SyllabuS
Cambridge IGCSE
®Combined Science
0653
For examination in June and November 2017 and 2018.
Also available for examination in March 2017 and 2018
for India only.
Cambridge International Examinations retains the copyright on all its publications. Registered Centres are permitted to copy material from this booklet for their own internal use. However, we cannot give permission
Changes to syllabus for 2017 and 2018
The syllabus has been revised. you are advised to read the whole of the syllabus before planning your teaching programme. The most signiicant changes are outlined below.
Signiicant changes to the syllabus are indicated by black vertical lines at the side of the text.
Changes to the structure of the assessment
The practical option Paper 4: Coursework has been withdrawn.
A new Multiple Choice paper for Extended candidates has been introduced. This paper is now Paper 2. Core candidates will now take Paper 1: Multiple Choice (Core), Paper 3: Theory (Core) and either Paper 5: Practical Test or Paper 6: Alternative to Practical.
Extended candidates will now take Paper 2: Multiple Choice (Extended), Paper 4: Theory (Extended), and either Paper 5: Practical Test or Paper 6: Alternative to Practical.
Changes to other sections of the syllabus
1. Introduction
In the introductory section, some small changes have been made to wording to align this syllabus with the equivalent section in the IGCSEs for Biology, Physics and Chemistry.
2. Syllabus content at a glance This section has been revised.
5. Syllabus aims and assessment objectives
This section has been updated to align this syllabus with the other science IGCSEs and are to ensure coherence across the IGCSE science suite.
The syllabus aims have been amended to more fully relect the skills and knowledge promoted by study of the course.
The assessment objectives have been revised slightly for clarity. The meaning of the assessment objectives remains unchanged.
7. Practical assessment
The wording of this section has been revised to align this syllabus with the equivalent sections in the other science IGCSEs.
8. Appendix
Contents
1. Introduction ... 2
1.1 Why choose Cambridge? 1.2 Why choose Cambridge IGCSE?
1.3 Why choose Cambridge IGCSE Combined Science? 1.4 Cambridge ICE (International Certiicate of Education) 1.5 How can I ind out more?
2. Teacher support ... 5
2.1 Support materials 2.2 Endorsed resources 2.3 Training
3. Syllabus content at a glance ... 6
4. Assessment at a glance ... 8
5. Syllabus aims and assessment objectives ... 10
5.1 Syllabus aims
5.2 Assessment objectives
5.3 Relationship between assessment objectives and components 5.4 Grade descriptions
5.5 Conventions (e.g. signs, symbols, terminology and nomenclature)
6. Syllabus content ... 15
6.1 Biology 6.2 Chemistry 6.3 Physics
7. Practical assessment ... 41
7.1 Teaching experimental skills
7.2 Description of Components, Paper 5: Practical Test and Paper 6: Alternative to Practical
8. Appendix ... 46
8.1 Symbols, units and deinitions of physical quantities 8.2 Electrical symbols
8.3 Safety in the laboratory
8.4 Notes for use in qualitative analysis 8.5 The Periodic Table of Elements 8.6 Mathematical requirements 8.7 Presentation of data
8.8 Glossary of terms used in science papers
Introduction
1. Introduction
1.1 Why choose Cambridge?
Cambridge International Examinations is part of the University of Cambridge. We prepare school students for life, helping them develop an informed curiosity and a lasting passion for learning. Our international qualiications are recognised by the world’s best universities and employers, giving students a wide range of options in their education and career. As a not-for-proit organisation, we devote our resources to delivering high-quality educational programmes that can unlock learners’ potential.
Our programmes set the global standard for international education. They are created by subject experts, are rooted in academic rigour, and provide a strong platform for progression. Over 10 000 schools in 160 countries work with us to prepare nearly a million learners for their future with an international education from Cambridge.
Cambridge learners
Cambridge programmes and qualiications develop not only subject knowledge but also skills. We encourage Cambridge learners to be:
• conident in working with information and ideas – their own and those of others
• responsible for themselves, responsive to and respectful of others
• relective as learners, developing their ability to learn
• innovative and equipped for new and future challenges
• engaged intellectually and socially, ready to make a difference.
Recognition
Cambridge IGCSE is recognised by leading universities and employers worldwide, and is an international passport to progression and success. It provides a solid foundation for moving on to higher level studies. Learn more at www.cie.org.uk/recognition
Support for teachers
A wide range of materials and resources is available to support teachers and learners in Cambridge schools. Resources suit a variety of teaching methods in different international contexts. Through subject discussion forums and training, teachers can access the expert advice they need for teaching our qualiications. More details can be found in Section 2 of this syllabus and at www.cie.org.uk/teachers
Support for exams oficers
Exams oficers can trust in reliable, eficient administration of exams entries and excellent personal support from our customer services. Learn more at www.cie.org.uk/examsoficers
Introduction
3
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
1.2 Why choose Cambridge IGCSE?
Cambridge IGCSEs are international in outlook, but retain a local relevance. The syllabuses provide
opportunities for contextualised learning and the content has been created to suit a wide variety of schools, avoid cultural bias and develop essential lifelong skills, including creative thinking and problem-solving.
