www.elsevier.com/locate/jinsphys
Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus
nematophilus, the symbiotic bacteria to the entomopathogenic
nematode Steinernema carpocapsae
Youngjin Park, Yonggyun Kim
*School of Bioresource Sciences, College of Natural Sciences, Andong National University, Andong 760-749, South Korea
Received 28 January 2000; accepted 4 April 2000
Abstract
Xenorhabdus nematophilus is a pathogenic bacterium causing insect haemolymph septicemia, which leads to host insect death.
To address the fundamental mechanisms underlying this haemolymph septicemia, or the immunodepressive response of the host insects following bacterial infection, we tested a hypothesis that the insect immune-mediating eicosanoid pathway is blocked by inhibitory action of the bacterium. Haemocoelic injection of the bacteria into the fifth instar larvae of Spodoptera exigua reduced the total number of living haemocytes with postinjection time and resulted in host death in 16 h at 25°C. The lethal efficacy, described by the median lethal bacterial dose (LD50), was estimated as 33 colony-forming units per fifth instar larva of S. exigua. The lethal effect of the bacteria on the infected larvae decreased significantly with the addition of exogenous arachidonic acid (10
µg), a precursor of eicosanoids. In comparison, injections of dexamethasone (10µg), a specific inhibitor of phospholipase A2, and other eicosanoid biosynthesis inhibitors elevated significantly the bacterial pathogenicity. Live X. nematophilus induced the infected larvae to form less nodules than did the heat-killed bacteria, but the addition of arachidonic acid increased the number of nodules formed significantly in response to live bacterial injection. The treatment with dexamethasone and other inhibitors, however, decreased the nodule formation after injection of heat-killed bacteria. These results indicate that eicosanoids play a role in the immune response of S. exigua, and suggest strongly that X. nematophilus inhibits its eicosanoid pathway, which then results in immunodepressive haemolymph septicemia.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Xenorhabdus nematophilus; Eicosanoid; Spodoptera exigua; Immune; Nodulation
1. Introduction
Insects are able to defend themselves against foreign organisms by their own immune response. This response is elicited by sequential reactions such as recognition of nonself, mediation of the immune signal, and cellular and humoral immune responses (reviewed in Gillespie and Kanost, 1997). Specific chemical structures in differ-ent organisms can be recognized as nonself by insects. This information is conveyed by localized mediators such as eicosanoids (Stanley-Samuelson, 1994) and bio-genic amines (Baines et al., 1992; Dunphy and Downer,
* Corresponding author. Tel.: +82-571-850-5638; fax: + 82-571-841-1628.
E-mail address: [email protected] (Y. Kim).
0022-1910/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 0 2 2 - 1 9 1 0 ( 0 0 ) 0 0 0 7 1 - 8
1994), and finally induces immunocompetent cells, hae-mocytes, to exhibit actual immune responses.
The cellular immune response represents rapid thera-peutic methods used by the haemocytes and includes encapsulation, phagocytosis, and nodule formation (Ratcliffe et al., 1985). The cellular response is then reinforced by the humoral immune response that inacti-vates or kills foreign organisms through the activities of polyphenoloxidase (Ashida and Yamazaki, 1990), lyso-zyme (Dunn, 1986), attacin, cecropins, and other anti-bacterial proteins (Boman and Hultmark, 1987).
insect host, and establish conditions for nematode repro-duction in the cadaver. These two mutualistic organisms invade the various insect hosts, cause significant lethal effects, and are thus used as biological control agents (Kaya and Gaugler, 1993). There is, however, little information available to understand the insecticidal mechanism of X. nematophilus whereby it causes infec-tivity and haemolymph septicemia. The cytotoxic activity exhibited by the bacteria has been generally regarded as the main cause of septicemia (Dunphy and Webster 1984, 1991; Ribeiro et al., 1999). In this hypothesis, after the bacteria enter the haemocoel of the host insects, they kill haemocytes which are the major cells exhibiting the immune responses. The cytotoxic effect on the haemocytes results in haemolymph septice-mia (Dunphy and Webster, 1984). But, the role of the haemocytes before they are hit by the bacterial virulence factors is questioned. Recent results by Forst et al. (1997) indicated that X. nematophilus inhibits the acti-vation of the insect enzyme, phenoloxidase, by using a lipopolysaccharide of the bacterium to tolerate or evade the humoral defense response. The active enzyme is believed to act crucially on various cellular and humoral immune responses to clear out foreign organisms (Brookman et al., 1989), in addition to its role in nonself recognition (So¨derha¨ll and Smith, 1986).
