Numerical Simulation of Organic Solar Cell TiO
2
Porosity-based Nano-Electrode
Irana Eka Putri
1, Anto Tri S
2Technical Implementation Unit for Instrumentation Development
Indonesia Institute of Science Bandung, Indonesia
1[email protected], 2[email protected]
Doty Dewi Risanti
Engineering Physics Department Institut Teknologi Sepuluh NopemberSurabaya, Indonesia [email protected]
Abstract—TiO2 porosity with anatase phase has attracted
much attention in the dye-sensitized solar cell (DSC) application because their crystalline structure can enhanced higher performance when applied in single DSC. TiO2 nanoparticle had
been sensitized by using co-precipitation method from TiCl3 +
HCl + NH4OH + aquades. The annealing treatment was given at
temperature 200 – 550oC for 4 and 10 hours. Then the obtained TiO2 powder were characterized by using XRD and BET. The
result from XRD and BET are TiO2 particle size, surface area,
pore volume and pore diameter with the highest value are 61.06 nm, 103.344 m2g-1, 0.317 cc.g-1, and 12.656 nm, respectively. They
are needed for numerical simulation to calculate TiO2 porosity
and diffusion coefficient. These parameters are used to determined DSC performance. Best performance is shown at porosity 0.389, with current density and voltage value at 16.02 mA.cm-2 and 0.53 volts, respectively.
Keywords—dssc; titanium dioxide; porosity.
I. INTRODUCTION
Photovoltaic device based on inorganic solid-state junction is being challenged to replace it with the organic. This offer a big benefit and good perspective, it is very low cost, feasible fabricate, pollution-free and colorful [1-2]. Organic solar cell or dye-sensitized solar cell (DSC) has been built by Grätzel, et. al. in 1991 used semiconductor oxide as photo electrode an dye ruthenium (Ru-dye) as photosensitized can obtain the greatest efficiency is about 11%. It is less than Si-based photovoltaic. Basic principle of DSC is the electron injection from the photo excited dyes into the conduction band under illumination. Meanwhile, the electrolyte reduces the oxidized dye and transfers it to the counter electrode [3]. The basic principle of DSC has been modeled by Södrgre et al. by the continuity equation that describe the transport (Je), recombination (Re), and generation (Ge) of electron within the nanoporous film in equation (1) below.
nt x G x R x Je e e
2 (1)
Then the effect of diffusion electron, lifetime electron, light intensity and light absorption coefficient is noticed, the equation (1) has solution as in equation (2),
t n x n
x n
x x n D
0 exp
2 2
(2)
The performance of DSC depends on electrode-conducting material; mostly it is used semiconductor-oxide. Titanium dioxide (TiO2) is chosen because it is known that TiO2 has better performance than the other semiconductor when applied on DSC [4]. And the other hand, it has larger hand0gap energy between 3.0 – 3.2 eV, can decrease the electron hole recombination chance, relatively inexpensive and nontoxic [5-6].
In numerical simulation, effect of internal and external module is noticed to estimate the current density and voltage. The internal properties of DSC module are diffusion coefficient (D), lifetime electron (τ), light absorption
coefficient (α), and ideality factor of TiO2 (m) [7-11]. Then, the external properties are light intensity (Φ) and operating temperature (T) [12-14]. Some numerical simulation research of DSC has been investigated by using steady-state method [15-17]. It has done to predict the best performance that could be getting from DSC module. Ni et al. modeled the effect of electrode porosity in DSC performance based on the fundamental structure of TiO2 by using one-dimensional second-order differential equation [18]. In that model, effect of temperature annealing and surface area of TiO2 did not take place to estimate the DSC performance. So, in this research include the effect of temperature annealing, pore volume and surface area of TiO2 nanoparticle to calculate the current density and voltage of DSC. In other hand, those parameters were determined by simple experiment of TiO2 nanoparticle synthesis by using co-precipitation.
II. EXPERIMENTAL
TiO2 or titania was synthesized from TiCl precursor. 10 ml of TiCl3 was reacted with 0.3 ml of HCl and 4.7 ml of aquades under continuous heating at 45oC by using magnetic stirrer. NH4OH 2M was added drop wise under stirring until the
solution became colorless. As the solution’s temperature
reached room temperature, it was washed successively with aquades to remove remaining unreacted material [19]. Then, the suspension was calcined subsequently at temperatures of
2015 International Conference on Automation, Cognitive Science, Optics, Micro Electro-Mechanical System, and Information Technology (ICACOMIT), Bandung, Indonesia, October 29–30, 2015
200, 400 and 550oC for 4 to 10 hours. The obtained powder samples were characterized using x-ray diffraction (XRD), Brunauer-Emmet-Teller (BET) and scanning electron microscope (SEM).