Our aim is to balance knowledge, understanding and skills in our programmes and qualiications to enable students to become effective learners and to provide a solid foundation for their continuing educational journey.
Through our professional development courses and our support materials for Cambridge IGCSEs, we provide the tools to enable teachers to prepare learners to the best of their ability and work with us in the pursuit of excellence in education.
Cambridge IGCSEsare considered to be an excellent preparation for Cambridge International AS and A Levels, the Cambridge AICE (Advanced International Certiicate of Education) Group Award, Cambridge Pre-U, and other education programmes, such as the US Advanced Placement program and the International Baccalaureate Diploma programme. Learn more about Cambridge IGCSEs at
www.cie.org.uk/cambridgesecondary2
Guided learning hours
Cambridge IGCSE syllabuses are designed on the assumption that learners have about 130 guided learning hours per subject over the duration of the course, but this is for guidance only. The number of hours required to gain the qualiication may vary according to local curricular practice and the learners’ prior experience of the subject.
1.3 Why choose Cambridge IGCSE Combined Science?
Cambridge IGCSE Combined Science gives learners the opportunity to study biology, chemistry and physics within a scientiically coherent syllabus and is accepted by universities and employers as proof of essential knowledge and ability. As well as a subject focus, the combined science syllabus enables learners to:
• better understand the technological world, with an informed interest in scientiic matters
• recognise the usefulness (and limitations) of scientiic method, and how to apply this to other disciplines and in everyday life
• develop relevant attitudes, such as a concern for accuracy and precision, objectivity, integrity, enquiry, initiative and inventiveness
• develop an interest in, and care for, the environment
• better understand the inluence and limitations placed on scientiic study by society, economy, technology, ethics, the community and the environment
• develop an understanding of the scientiic skills essential for both further study and everyday life.
Prior learning
Introduction
Progression
Cambridge IGCSE Certiicates are general qualiications that enable candidates either to progress directly to employment, or to proceed to further qualiications.
1.4 Cambridge ICE (International Certiicate of Education)
Cambridge ICE is a group award for Cambridge IGCSE. It gives schools the opportunity to beneit from offering a broad and balanced curriculum by recognising the achievements of learners who pass examinations in a number of different subjects.
Learn more about Cambridge ICE at www.cie.org.uk/cambridgesecondary2
1.5 How can I ind out more?
If you are already a Cambridge school
You can make entries for this qualiication through your usual channels. If you have any questions, please contact us at [email protected]
If you are not yet a Cambridge school
Learn about the beneits of becoming a Cambridge school at www.cie.org.uk/startcambridge. Email us at
Teacher support
5
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
2.
Teacher support
2.1 Support materials
We send Cambridge syllabuses, past question papers and examiner reports to cover the last examination series to all Cambridge schools.
You can also go to our public website at www.cie.org.uk/igcse to download current and future syllabuses together with specimen papers or past question papers and examiner reports from one series.
For teachers at registered Cambridge schools a range of additional support materials for speciic syllabuses is available from Teacher Support, our secure online support for Cambridge teachers. Go to
http://teachers.cie.org.uk (username and password required).
2.2 Endorsed resources
We work with publishers providing a range of resources for our syllabuses including print and digital materials. Resources endorsed by Cambridge go through a detailed quality assurance process to ensure they provide a high level of support for teachers and learners.
We have resource lists which can be iltered to show all resources, or just those which are endorsed by Cambridge. The resource lists include further suggestions for resources to support teaching.
2.3 Training
Syllabus content at a glance
3.
Syllabus content at a glance
The syllabus content that follows is divided into three sections: Biology (B1–B10), Chemistry (C1–C12) and Physics (P1–P12). Candidates must study all three sections.
Candidates can either follow the Core syllabus only, or they can follow the Extended syllabus which includes both the Core and the Supplement. Candidates aiming for grades A* to C should follow the Extended syllabus.
It is important that, throughout this course, teachers should make candidates aware of the relevance of the concepts studied to everyday life, and to the natural and man-made worlds.
biology
B1. Characteristics of living organisms B2. Cells
B3. Enzymes B4. Nutrition B5. Transportation B6. Respiration
B7. Co-ordination and response B8. Reproduction
B9. Energy low in ecosystems
B10. Human inluences on the ecosystem
Chemistry
C1. The particulate nature of matter C2. Experimental techniques
C3. Atoms, elements and compounds C4. Stoichiometry
C5. Electricity and chemistry
C6. Energy changes in chemical reactions C7. Chemical reactions
C8. Acids, bases and salts C9. The Periodic Table C10. Metals
Syllabus content at a glance
7
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Physics
P1. Motion
P2. Matter and forces P3. Energy, work and power
P4. Simple kinetic molecular model of matter P5. Matter and thermal properties
P6. Transfer of thermal energy P7. Waves
P8. Light
P9. Electromagnetic spectrum P10. Sound
Assessment at a glance
4.
assessment at a glance
All candidates must enter for three papers.