Eicosanoids and some aminergic compounds also play roles in the insect immune cascade (Stanley-Samuelson et al., 1991; Baines et al., 1992). Stanley-Samuelson et al. (1991) proposed an eicosanoid role in mediating insect immune responses. Arachidonic acid, a precursor of various eicosanoids, rescued the insects infected with pathogenic bacteria in Lepidoptera, Coleoptera, and Hemiptera (Miller et al. 1994, 1996; Tunaz et al., 1999). Injected arachidonic acid strengthened the immune response by activating phagocytosis and prophenoloxi-dase (Mandato et al., 1997).
We investigated the action of the virulent factor(s) secreted by X. nematophilus on the insect immune-mediating process, and proposed a hypothesis that the bacteria exerts an inhibitory effect on eicosanoid-mediating immune signaling, thus resulting in an immu-nodepressive condition in the infected insect host. To test this hypothesis, the effect of arachidonic acid was evaluated on rescuing X. nematophilus-infected insects, and specific eicosanoid inhibitors were used to simulate the inhibitory effect of the bacteria.
2. Materials and methods
2.1. Insects and bacterial cultures
The beet armyworm, Spodoptera exigua (Hu¨bner), was used as the insect host species. The larvae were reared on an artificial diet (Gho et al., 1990) at 25±1°C,
a photoperiod of 16:8 (L:D) h, and RH 60±5%. Adults were fed a 10% sucrose solution. Two-day-old fifth instar larvae were used as test insects for bacterial patho-genicity.
The entomopathogenic bacteria, Xenorhabdus nema-tophilus, were isolated from the haemolymph of the fifth instar larvae of S. exigua infected with Steinernema car-pocapsae collected in Pochon, Korea (Park et al., 1999). The bacteria were cultured in tryptic soy agar (TSA) medium (Difco, USA) at 28°C. The bacterial colonies used in this study were phase I variant because they showed a red color on NBTA (nutrient agar+0.0025% bromothymol blue+0.004% triphenyltetrazolium chloride) medium (Akhurst, 1980).
2.2. In vivo bacterial pathogenicity
The pathogenicity was described as a median lethal bacterial dose (LD50) against the test insects. The
bac-teria were cultured in tryptic soy broth (Difco, USA) at 25°C for 48 h. After washing the cultured bacterial cells three times with sterilized water and centrifuging the cul-ture medium at 4000g for 2 min at 4°C, the cells were resuspended in sterilized insect Ringer’s solution (Humason, 1972) for the pathogenic assay. After several tenfold dilutions of the stock bacterial solution with ster-ilized Ringer’s solution, the bacterial samples were grown on nutrient agar (Difco, USA) and counted to esti-mate the mean colony forming units (cfu). The bacterial test doses were prepared by diluting the estimated stock suspension with Ringer’s solution.
The fifth instar larvae were surface-sterilized with 95% ethanol. One micoliter of the bacterial suspension was administered to each test larva through the first abdominal proleg by 10 µl Hamilton micro-syringe (Hamilton, Nevada, USA). The infected larvae were incubated at 25°C. After 24 h, the dead larvae were scored by the absence of voluntary movement when each of three parts (head, thorax, and abdomen) of the body was prodded once with a blunt needle. Each test dose was replicated three times and each replicate consisted of 12 larvae. LD50was estimated by probit analysis.
2.3. Change in total live haemocyte counts
After being injected with an LD80bacterial dose, the
larvae were kept at 25°C for predetermined times between 0 and 24 h. At each test time, the treated larvae were bled by cutting off the abdominal prolegs with a pair of sterile scissors. The exuded haemolymph was collected in a 1.5 ml cold (|5°C) tube, mixed with
0.04% trypan blue dye (1:1, v/v), and incubated for 5 min at 25°C. The haemocytes, which did not absorb the dye, were regarded as living cells (Ribeiro et al., 1999)
Germany) under a phase contrast microscope. Three lar-vae were used at each time per treatment.