and the current density is in equation (4) below [18],
ECB-Eredox : difference between Fermi level of TiO2
nanoparticle and electrolyte redox energy (eV), k : Boltzman constant (JK-1),
L : diffusion length (cm), m : ideality factor of TiO2,
n0 : electron concentration under a dark condition (cm-3),
NCB : electron density coefficient in conduction band of TiO2 (cm-3),
neq : electron density in dark condition (cm -3
),
oc I
n
: concentration of iodide in open-circuit condition(cm-3),
oc I
n
3: concentration of iodide in open-circuit condition
(cm-3),
q : charge of an electron (C), T : operating temperature (K),
α : absorption coefficient of TiO2 (cm-1), pt : efficiency of counter electrode-Pt,
Φ : light intensity (cm-2s-1).
III. RESULT AND DISCUSSION
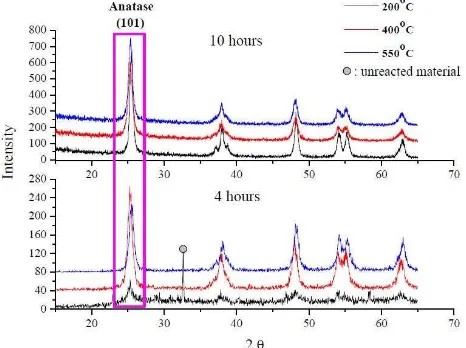
XRD spectra. The XRD spectra of all samples are given
in Figure 1. The result indicates that they are TiO2 crystalline particles. The particle size can be estimated by using Scherrer method in equation (5) below [19,22-23].
obtained for every samples is shown Table 1.TABLE I. PARTICLE SIZE FROM XRD RECULT from anatase-to-rutile process induces the particle growth [24]. The particle growth is determined by diffusion electron from smaller particle to the larger particle in equation (6).
t in 101 tetragonal crystal structures. TiO2 that was annealed in temperature 200oC for 4 hours indicates the unreacted material. It is assumed as Ti3O5 [22]. Figure 2 shows the crystal structure of tetragonal TiO2-anatase.
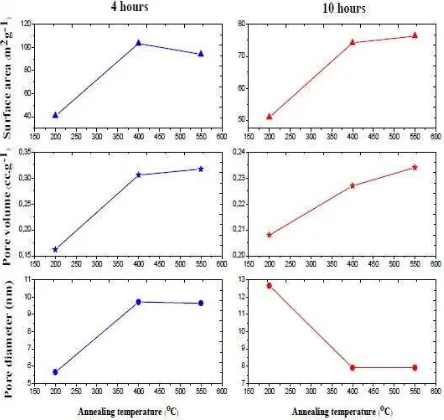
BET result. Figure 3 shows the BET result of TiO2 nanoparticle. Those samples deliver higher surface area when particle size increases. The highest surface area is 103.344 m2g-1 at particle size 10.71 nm, and the lowest surface area is 40.572 m2g-1 at 6.38 nm. In DSC, the higher surface area can enhanced the light harvesting and increase the performance [26]. In the other hand, dye loading can more absorb by increasing the surface area [27].
Meanwhile, pore diameter and pore volume area linearly increase when the particle size is growing. It means when the temperature increase, it leads to a decrease in defect of crystallization in TiO2 nanoparticle. So the pore becomes higher [28]. The TiO2 nanoparticle porosity can be determined by using the equation (7) below [18].
por e bulk
por e
Vol Vol
Vol P
(7)
where
2
1
TiO bulk
V
and2 TiO
= 3.93 g/cm3.
Figure 4 shows of TiO2 nanoparticle porosity. The highest porosity is 0.555 at temperature 550oC for 4 hours, and the
lowest porosity is 0.389 at temperature 200oC for 4 hours. The porosity is bigger by the increasing of annealing temperatures.
Theoretically, the higher porosity can improve dye loading to increase the performance [29], But Ni et al. result’s indicates that there is optimum porosity value of nanoparticle-electrode when applied in DSC thin layer [18].
Current density-voltage (J-V) characterization. Figure 5
shows current density-voltage curve in various porosity value. In equation (4), there are effect of diffusion coefficient (D) and diffusion length of electron (L) that can be calculated by using equation (8) and (9), respectively.
C
P P a
D (8)
and,
por e bulk
por e
Vol Vol
Vol P
(7)
where, critical porosity (PC), a, and µ are 4x10-4 cm2s-1, 0.82 and 0.76. In this paper, lifetime electron is determined by some paper about 10 ms [31].
From Figure 5, the best performance is in porosity 0.389. It has current density and voltage is 16.02 mA.cm-2 and 0.53 volts, respectively. While, the lowest performance is at porosity 0.555. It has current density and voltage area 13.82
Fig. 5. Current density and voltage in various TiO2 porosity.