Core candidates take: Extended candidates take:
Paper 1 45 minutes A multiple-choice paper consisting of 40 items of the four-choice type.
This paper will test assessment objectives AO1 and AO2. Questions will be based on the Core syllabus content.
This paper will be weighted at 30% of the inal total mark.
Paper 2 45 minutes A multiple-choice paper consisting of 40 items of the four-choice type.
This paper will test assessment objectives AO1 and AO2. Questions will be based on the Extended syllabus content (Core and Supplement).
This paper will be weighted at 30% of the inal total mark.
and: and:
Paper 3 1 hour 15 minutes A written paper consisting of short-answer and structured questions.
This paper will test assessment objectives AO1 and AO2. Questions will be based on the Core syllabus content.
80 marks
This paper will be weighted at 50% of the inal total mark.
Paper 4 1 hour 15 minutes A written paper consisting of short-answer and structured questions.
This paper will test assessment objectives AO1 and AO2. Questions will be based on the Extended syllabus content (Core and Supplement).
80 marks
This paper will be weighted at 50% of the inal total mark.
all candidates take:
either: or:
Paper 5 1 hour 30 minutes Practical Test
This paper will test assessment objective AO3. Questions will be based on the experimental skills in Section 7.
The paper is structured to assess grade ranges A*–G.
30 marks
Paper 6 1 hour Alternative to Practical
This paper will test assessment objective AO3. Questions will be based on the experimental skills in Section 7.
The paper is structured to assess grade ranges A*–G.
Assessment at a glance
9
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Candidates who have studied the Core syllabus content, or who are expected to achieve a grade D or below, should be entered for Paper 1, Paper 3 and either Paper 5 or Paper 6. These candidates will be eligible for grades C to G.
Candidates who have studied the Extended syllabus content (Core and Supplement), and who are expected to achieve a grade C or above, should be entered for Paper 2, Paper 4 and either Paper 5 or Paper 6. These candidates will be eligible for grades A* to G.
Availability
This syllabus is examined in the June and November examination series. This syllabus is also available for examination in March for India only.
This syllabus is available to private candidates.
Detailed timetables are available from www.cie.org.uk/examsoficers
Combining this with other syllabuses
Candidates can combine this syllabus in an examination series with any other Cambridge syllabus, except:
• syllabuses with the same title at the same level
• 0610 Cambridge IGCSE Biology
• 0620 Cambridge IGCSE Chemistry
• 0625 Cambridge IGCSE Physics
• 0652 Cambridge IGCSE Physical Science
• 0654 Cambridge IGCSE Co-ordinated Sciences (Double Award)
• 5054 Cambridge O Level Physics
• 5070 Cambridge O Level Chemistry
• 5090 Cambridge O Level Biology
• 5129 Cambridge O Level Combined Science
Syllabus aims and assessment objectives
5.
Syllabus aims and assessment objectives
5.1 Syllabus aims
The syllabus aims listed below describe the educational purposes of a course based on this syllabus. These aims are not intended as assessment criteria but outline the educational context in which the syllabus content should be viewed. These aims are the same for all learners and are not listed in order of priority. Some of these aims may be delivered by the use of suitable local, international or historical examples and applications, or through collaborative experimental work.
The aims are:
1. to provide an enjoyable and worthwhile educational experience for all learners, whether or not they go on to study science beyond this level
2. to enable learners to acquire suficient knowledge and understanding to:
• become conident citizens in a technological world and develop an informed interest in scientiic
matters
• be suitably prepared for studies beyond Cambridge IGCSE
3. to allow learners to recognise that science is evidence-based and understand the usefulness, and the limitations, of scientiic method
4. to develop skills that:
• are relevant to the study and practice of science • are useful in everyday life
• encourage a systematic approach to problem-solving • encourage eficient and safe practice
• encourage effective communication through the language of science
5. to develop attitudes relevant to science such as:
• concern for accuracy and precision • objectivity
• integrity • enquiry • initiative • inventiveness
6. to enable learners to appreciate that:
• science is subject to social, economic, technological, ethical and cultural inluences and limitations • the applications of science may be both beneicial and detrimental to the individual, the community
Syllabus aims and assessment objectives
11
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
5.2 Assessment objectives
AO1: Knowledge with understanding
Candidates should be able to demonstrate knowledge and understanding of: 1. scientiic phenomena, facts, laws, deinitions, concepts and theories
2. scientiic vocabulary, terminology and conventions (including symbols, quantities and units) 3. scientiic instruments and apparatus, including techniques of operation and aspects of safety 4. scientiic and technological applications with their social, economic and environmental implications.