2.4. Nodulation assay
At predetermined times after bacterial injection, the test larvae were killed and kept at270°C until assessed. For the nodulation assay, larvae were dissected by open-ing the haemoceol. Melanized and dark nodules were counted under a stereomicroscope at 50-fold magnifi-cation. Even though their sizes varied, the nodules were distinct enough to determine their locations.
To determine the time course of nodule formation, the test larvae were injected with 106 cells of heat-killed
bacteria (60°C for 20 min) and kept at 25°C for seven different incubation times. Control larvae were injected with 2 µl of sterile Ringer’s solution. Each treatment consisted of nine test larvae.
To determine the dose response of nodule formation, the test larvae were injected with different doses of live or heat-killed bacteria and incubated for 16 h at 25°C. Each treatment consisted of nine test larvae.
2.5. Pharmacological treatment
Test insects were injected with 2µl containing 10 µg of each pharmaceutical agent. Injection was conducted with a 10 µl Hamilton micro-syringe through the abdominal proleg which was on the opposite side to that of the bacterial injection. Control larvae were injected with 95% ethanol.
All test chemicals were purchased from Sigma (St Louis, MO, USA). They include arachidonic acid (5,8,11,14-eicosatetraenoic acid), the specific phospho-lipase A2 inhibitor dexamethasone ((11b,16a
)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-dione), the cyclooxygenase inhibitor naproxen (d -2-(6-methoxy-2-naphthyl) propionic acid), the lipoxygenase inhibitor esculetin (6,7-dihydroxycoumarin) and the dual cycloox-ygenase and lipoxcycloox-ygenase inhibitor phenidone (1-phe-nyl-3-pyrazolidinone).
2.6. Data analysis
Survival data were transformed by the square root and arcsine method for normalization. Treatment means and variances of the transformed data were analyzed by PROC GLM of the SAS program (SAS Institute Inc., 1988).
3. Results
3.1. Pathogenicity of X. nematophilus to S. exigua
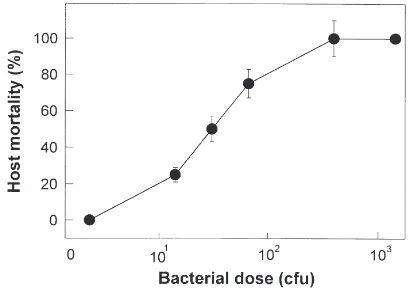
The insecticidal effect of X. nematophilus was ana-lyzed in vivo in the fifth instar larvae of S. exigua (Fig.
Fig. 1. Pathogenicity of Xenorhabdus nematophilus to the fifth instar larvae of Spodoptera exigua. The larvae injected intrahaemocoelically with different colony forming units (cfu) of the bacteria were kept at 25°C for 24 h and then assayed for mortality. Each measurement con-sisted of 36 larvae with three replications.
1). Mortalities were dependent on the bacterial doses. The slope of the dose–mortality regression was 2.6±0.8 and the median lethal dose of the bacteria (LD50) was
estimated as 33 cfu per larva. To give 100% mortality to the host insects, more than 325 live bacterial cells were required to be injected per larva.
Change in the total numbers of live haemocytes in fifth instar larvae of S. exigua was traced with different time intervals after bacterial injection (Fig. 2). Nonim-munized larvae had |970 000 live haemocytes per ml of haemolymph. The bacteria had a significant cytotoxic effect on the haemocytes (F=229.31; df=8, 18; P=0.0001). After 24 h post-injection, the larvae had only 55,000 live haemocytes per ml haemolymph, which rep-resented ca. a 94% haemocyte density reduction. In con-trast, did not change the total numbers of live haemo-cytes in the controls (F=0.43; df=8, 18; P=0.8903). Significant cytotoxicity (t=17.64; df=4; P=0.0001) was found 4 h postinjection, which was the earliest
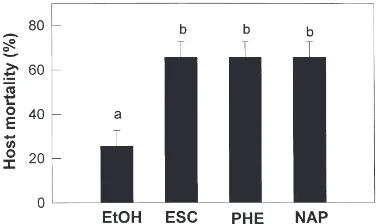
Fig. 3. Arachidonic acid (ARA, 10µl/larva) rescues the fifth instar larva of Spodoptera exigua infected with Xenorhabdus nematophilus at a dose of LD80. Controls were injected with 95% ethanol (EtOH) instead of arachidonic acid. Mortality was measured at 24 h after the haemocoelic injection of the bacteria. Each treatment consisted of 20 larvae with two replications. The error bars represent the standard devi-ations. Different letters above the error bars were significantly different ata=0.05.
ment time in this assay. All treated larvae had died by 16 h after the bacterial injection.