Fig. 4. TiO2 porosity in various temperature and time annealing
Fig. 3. BET result of TiO2 nanoparticles.
mA.cm-2 and 0.51 volts, respectively. When applied in single layer DSC, its performance decreases by the porosity increasing.
IV. CONCLUSSION
From BET results indicates when temperatures annealing is higher so the pore volume, pore diameter and surface area are increased linearly. TiO2 nanoparticles that have been synthesized using co-precipitation method deliver porosity value is about 0.389 to 0.555. By using numerical model, the highest performance is shown at porosity 0.389 with current density and voltage is 16.02 mA.cm-2 and 0.53 volts, respectively.
V. ACKNOWLADGEMENT
This research was supported by the thematic program through the Bandung Technical Management Unit for Instrumentation Development (Deputy for Scientific Services) and the flagship program through in Research Center for Physics (Deputy for Engineering Sciences) funded by Indonesian Institut of Sciences, Indonesia.
References
[1] M. Grätzel, Review: Dye-sensitized Solar Cells, Journal of
Photochemistry and Photobiology C: Photochemistry Reviews 4, 2003, pp. 145-153.
[2] Q. Miao, M. Wu, W. Guo, T. Ma, Studies of High-efficient and
Low-cost Dye-sensitized Solar Cells, Front. Optoelectron. China vol. 4 issue 1, 2011, pp. 103-107.
[3] B. O’Regan and M. Grätzel, A Low-cost, High-efficiency Solar Cell Based on Dye Sensitized Solar Colloidal TiO2 Films, Nature vol. 353,
1991, pp. 737-740.
[4] A. K. Chandiran, M. A. Jalebi, M. K. Nazeeruddin, M. Grätzel, Analysis
of Electron Transfer Properties of ZnO and TiO2 Photioanodes for
Dye-Sensitized Solar Cells, ACS nano vol. 8(3), 2014, pp. 2261-2268.
[5] A. J. Cowan, J. Tang, W. Leng, J. R. Durrant, D. R. Klug, Water
Splitting by Nanocrystalline TiO2 in a Complete Photoelectrochemical
Cell Exhibits Efficiencies Limited by Charge Recombination, J. Phys. Chem. C vol. 114(9), 2010, pp. 4208-4214.
[6] F. M. Pesci, G. Wang, D. R. Klug, Y. Li, A. J. Cowan, Efficient
Suspenssion of Electron-Hole Recombination in Oxygen-Deficient
Hydrogen-Treated TiO2 Nanowires for Photoelectrochemical Water
Splitting, J. Phys. Chem. C vol. 117, 2013, pp. 25837-25844.
[7] J. Villanueva-Cab, H. Wang, G. Oskam, L. M. Peter, Electron Diffusion
and Black Reaction in Dye-Sensitized Solar Cells: The Effect of Nonlinear Recombination on Kinetics, J. Phys. Chem. Lett. Vol. 1(4), 2010, pp. 748-751.
[8] C. He, L. Zhao, Z. Zheng, F. Lu, Determination of Electron Diffusion Coefficient and Lifetime in Dye-Sensitized Solar Cells by Electrochemical Impedance Spectroscopy at High Fermi Level Condition, J. Phys. Chem. C vol. 112(48), 2008, pp. 18730-18733.
[9] D. W. Liu, I. C. Cheng, J. Z. Chen, H. W. Chen, K. C. Ho, C. C. Chiang,
Enhanced Optical Absorption of Dye-sensitzed Solar Cells with
Microactivity-embedded TiO2 Photoanodes, Optics Express A171 vol.
20 no. S2, 2012, pp. 1-9.
[10] Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada, A. Shinpo, A
High-Light-Harvesting-Efficiency Coumarin Dye fro Stable Dye-Sensitized Solar Cells, Adv. Mater. Vol. 19, 2007, pp. 1138-1141.
[11] H. S. Hafez, I. S. Yahia, G. B. Sakr, M. S. A. Abdel-Mottaleb, F.
Yakuphanoglu, Extraction of the DSC Parameters Based TiO2 under
Dark and Illumination Condition, Advances in Materials and Corrosion vol. 1, 2012, pp. 8-13.
[12] J. H. Kim, K. J. Moon, J. M. Kim, D. Lee, S. H. Kim, Effect of Various
Light-Intensity and Temperature Environments on the Photovoltaic Performance of Dye-Sensitized Solar Cells, Solar Energy vol. 113, 2015, pp. 251-257.
[13] M. Berginc, U. O. Krašovec, M. Jankovec, M. Topič, The Effect of Temperature on the Performance of Dye-Sensitized Solar Cells Based on a Propyl-Methyl-Imidazolium Iodide Electrolyte, Solar Energy Materials and Solar Cells vol. 91, 2007, pp. 821-828.