Syllabus content deines the factual material that candidates may be required to recall and explain.
Candidates will also be asked questions which require them to apply this material to unfamiliar contexts and to apply knowledge from one area of the syllabus to another.
Questions testing this assessment objective will often begin with one of the following words: deine, state,
describe, explain (using your knowledge and understanding) or outline (see the Glossary of terms used in
science papers).
AO2: Handling information and problem solving
Candidates should be able, in words or using other written forms of presentation (i.e. symbolic, graphical and numerical), to:
1. locate, select, organise and present information from a variety of sources 2. translate information from one form to another
3. manipulate numerical and other data
4. use information to identify patterns, report trends and draw inferences 5. present reasoned explanations for phenomena, patterns and relationships 6. make predictions and hypotheses
7. solve problems, including some of a quantitative nature.
Questions testing these skills may be based on information that is unfamiliar to candidates, requiring them to apply the principles and concepts from the syllabus to a new situation, in a logical, deductive way.
Questions testing these skills will often begin with one of the following words: predict, suggest, calculate or
determine (see the Glossary of terms used in science papers).
AO3: Experimental skills and investigations
Candidates should be able to:
1. demonstrate knowledge of how to safely use techniques, apparatus and materials (including following a sequence of instructions where appropriate)
2. plan experiments and investigations
Syllabus aims and assessment objectives
5.3 Relationship between assessment objectives and components
The approximate weightings allocated to each of the assessment objectives are summarised in the table below.
assessment objective Papers
1 and 2
Papers 3 and 4
Papers 5 and 6
Weighting of aO in overall qualiication
AO1: Knowledge with
understanding 63% 63% – 50%
AO2: Handling information
and problem solving 37% 37% – 30%
AO3: Experimental skills and
investigations – – 100% 20%
Weighting of paper in
overall qualiication 30% 50% 20%
5.4 Grade descriptions
The scheme of assessment is intended to encourage positive achievement by all candidates.
A Grade a candidate will be able to:
• recall and communicate precise knowledge and display comprehensive understanding of scientiic phenomena, facts, laws, deinitions, concepts and theories
• apply scientiic concepts and theories to present reasoned explanations of familiar and unfamiliar phenomena, to solve complex problems involving several stages, and to make reasoned predictions and hypotheses
• communicate and present complex scientiic ideas, observations and data clearly and logically, independently using scientiic terminology and conventions consistently and correctly
• independently select, process and synthesise information presented in a variety of ways, and use it to draw valid conclusions and discuss the scientiic, technological, social, economic and environmental implications
• devise strategies to solve problems in complex situations which may involve many variables or complex manipulation of data or ideas through multiple steps
• analyse data to identify any patterns or trends, taking account of limitations in the quality of the data and justifying the conclusions reached
Syllabus aims and assessment objectives
13
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
A Grade C candidate will be able to:
• recall and communicate secure knowledge and understanding of scientiic phenomena, facts, laws, deinitions, concepts and theories
• apply scientiic concepts and theories to present simple explanations of familiar and some unfamiliar phenomena, to solve straightforward problems involving several stages, and to make detailed predictions and simple hypotheses
• communicate and present scientiic ideas, observations and data using a wide range of scientiic terminology and conventions
• select and process information from a given source, and use it to draw simple conclusions and state the scientiic, technological, social, economic or environmental implications
• solve problems involving more than one step, but with a limited range of variables or using familiar methods
• analyse data to identify a pattern or trend, and select appropriate data to justify a conclusion
• select, describe and evaluate techniques for a range of scientiic operations and laboratory procedures.
A Grade F candidate will be able to:
• recall and communicate limited knowledge and understanding of scientiic phenomena, facts, laws, deinitions, concepts and theories
• apply a limited range of scientiic facts and concepts to give basic explanations of familiar phenomena, to solve straightforward problems and to make simple predictions
• communicate and present simple scientiic ideas, observations and data using a limited range of scientiic terminology and conventions
• select a single piece of information from a given source, and use it to support a given conclusion and to make links between scientiic information and its scientiic, technological, social, economic or environmental implications
• solve problems involving more than one step if structured help is given
• analyse data to identify a pattern or trend
Syllabus aims and assessment objectives
5.5 Conventions (e.g. signs, symbols, terminology and
nomenclature)
Syllabuses and question papers will conform with generally accepted international practice.
In particular, attention is drawn to the following documents, published in the UK, which will be used as guidelines.
(a) Reports produced by the Association for Science Education (ASE):
• SI Units, Signs, Symbols and Abbreviations (1981)
• Chemical Nomenclature, Symbols and Terminology for use in school science (1985)
• Signs, Symbols and Systematics: The ASE Companion to 16–19 Science (2000)
(b) Reports produced by the Society of Biology (in association with the ASE):
• Biological Nomenclature, Standard terms and expressions used in the teaching of biology,
fourth edition (2009)
litre/dm3
To avoid any confusion concerning the symbol for litre, dm3
will be used in place of l or litre.