3.2. Effect of arachidonic acid on the pathogenicity of X. nematophilus
The importance of arachidonic acid was analyzed in the immunity of fifth instar larvae of S. exigua against X. nematophilus. The insecticidal action of X. nematophilus decreased significantly by the addition of 10µg of arach-idonic acid injected into bacterial-infected larvae (Fig. 3).
To test the dose effect of arachidonic acid on the bac-terial insecticidal action, 10-fold dilutions of arachidonic acid stock were prepared and compared (Fig. 4). There was a dose-dependent response up to 10 µg of arachi-donic acid. The higher dose (100 µg) did not, however, have a significant effect on rescuing the infected larvae.
Fig. 4. Dose effect of arachidonic acid on the pathogenicity of
Xenor-habdus nematophilus to the fifth instar larvae of Spodoptera exigua.
All larvae were injected with an LD80dose of bacteria after arachidonic acid treatments. Mortality was measured 24 h after the bacterial injec-tion. Each measurement consisted of 20 larvae with two replications. The error bars represent the standard deviations. Different letters above the error bars were significantly different ata=0.05.
Fig. 5. Synergistic effect of dexamethasone (DEX), a specific inhibi-tor of phospholipase A2, on the pathogenicity of Xenorhabdus
nemato-philus to the fifth instar larvae of Spodoptera exigua. All larvae were
injected with an LD20dose of bacteria after being treated with different inhibitors. Mortality was measured 24 h after the bacterial injection. Each measurement consisted of 20 larvae with two replications. The error bars represent the standard deviations. Different letters above the error bars were significantly different ata=0.05.
3.3. Effect of eicosanoid biosynthesis inhibitors on the pathogenicity of X. nematophilus
The effects of eicosanoid biosynthesis inhibitors were examined to test the importance of eicosanoids in insect immunity and to determine whether the insecticidal action of X. nematophilus inhibits the eicosanoid path-way.
Dexamethasone (DEX), a specific inhibitor of phos-pholipase A2, enhanced the insecticidal effect of X.
nem-atophilus on the fifth instar larvae of S. exigua (Fig. 5). This synergistic DEX effect on X. nematophilus was dose-dependent, but did not change above 10 µg.
All the other eicosanoid biosynthesis inhibitors tested in this study also increased the lethal effect of X. nema-tophilus (Fig. 6). There were no significant differences among the effects of the individual inhibitors such as esculetin, phenidone, and naproxen. The effects of all
Fig. 6. Effect of other eicosanoid biosynthesis inhibitors (all 10
Fig. 7. Time-course of nodule formation in the fifth instar larvae of
Spodoptera exigua in response to haemocoelic injection of heat-killed Xenorhabdus nematophilus (106 cells/larva). Controls were injected with sterile Ringer’s solution. Each measurement consisted of five lar-vae. The vertical bars represent the standard deviations. Different let-ters above the error bars were significantly different ata=0.05.
other inhibitors were lower than that of DEX on the lar-vae at the same pharmaceutical dose (10 µg).
3.4. Immunodepressive effect caused by X. nematophilus
The lethal effect of X. nematophilus on the larvae of S. exigua was further analyzed by measuring a cellular immune response, nodule formation, since the bacteria had significant cytotoxic effect on the haemocytes, immunocompetent cells.
The fifth instar larvae of S. exigua were able to form nodules in response to bacterial infection temporally and quantitatively (Figs. 7 and 8). When the larvae were injected with 106cells of heat-killed bacteria, they began
to form nodules (t=2.89; df=9; P=0.0203) 1 h postinjec-tion, the earliest time measured (Fig. 7). They formed nodules in a time-dependent manner and had maximal capacity (|33 nodules) 16 h postinjection. We used the 16 h incubation period for the subsequent nodule assays.