[14] B. Tripathi, P. Yadav, M. Kumar, Effect of Varying Illumination and Temperature on Steady-State and Dynamic Parameters on Dye-Sensitized Solar Cell Using AC Impedance Modeling International Journal of Photoenergy, Hindawi Publishing Corporation, 2013, doi: http://dx.doi.org/10.115/2013/646407.
[15] M. Ni, M. K. H. leung, D. Y. C. Leung, K. Sumanthy, Theoretical
Modeling of TiO2/TCO Interfacial Effect on Dye-sensitized Solar Cell
Performance, Solar Energy Materials and Solar Cells vol. 90, 2006, pp. 2000-2009.
[16] L. Andrade, J. Sousa, H. A. Ribeiro, A. Mendes, Phenomenological
Modeling of Dye-sensitized Solar Cells under Transdient Conditions, Solar Energy vol. 85, 2011, pp. 781-793.
[17] M. Belarbi, A. Benyoucef, H. A. Ribeiro, A. Mendes, Phenomenological
Modeling of Dye-sensitized Solar Cells underTransdient Conditions, Solar Energy vol. 85, 2011, pp. 781-793.
[18] M. Ni, M. K. H. leung, D. Y. C. Leung, K. Sumanthy, An Analytical Study of the Porosity Effect on Dye-sensitized Solar Cell Performance, Solar Energy Materials and Solar Cells vol. 90, 2006b, pp. 1331-1344.
[19] I. E. Putri, M. L. Sidik, R. A. Wahyuono, D. Sawitri, D. D. Risanti,
Co-Sensitized Promoted Light Harvesting Capability of Dye-Co-Sensitized Solar Cell (DSC) using Anthocyanin-Based Dye, Advanced Materials Research vol. 1123, 2015, pp. 325-328.
[20] A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson,
Dye-Sensitized Solar Cells, Chem. Rev. vol. 110(11), 2010, pp. 6595-6663. [21] J. Maҫaira, L. Andrade, A. Mendes, Modeling Simulation and Design of
Dye Sensitized Solar Cells, RSC Adv. Vol. 4, 2014, pp. 2830-2844.
[22] I. E. Putri, H. A. Budiarti, D. Sawitri, D. D. Risanti, On the Role of naCl
Addition to Phase Transformation of TiO2 from TiCl3, Advanced
Materials Research vol. 112, 2015, pp. 313-316.
[23] D. Reyes-coronado, F. Rodríguea-Gattorno, M. E. Espinosa-Pesquiera,
C. Cab, R. De Coss, G. Oskam, Phase-pure TiO2 nanoparticles: Anatase,
Brookite and Rutile, nanotechnology vol. 19, 2008, pp. 145605-145614.
[24] M. R. Renade, A. Navrotsky, H. Z. Zhang, J. F. Banfield, S. H. Elder, A.
Zaban, P. H. Borse, S. K. Kulkarni, G. S. Doran, H. J. Whitfield,
Energetics of Nanocrystalline TiO2, PNAS vol. 99, 2002, pp. 6476-6481.
[25] R. Riedel, Handbook of Ceramic Hard Materials, vol. 1, 2008, doi:
10.1002/9783527618217.
[26] M. Pan, N. Huang, X. Zhao, J. Fu, X. Zhong, Enhanced Efficiency of
Dye-Sensitized Solar cell by High Surface Area Anatase-TiO2-Modified
P25 Paste, Journal of nanomaterials, Hindawi Publishing corporation, 2013, doi: http//dx.doi.org/10.1155/2013/760685.
[27] H. Wang, B. Wang, J. Yu, Y. Hu, C. Xia, J. Zhang, R. Liu, Significat Enhancement of Power conversion Efficiency for Dye Sensitized Solar cell using 1D/3D network nanostructures as Photoanodes, Sci. Rep. vol. 5, 2015, pp. 9305-9313.
[28] A. Gaber, M. A. Abdel-Rahim, A. Y. Abdel-Latief, M. N. Abdel-Salam,
Influence of Calcination Temperature on the Structure and Porosity of
Nanocrystalline SnO2 Synthesized by a Conventional Precipitation
Method, Int. J. Electrochem. Sci. vol. 9, 2014, pp. 81-95.
[29] D. Zhang, T. Yishida, T. Oekermann, K. Furuta, H. Minoura,
Room-Temperature Synthesis of Porous Nanoparticulate TiO2 Films for
Flexible Dye-Sensitized Solar Cells, Advanced Functional Materials vol. 16 issue 9, 2006, pp. 1228-1234.
[30] A. A. El Tayyan, dye Sensitized Solar Cell: Parameters Calculation and