Decimal markers
In accordance with current ASE convention, decimal markers in examination papers will be a single dot on the line. Candidates are expected to follow this convention in their answers.
Numbers
Syllabus content
15
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
6.
Syllabus content
The syllabus content that follows is divided into three sections: Biology (B1–B10), Chemistry (C1–C12) and Physics (P1–P12). Candidates must study all three sections.
Candidates can either follow the Core syllabus only, or they can follow the Extended syllabus which includes both the Core and the Supplement. Candidates aiming for grades A* to C should follow the Extended syllabus.
Note:
1. The syllabus content is designed to provide guidance to teachers as to what will be assessed in the overall evaluation of the candidate. It is not meant to limit, in any way, the teaching programme of any particular school or college.
2. The content is set out in topic areas within biology, chemistry and physics. Each topic area is divided into a number of sections. The left-hand column provides ampliication of the Core content, which all candidates must study. The right-hand column outlines the Supplement content, which should be studied by candidates following the Extended syllabus.
The syllabus content below is a guide to the areas on which candidates are assessed.
It is important that, throughout this course, teachers should make candidates aware of the relevance of the concepts studied to everyday life, and to the natural and man-made worlds.
In particular, attention should be drawn to:
• the inite nature of the world’s resources, the impact of human activities on the environment, and the need for recycling and conservation
• economic considerations for agriculture and industry, such as the availability and cost of raw materials and energy
• the importance of natural and man-made materials, including chemicals, in both industry and everyday life.
Syllabus content
6.1 Biology
Core Supplement
b1. Characteristics of living organisms
1 List and describe the characteristics of living organisms.
b2. Cells
2.1 Cell structure and organisation
1 State that living organisms are made of cells. 2 Identify and describe the structure of a plant
cell (palisade cell) and an animal cell (liver cell), as seen under a light microscope. 4 Describe the differences in structure
between typical animal and plant cells. 5 Calculate magniication and size of biological
specimens using millimetres as units.
3 Relate the structures seen under the light microscope in the plant cell and in the animal cell to their functions.
2.2 Movement in and out of cells
1 Deine diffusion as the net movement of molecules from a region of their higher concentration to a region of their lower concentration down a concentration gradient, as a result of their random movement. 2 Describe the importance of diffusion of
gases and solutes and of water as a solvent.
b3. Enzymes
1 Deine enzymes as proteins that function as biological catalysts.
2 Investigate and describe the effect of changes in temperature and pH on enzyme activity.
3 Explain the effect of changes in
Syllabus content
17
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
b4. Nutrition
4.1 Nutrients
1 List the chemical elements that make up:
• carbohydrates • fats
• proteins.
2 Describe the structure of large molecules made from smaller basic units, i.e.
• simple sugars to starch and glycogen • amino acids to proteins
• fatty acids and glycerol to fats and oils.
3 Describe tests for:
• starch (iodine solution)
• reducing sugars (Benedict’s solution) • protein (biuret test)
• fats (ethanol).
4 List the principal sources of, and describe the importance of:
• carbohydrates • fats
• proteins
• vitamins (C and D only)
• mineral salts (calcium and iron only) • ibre (roughage)
• water.
5 Describe the deiciency symptoms for:
• vitamins (C and D only)
• mineral salts (calcium and iron only).
Syllabus content
Core Supplement
4.2 Plant nutrition
1 Deine photosynthesis as the fundamental process by which plants manufacture carbohydrates from raw materials using energy from light.
3 State the word equation for the production of simple sugars and oxygen.
5 Investigate the necessity for chlorophyll, light and carbon dioxide for photosynthesis, using appropriate controls.
7 Describe the intake of carbon dioxide and water by plants.
8 Identify and label the cuticle, cellular and tissue structure of a dicotyledonous leaf, as seen in cross-section under the light microscope.
2 Explain that chlorophyll traps light energy and converts it into chemical energy for the formation of carbohydrates and their subsequent storage.
4 State the balanced equation for photosynthesis in symbols 6CO2 + 6H2O
light
chlorophyll C6H12O6 + 6O2
6 Investigate and state the effect of varying light intensity on the rate of photosynthesis (e.g. in submerged aquatic plants).
4.3 animal nutrition
1 State what is meant by the term balanced diet and describe a balanced diet related to age, sex and activity of an individual. 3 Identify the main regions of the alimentary
canal and associated organs, including mouth, salivary glands, oesophagus, stomach, small intestine: duodenum and ileum, pancreas, liver, gall bladder, large intestine: colon and rectum, anus.
4 Describe the functions of the regions of the alimentary canal listed above, in relation to ingestion, digestion, absorption, assimilation and egestion of food.
5 Deine digestion as the break down of large, insoluble food molecules into small, water-soluble molecules using mechanical and chemical processes.