Fig. 8. Inhibitory effect of Xenorhabdus nematophilus on nodule for-mation of the fifth instar larvae of Spodoptera exigua. Bacteria-injected larvae were incubated for 16 h at 25°C. Each measurement consisted of five larvae. The vertical bars represent the standard deviations.
All treated larvae injected with the heat-killed bacteria were alive and pupated successfully.
The number of nodules formed by the larvae increased with the bacterial doses for both live and heat-killed bac-teria (Fig. 8). The bacbac-terial concentrations above 105
cells did not, however, change nodule formation in both treatments. The maximum number of nodules was 12 and 31 for live and heat-killed bacterial injections, respectively. There was a significant reduction in nodule formation for live bacterial injections at all doses (F=78.45; df=1, 13; P=0.0001).
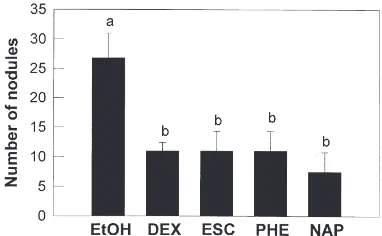
3.5. Comparison of the effects of arachidonic acid and eicosanoid biosynthesis inhibitors on the action of X. nematophilus in nodule formation
The pharmaceutical effects on the lethal action of X. nematophilus were analyzed by nodule assays. Appli-cation of arachidonic acid significantly increased nodule formation in larvae which were injected with 106 cells
of live bacteria (t=22.95; df=5; P=0.0087). The infected larvae treated with arachidonic acid had fewer nodules (8.4±4.5 per larva) than the control infected larvae (26.0±4.5 per larva). In comparison, DEX and other eicosanoid biosynthesis inhibitors reduced the nodule formation of larvae injected with heat-killed bacteria (106 cells) (Fig. 9). There was no significant variation
among the inhibitors in their inhibitory action on nod-ule formation.
4. Discussion
Entomopathogenic bacteria, Xenorhabdus nemato-philus, are transported into insect haemocoel by the help
of mutualistic nematodes, Steinernema spp. (Akhurst, 1982). The bacteria then secrete insecticidal toxin(s) to kill the host insects and produce other antibiotics to transform the host internal environment into favorable conditions for the proliferation of the nematodes and symbiotic bacteria (Poinar and Thomas, 1966). Insecti-cidal toxins secreted by the bacteria have not been ident-ified even though there are many reports of antimicrobial agents synthesized by bacteria (Thaler et al., 1998; Webster et al., 1998). Also, little information has been documented on insecticidal mechanisms of the bacteria. X. nematophilus was highly toxic to S. exigua, death occurring within 16 h. Our estimate of LD50of X.
nema-tophilus to the larvae was |33 cfu.
In contrast, Dunphy and Webster (1984) reported in Galleria mellonella an LD50of only|1 cfu in both
pri-mary and secondary X. nematophilus. This suggests there may be a difference in the immune capacity between insect species. Similar host insect variation was reported in X. bovienii between Tipula oleracea and G. mellonella (Ehlers et al., 1997). Here again, G. mel-lonella was about 2000-fold less susceptible than T. oler-acea.
The in vivo lethal effect was also shown in the cyto-toxicity of the bacteria against haemocytes of S. exigua. There was approximately a 94% reduction in total live haemocyte counts at 24 h postinjection. Dunphy and Webster (1984) reported a total loss of haemocytes after the injection of X. nematophilus into G. mellonella, fol-lowing an initial increase. In our data, the number of total haemocytes decreased just after the bacterial injec-tion. This difference may be due to different insect hosts or the nature of the haemocytes. We measured only liv-ing haemocytes differentiated by trypan blue dye (Ribeiro et al., 1999).
We could demonstrate the cellular immune response of S. exigua by observing nodule formation. The larvae could form about 33 nodules maximally in response to the heat-killed X. nematophilus in a dose–response experiment. This nodule number is smaller than those of other insect species: about 50 nodules in Agrotis ipsilon, Pseudaletia unipuncta (Jurenka et al., 1997) and Zophobas atratus (Miller et al., 1996) and over 100 nod-ules in Manduca sexta (Miller et al., 1994) and Galleria mellonella (Mandato et al., 1997). This variation in nod-ule formation capacity among insect species may come from different pathogens and variation in circulating haemocyte concentrations and haemolymph volumes as suggested by Miller et al. (1996).
