow, Idaho, 57 p.
Govindaraju, D.R. 1988. Life histories, neighbourhood sizes and vari-ance structure in some North American conifers. Biol. J. Linn. Soc. 35:69--78.
Gresshoff, D.M. and C.H. Doy. 1972. Development and differentiation of haploid Lycopersicum esculentum. Planta 107:161--170.
Gupta, P.K. and D.J. Durzan. 1985. Shoot multiplication from mature trees of Douglas fir, Pseudotsuga menziesii, and sugar pine, Pinus lambertiana. Plant Cell Rep. 4:177--179.
Hagle, S.K., G.I. McDonald and E.A. Norby. 1989. White pine blister rust in northern Idaho and western Montana: alternatives for inte-grated management. USDA Forest Service, Intermountain Forest and Range Expt. Stn., General Tech. Rep. INT-261, 35 p. Hoff, R.J., and G.I. McDonald. 1968. Rooting of needle fascicles from
western white pine. USDA Forest Service, Intermountain Forest and Range Expt. Stn., Res. Note INT-80, 6 p.
Hunt, R.S. 1988. White pine tree improvement in British Columbia, Nakusp, B.C. In Proc. Western White Pine Management Symp. Ed. R.S. Hunt. Pacific Forestry Centre, Can. For. Serv., Victoria, B.C., pp 32--36.
Hunt, R.S., E. von Rudloff, M.S. Lapp and J.F. Manville. 1985. White pine blister rust in British Columbia. III. Effects on the gene pool of western white pine. For. Chron. 61:484--488.
Lapp, M.S., J. Malinek and M. Coffey. 1995. Micropropagation of western white pine (Pinus monticola) starting with mature em-bryos. Plant Cell Rep. 15:264--267.
Litvay, J.D., M.A. Johnson, D.C. Verma, D.W. Einspahr and K. Weyrauch. 1981. Conifer suspension culture medium development using analytical data from developing seeds. Inst. Paper Chemistry, Tech. Paper Ser. No. 115, 17 p.
McAfee, B.J., M.S.C. Pedras and M.S. Lapp. 1993a. Effects of
Pisolithus arhizus and other ectomycorrhizal fungi on in vitro
rooting of Pinus monticola. Proc. N.S. Inst. Sci. 40:1--10.
McAfee, B.J., E.E. White, L.E. Pelcher and M.S. Lapp. 1993b. Root
induction in pine (Pinus) and larch (Larix) spp. using Agrobac-terium rhizogenes. Plant Cell Tissue Organ Cult. 34:53--62. McDonald, G.I. and R.J. Hoff. 1969. Effect of rooting medium and
hormone application on rooting of western white pine needle fasci-cles. USDA Forest Service, Intermountain Forest and Range Expt. Stn., Res. Note INT-101, 6 p.
McDonald, G.I. and R.J. Hoff. 1970. Effects of Cronartium ribicola
on rooting potential of detached Pinus monticola needle bundles. Can. J. Bot. 48:1943--1945.
Mize, C.W. and Y.W. Chun. 1988. Analyzing treatments means in plant tissue culture research. Plant Cell Tissue Organ Cult. 13:201--217. Mott, R.L. and H.V. Amerson. 1981. Tissue culture plantlets produced
from Pinus monticola embryonic materials. For. Sci. 27:299--304.
Patel, K.R. and T.A. Thorpe. 1984. In vitro differentiation of plantlets from embryonic explants of lodgepole pine (Pinus contorta Dougl.
ex Loud.) Plant Cell Tissue Organ Cult. 3:131--142.
Quoirin, M. and P. Lepoivre. 1977. Étude de milieux adaptés aux cultures in vitro de Prunus. Acta Hortic. 78:437--442.
Schenk, R.U. and A.C. Hildebrandt. 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50:199--204.
Stiff, C.M., D.L. Wenny, R.K. Dumroese, L.W. Roberts and C.T. Stiff. 1989. Establishment of western white pine shoots in vitro. Can. J. For. Res. 19:1330--1333.
Thorpe, T.A., I.S. Harry and P.P. Kumar. 1991. Application of micro-propagation to forestry, In Micropropagation: Technology and
Ap-plication. Eds. P.C. Debergh and R.H. Zimmerman. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 311--336. von Arnold, S. and T. Eriksson. 1981. In vitro studies of adventitious
shoot formation in Pinus contorta. Can. J. Bot. 59:870--874.