6 Identify the types of human teeth and describe their structure and functions. 7 State the causes of dental decay and
Syllabus content
19
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
9 Deine absorption as movement of digested food molecules through the wall of the intestine into the blood.
10 Identify the small intestine as the region for the absorption of digested food.
b5. Transportation
5.1 Transport in plants
1 State the functions of xylem and phloem. 2 Identify the positions of xylem tissues as
seen in transverse sections of unthickened, herbaceous, dicotyledonous roots, stems and leaves.
3 Identify root hair cells, as seen under the light microscope, and state their functions. 5 Investigate, using a suitable stain, the
pathway of water through the above-ground parts of a plant.
6 Deine transpiration as evaporation of water at the surfaces of the mesophyll cells followed by loss of water vapour from plant leaves, through the stomata.
7 Describe the effects of variation of
temperature, humidity and light intensity on transpiration rate.
Syllabus content
Core Supplement
5.2 Transport in humans
1 Describe the circulatory system as a system of tubes with a pump and valves to ensure one-way low of blood.
3 Describe the structure of the heart, including the muscular wall and septum, atria,
ventricles, valves and associated blood vessels.
5 Describe the function of the heart in terms of muscular contraction and the working of the valves.
6 Investigate the effect of physical activity on pulse rate.
8 Identify red and white blood cells as seen under the light microscope on prepared slides, and in diagrams and photomicrographs.
9 Describe the structure and functions of arteries, veins and capillaries.
11 List the components of blood as red blood cells, white blood cells, platelets and plasma. 12 State the functions of blood:
• red blood cells – haemoglobin and oxygen transport
• white blood cells – phagocytosis and antibody formation
• platelets – causing clotting (no details)
• plasma – transport of blood cells, ions, soluble nutrients, hormones and carbon dioxide.
2 Describe the double circulation in terms of a low-pressure circulation to the lungs and a high-pressure circulation to the body tissues, and relate these differences to the different functions of the two circuits. 4 Describe coronary heart disease in terms of the blockage of coronary arteries and state the possible causes (diet, stress and smoking) and preventive measures.
7 Investigate, state and explain the effect of physical activity on pulse rate.
Syllabus content
21
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
b6. Respiration
6.1 Respiration and energy
1 Deine respiration as the chemical reactions that break down nutrient molecules in living cells to release energy.
2 State the uses of energy in the body of humans: muscle contraction, protein synthesis, cell division, growth, the passage of nerve impulses and the maintenance of a constant body temperature.
3 State the word equation for aerobic respiration.
4 Deine aerobic respiration as the release of a relatively large amount of energy in cells by the breakdown of food substances in the presence of oxygen. 5 State the equation for aerobic respiration
using symbols
(C6H12O6 + 6O2→ 6CO2 + 6H2O). 6.2 Gas exchange
1 Identify on diagrams and name the larynx, trachea, bronchi, bronchioles, alveoli and associated capillaries.
5 State the differences in composition between inspired and expired air.
6 Use limewater as a test for carbon dioxide to investigate the differences in composition between inspired and expired air.
7 Investigate and describe the effects of physical activity on rate and depth of breathing.
2 List the features of gas exchange surfaces in animals.
3 Explain the role of mucus and cilia in protecting the gas exchange system from pathogens and particles.
4 Describe the effects of tobacco smoke and its major toxic components (tar, nicotine, carbon monoxide, smoke particles) on the gas exchange system.
Syllabus content
Core Supplement
b7. Co-ordination and response
7.1 Hormones
1 Deine a hormone as a chemical substance, produced by a gland, carried by the blood, which alters the activity of one or more speciic target organs and is then destroyed by the liver.
2 State the role of the hormone adrenaline in chemical control of metabolic activity, including increasing the blood glucose concentration and pulse rate.
3 Give examples of situations in which adrenaline secretion increases.
7.2 Tropic responses
1 Deine and investigate geotropism (as a response in which a plant grows towards or away from gravity) and phototropism (as a response in which a plant grows towards or away from the direction from which light is coming).
2 Explain the chemical control of plant growth by auxins including geotropism and phototropism in terms of auxins regulating differential growth.
b8. Reproduction
8.1 asexual and sexual reproduction
1 Deine asexual reproduction as the process resulting in the production of genetically identical offspring from one parent.
Syllabus content
23
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
8.2 Sexual reproduction in plants
1 Identify and draw, using a hand lens if necessary, the sepals, petals, stamens, anthers, carpels, ovaries and stigmas of one locally available, named, insect-pollinated, dicotyledonous lower, and examine the pollen grains under a light microscope or in photomicrographs.
3 State the functions of the sepals, petals, anthers, stigmas and ovaries.
4 Candidates should expect to apply their understanding of the lowers they have studied to unfamiliar lowers.
5 Deine pollination as the transfer of pollen grains from the male part of the plant (anther or stamen) to the female part of the plant (stigma).