X. nematophilus caused the infected larvae to be in immunodepression. The larvae injected with live bac-teria showed only about 30% of the maximal nodule capacity. The nodulation process is generally divided into two steps of haemocyte degranulation and activation of prophenoloxidase cascade (Mandato et al., 1997). Most haemolymph phenoloxidase activity is localized in
the haemocytes in S. exigua (Hung and Boucias, 1996). Dunphy and Webster (1991) demonstrated that X. nema-tophilus inhibited the activation of prophenoloxidase to phenoloxidase but did not inhibit preactivated phenol-oxidase. This suggests that X. nematophilus can downre-gulate the insect immune response and that the reduced but still significant nodule formation of the infected insects may be due to the pre-existing active phenoloxi-dase.
The addition of arachidonic acid, a precursor of eicos-anoids, reversed the lethal and inhibitory actions of X. nematophilus on the infected larvae. The effect was dose-dependent except at an extraordinarily high dose (100 µg) at which arachidonic acid did not have any therapeutic effect and seemed to be out of the physio-logical dose range. Direct and indirect evidence for immune response mediation by eicosanoids is accumu-lating for other insects infected with another pathogenic bacteria, Serretia marsescens (Stanley-Samuelson, 1994; Miller et al. 1994, 1996; Jurenka et al., 1997). Arachi-donic acids, 20-carbon polyunsaturated fatty acids, are released from phospholipids, mostly located in the mem-brane, by the action of phospholipase A2 (PLA2) and
used as the precursors for biosynthesis of the various eicosanoids (Stanley-Samuelson, 1994). Most insects possess these C20 polyunsaturated fatty acids in low pro-portions (Uscian and Stanley-Samuelson, 1994).
Although we did not measure the arachidonic acid content and PLA2 activity of S. exigua, the results
sug-gest that the target of the toxin(s) of X. nematophilus is PLA2. This was demonstrated indirectly by the use of
dexamethasone, the specific inhibitor of PLA2.
Treat-ment of dexamethasone reinforced the lethal and tory action of X. nematophilus. Other eicosanoid inhibi-tors, which act on different enzymes catalyzing eicosanoid biosynthesis, also had effects similar to that of dexamethasone. The inhibitory action on any type of eicosanoids could give a significant synergistic effect on the pathogenicity of X. nematophilus. This suggests the importance of various eicosanoids in the cellular immune reactions in S. exigua to defend against the pathogenicity of X. nematophilus.
PGD2which may be responsible for depression of
endo-thelium-dependent relaxation to facilitate the filarial pathogenicity (Kaiser et al., 1992). Prostaglandins such as PGE2and PGI are found in the saliva of Ixodes
dam-mini and their activity may help tick feeding by depress-ing host refractory responses and promotdepress-ing hyperemia (Ribeiro et al., 1988). Insect defensive secretions have been speculated to have immunodepressive materials. Howard et al. (1986) demonstrated that the defensive secretions of Tribolium castaneum inhibit prostaglandin biosynthesis. Jurenka et al. (1989) also found prostaglan-din biosynthetic inhibitors in the exocrine secretions of Stephanitis sp. and speculated that the lace bug secretions may be responsible for an unusual escape from the attack of predators and parasites. The adaptive significance of the inhibitors may be considered as an additive effect on the defensive materials by inhibiting the immune reaction of the recipients (Stanley, 2000). We are currently trying to obtain direct evidence of the inhibitory action of X. nematophilus on the eicosanoid pathway by comparing the amount of eicosanoids and PLA2 activities present in infected and non-infected
insect hosts.
Acknowledgements
We thank Dr Sungsik Han, Korea University, South Korea, for technical guidance and critical comments on haemocyte examination. We also thank Doyoung Kim for rearing and supplying the test insects. This work was funded by the Special Grants Research Program of the Korea Ministry of Agriculture, Forestry and Fisheries.
References
Akhurst, R.J., 1980. Morphological and functional dimorphism in
Xenorhabdus spp., bacteria symbiotically associated with the insect
pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology 121, 303–309.
Akhurst, R.J., 1982. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterohabditidae and Steinernematidae. Journal of General Microbiology 128, 3061–3065.