6 Name the agents of pollination.
8 Investigate and state the environmental conditions that affect germination of seeds: requirement for water and oxygen, suitable temperature.
2 Use a hand lens to identify and describe the anthers and stigmas of one locally available, named, wind-pollinated lower.
Syllabus content
Core Supplement
8.3 Sexual reproduction in humans
1 Identify on diagrams of the male
reproductive system: the testes, scrotum, sperm ducts, prostate gland, urethra and penis, and state the functions of these parts. 3 Identify on diagrams of the female
reproductive system: the ovaries, oviducts, uterus, cervix and vagina, and state the functions of these parts.
4 Describe the menstrual cycle in terms of changes in the uterus and ovaries.
5 Describe fertilisation in terms of the joining of the nuclei of male gamete (sperm) and the female gamete (egg).
6 Outline early development of the zygote simply in terms of the formation of a ball of cells that becomes implanted in the wall of the uterus.
10 Describe the methods of transmission of human immunodeiciency virus (HIV), and the ways in which HIV
/
AIDS can be prevented from spreading.
2 Compare male and female gametes in terms of size, numbers and mobility.
7 Indicate the functions of the amniotic sac and amniotic luid.
8 Describe the function of the placenta and umbilical cord in relation to exchange of dissolved nutrients, gases and excretory products (no structural details are required).
9 Describe the advantages and
disadvantages of breast-feeding compared with bottle-feeding using formula milk. 11 Outline how HIV affects the immune
system in a person with HIV
/
Syllabus content
25
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
b9. Energy low in ecosystems
1 State that the Sun is the principal source of energy input to biological systems.
2 Deine the terms:
• food chain as showing the low of
energy (food) from one organism to the next, beginning with a producer (e.g. mahogany tree → caterpillar → song bird → hawk)
• food web as a network of interconnected
food chains showing the energy low through part of an ecosystem
• producer as an organism that makes
its own organic nutrients, usually using energy from sunlight, through photosynthesis
• consumer as an organism that gets its
energy by feeding on other organisms
• herbivore as an animal that gets its
energy by eating plants
• carnivore as an animal that gets its
energy by eating other animals. 6 Describe the carbon cycle.
3 Describe energy losses between trophic levels.
4 Deine the terms:
• decomposer as an organism that gets
its energy from dead or waste organic matter
• ecosystem as a unit containing all of
the organisms and their environment, interacting together, in a given area, e.g. decomposing log or a lake
• trophic level as the position of an
organism in a food chain or food web. 5 Explain why food chains usually have
fewer than ive trophic levels.
7 Discuss the effects of the combustion of fossil fuels and the cutting down of forests on the oxygen and carbon dioxide concentrations in the atmosphere.
b10. Human inluences on the ecosystem
1 List the undesirable effects of deforestation (to include extinction, loss of soil, looding, carbon dioxide build-up).
2 Describe the undesirable effects of pollution to include:
• water pollution by sewage and chemical waste
• air pollution by greenhouse gases (carbon dioxide and methane) contributing to global warming. 6 Describe the need for conservation of:
• species and their habitats
• natural resources (limited to water and non-renewable materials including fossil fuels).
3 Describe the undesirable effects of overuse of fertilisers (to include eutrophication of lakes and rivers). 4 Discuss the causes and effects on
the environment of acid rain, and the measures that might be taken to reduce its incidence.
Syllabus content
6.2 Chemistry
Core Supplement
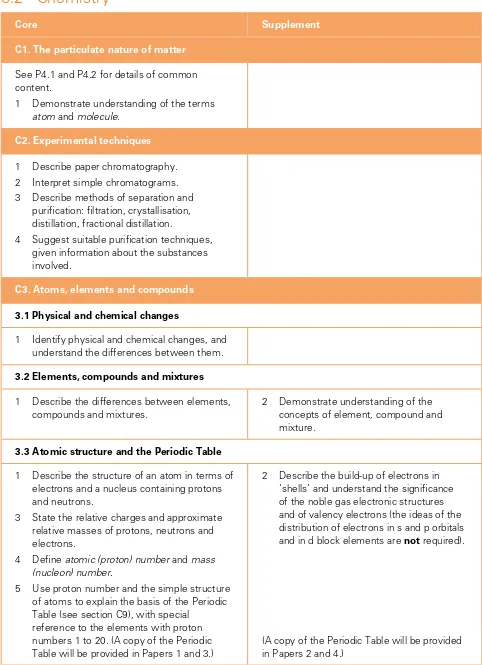
C1. The particulate nature of matter
See P4.1 and P4.2 for details of common content.
1 Demonstrate understanding of the terms
atom and molecule.
C2. Experimental techniques
1 Describe paper chromatography. 2 Interpret simple chromatograms. 3 Describe methods of separation and
puriication: iltration, crystallisation, distillation, fractional distillation.