Ashida, M., Yamazaki, H.I., 1990. Biochemistry of phenoloxidase sys-tem in insects: with special reference to its activation. In: Ohnishi, E., Ishizaki, H. (Eds.), Molting and Metamorphosis. Japan Sci. Soc. Press, Tokyo, pp. 239–269.
Baines, D., Desantis, T., Downer, R.G.H., 1992. Octopamine and 5-hydroxytryptamine enhance the phagocytic and nodule formation activities of cockroach (Periplaneta americana) haemocytes. Jour-nal of Insect Physiology 38, 905–914.
Boman, H.G., Hultmark, D., 1987. Cell-free immunity in insects. Annual Review of Microbiology 41, 103–126.
Brookman, J.L., Ratcliffe, N.A., Rowley, A.F., 1989. Studies on the activation of the prophenoloxidase system of insects by bacterial cell wall components. Insect Biochemistry 19, 47–57.
Dunn, P.E., 1986. Biochemical aspects of insect immunity. Annual Review of Entomology 31, 321–339.
Dunphy, G.B., Downer, R.G.H., 1994. Octopamine, a modulator of the haemocytic nodulation response of non-immune Galleria
mel-lonella larvae. Journal of Insect Physiology 40, 267–272.
Dunphy, G.B., Webster, J.M., 1984. Interaction of Xenorhabdus
nema-tophilus subsp. Nemanema-tophilus with the haemolymph of Galleria mellonella. Journal of Insect Physiology 30, 883–889.
Dunphy, G.B., Webster, J.M., 1991. Antihemocytic surface compo-nents of Xenorhabdus nematophilus var. dutki and their modifi-cation by serum of nonimmune larvae of Galleria mellonella. Jour-nal of Invertebrate Pathology 58, 40–51.
Ehlers, R.-U., Wulff, A., Peters, A., 1997. Pathogenicity of axenic
Stei-nernema feltiae, Xenorhabdus bovienii, and the bacto-helminthic
complex to larvae of Tipula oleracea (Diptera) and Galleria
mel-lonella (Lepidoptera). Journal of Invertebrate Pathology 69, 212–
217.
Forst, S.B., Dows, N., Bocmare, N., Stackebrandt, E., 1997.
Xeno-rhabdus and HeteroXeno-rhabdus spp: Bugs that kill bugs. Annual
Review of Microbiology 51, 47–72.
Gho, H.G., Lee, S., Lee, B., Choi, K., Kim, J., 1990. Simple mass-rearing of beet armyworm, Spodoptera exigua (Hu¨bner) (Lepidoptera: Noctuidae), on an artificial diet. Korean Journal of Applied Entomology 29, 180–183.
Gillespie, J.P., Kanost, M.R., 1997. Biological mediators of insect immunity. Annual Review of Entomology 42, 611–643.
Howard, R.W., Jurenka, R.A., Blomquist, G.J., 1986. Prostaglandin synthetase inhibitors in the defensive secretion of the red flour beetle Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Insect Biochemistry 16, 757–760.
Humason, G.L., 1972. Animal Tissue Technique. 3rd. ed., W.H. Free-man and Company, San Francisco.
Hung, S.Y., Boucias, D.G., 1996. Phenoloxidase activity in hemo-lymph of naive and Beauveria bassiana-infected Spodoptera
exigua larvae. Journal of Invertebrate Pathology 67, 35–40.
Jurenka, R.A., Neal, J.W. Jr., Howard, R.W., Oliver, J.E., Blomquist, G.J., 1989. In vitro inhibition of prostaglandin H synthase by com-pounds from the exocrine secretions of lace bugs. Comparative Biochemistry and Physiology 93C, 253–255.
Jurenka, R.A., Miller, J.S., Pedibhotla, V.K., Rana, R.L., Stanley-Samuelson, D., 1997. Eicosanoids mediate microaggregation and nodulation responses to bacterial infections in black cutworms,
Agrotis ipsilon, and true armyworms, Pseudaletia unipuncta.
Jour-nal of Insect Physiology 43, 125–133.
Kaiser, L., Lamb, V.L., Tithof, P.K., Gage, D.A., Chamberlin, B.A., Watson, J.T. et al., 1992. Dirofilaria immitis: do filarial cyclooxyg-enase products depress endothelium-dependent relaxation in the in vitro rat aorta? Experimental Parasitology 75, 159–167.
Kaya, H.K., Gaugler, R., 1993. Entomopathogenic nematodes. Annual Review of Entomology 38, 181–206.
Liu, L.X., Weller, P.F., 1992. Intravascular filarial parasites inhibit pla-telet aggregation—role of parasite-derived prostanoids. The Journal of Clinical Investigation 89, 1113–1120.
Mandato, C.A., Diehl-Jones, L., Moore, S., Downer, R., 1997. The effects of eicosanoid biosynthesis inhibitors on prophenoloxidase activation, phagocytosis and cell spreading in Galleria mellonella. Journal of Insect Physiology 43, 1–8.
Miller, J.S., Nguyen, T., Stanley-Samuelson, D.W., 1994. Eicosanoids mediate insect nodulation responses to bacterial infections. Pro-ceedings of the National Academy of Sciences USA 91, 12418– 12422.
Miller, J.S., Howard, R.W., Nguyen, T., Nguyen, A., Rosario, R.M.T., Stanley-Samuelson, D.W., 1996. Eicosanoids mediate nodulation responses to bacterial infections in larvae of the tenebrionid beetle,
Zophobas atratus. Journal of Insect Physiology 42, 3–12.
Park, Y., Kim, Y., Lee, Y., 1999. Identification and characterization of a symbiotic bacterium associated with Steinernema carpocapsae in Korea. Journal of Asia–Pacific Entomology 2, 105–111. Poinar, G.O., Thomas, G.M., 1966. Significance of Achromobacter
nematophilus Poinar et Thomas (Achromobacteriaceae: Eubacteriales) in the development of the nematode DD136 (Neoaplectana sp.: Steinernematidae). Parasitology 56, 385–390. Ratcliffe, N.A., Rowley, A.F., Fitzgerald, S.W., Rhodes, C.P., 1985.
Invertebrate immunity—basic concepts and recent advances. Inter-national Review of Cytology 97, 183–350.
Ribeiro, C., Duvic, B., Oliveira, P., Givaudan, A., Palha, F., Simoes, N. et al., 1999. Insect immunity—effects of factors produced by a nematobacterial complex on immunocompetent cells. Journal of Insect Physiology 45, 677–685.
Ribeiro, J.M.C., Makoul, G.T., Robinson, D.R., 1988. Ixodes dammini: evidence for salivary prostacyclin secretion. Journal of Parasitology 74, 1068–1069.
SAS Institute Inc., 1988. SAS/STAT user’s guide. SAS Institute Inc., Cary, NC.
So¨derha¨ll, K., Smith, V.J., 1986. Prophenoloxidase-activating cascade as a recognition and defence system in arthropods. In: Gupta, A.P. (Ed.), Hemocytic and Humoral Immunity in Arthropods. Wiley, New York, pp. 251–286.
Stanley, D.W., 2000. Eicosanoids in Invertebrate Signal Transduction Systems. Princeton University Press, Princeton, NJ.
Stanley-Samuelson, D.W., 1994. Prostaglandins and related eicosano-ids in insects. Advanced Insect Physiology 24, 115–212. Stanley-Samuelson, D.W., Jensen, E., Nickerson, K.W., Tiebel, K.,
Ogg, C.L., Howard, R.W., 1991. Insect immune response to bac-terial infection is mediated by eicosanoids. Proceedings of the National Academy of Sciences USA 86, 1064–1068.
Thaler, J.-O., Duvic, B., Givaudan, A., Boemare, N., 1998. Isolation and entomotoxic properties of the Xenorhabdus nematophilus F1 lecithinase. Applied and Environmental Microbiology 64, 2367– 2373.
Tunaz, H., Bedick, J.C., Miller, J.S., Hoback, W.W., Rana, R.L., Stan-ley, D.W., 1999. Eicosanoids mediate nodulation reactions to bac-terial infections in adults of two 17-year periodical cicadas,
Magic-icada septendecim and M. cassini. Journal of Insect Physiology 45,
923–931.
Uscian, J.M., Stanley-Samuelson, D.W., 1994. Fatty acid compositions of phospholipids and triacylglycerols from selected terrestrial arthropods. Comparative Biochemistry and Physiology 107B, 371–379.