4 Suggest suitable puriication techniques, given information about the substances involved.
C3. atoms, elements and compounds
3.1 Physical and chemical changes
1 Identify physical and chemical changes, and understand the differences between them.
3.2 Elements, compounds and mixtures
1 Describe the differences between elements, compounds and mixtures.
2 Demonstrate understanding of the concepts of element, compound and mixture.
3.3 atomic structure and the Periodic Table
1 Describe the structure of an atom in terms of electrons and a nucleus containing protons and neutrons.
3 State the relative charges and approximate relative masses of protons, neutrons and electrons.
4 Deine atomic (proton) number and mass (nucleon) number.
Syllabus content
27
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
3.4 Ions and ionic bonds
1 Describe the formation of ions by electron loss or gain.
2 Describe the formation of ionic bonds between elements from Groups I and VII.
3 Explain the formation of ionic bonds between metallic and non-metallic elements.
3.5 Molecules and covalent bonds
1 State that metallic elements form non-ionic compounds using a different type of bonding called covalent bonding involving shared pairs of electrons.
2 Draw dot-and-cross diagrams to represent the sharing of electron pairs to form single covalent bonds in simple molecules, exempliied by H2, Cl2, H2O, CH4 and HCl.
3 Draw dot-and-cross diagrams to represent the multiple bonding in N2, C2H4 and CO2.
C4. Stoichiometry
1 Use the symbols of the elements to write the formulae of simple compounds.
2 Deduce the formula of a simple compound from the relative numbers of atoms present. 3 Deduce the formula of a simple compound
from a model or a diagrammatic representation.
5 Construct and use word equations.
4 Determine the formula of an ionic compound from the charges on the ions present.
6 Construct and use symbolic equations with state symbols.
7 Deduce the balanced equation for a chemical reaction, given relevant information.
C5. Electricity and chemistry
1 State that electrolysis is the chemical effect of electricity on ionic compounds, causing them to break up into simpler substances, usually elements.
2 Use the terms electrode, electrolyte, anode
and cathode.
4 Describe the electrode products, using inert electrodes, in the electrolysis of:
• molten lead(II) bromide
• aqueous copper chloride.
3 Describe electrolysis in terms of the ions present and the reactions at the electrodes.
Syllabus content
Core Supplement
C6. Energy changes in chemical reactions
1 Relate the terms exothermic and
endothermic to the temperature changes
observed during chemical reactions.
2 Demonstrate understanding that exothermic and endothermic changes relate to the transformation of chemical energy to heat (thermal energy), and vice versa.
C7. Chemical reactions
7.1 Rate of reaction
1 Describe the effect of concentration, particle size, catalysis and temperature on the rate of reaction.
2 Describe a practical method for investigating the rate of a reaction involving gas evolution.
5 Deine catalyst as an agent which increases rate of reaction but which remains
unchanged.
3 Interpret data obtained from experiments concerned with rate of reaction.
4 Describe and explain the effects of
temperature and concentration in terms of collisions between reacting particles (the concept of activation energy will not be examined).
7.2 Redox
1 Deine oxidation and reduction in terms of oxygen loss / gain, and identify such reactions from given information.
C8. acids, bases and salts
8.1 The characteristic properties of acids and bases
1 Describe neutrality and relative acidity and alkalinity in terms of pH (whole numbers only) measured using full-range indicator and litmus.
2 Describe the characteristic reactions of acids with metals, bases (including alkalis) and carbonates.
Syllabus content
29
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
8.2 Preparation of salts
1 Describe the preparation, separation and puriication of salts using techniques
selected from section C2.1 and the reactions speciied in section C8.1.
2 Suggest a method of making a given salt from suitable starting material, given appropriate information.
8.3 Identiication of ions and gases
1 Use the following tests to identify:
aqueous cations:
• ammonium, copper(II), iron(II), iron(III)
and zinc by means of aqueous sodium hydroxide and aqueous ammonia as appropriate (formulae of complex ions are not required)
anions:
• carbonate by means of dilute acid and
then limewater
• chloride by means of aqueous silver
nitrate under acidic conditions
• nitrate by reduction with aluminium
• sulfate by means of aqueous barium ions
under acidic conditions
gases:
• ammonia by means of damp red litmus
paper
• carbon dioxide by means of limewater
• chlorine by means of damp litmus paper
• hydrogen by means of a lighted splint
• oxygen by means of a glowing splint.
C9. The Periodic Table
1 Describe the way the Periodic Table classiies elements in order of atomic (proton) number.
2 Use the Periodic Table to predict properties of elements by means of groups and periods.
9.1 Periodic trends
1 Describe the change from metallic to non-metallic character across a period.
Syllabus content
Core Supplement
9.2 Group properties
1 Describe lithium, sodium and potassium in Group I as a collection of relatively soft metals showing a trend in melting point and reaction with water.
3 Describe the trends in properties of chlorine, bromine and iodine in Group VII, including colour, physical state and reactions with other halide ions.
2 Predict the properties of other elements in Group I, given data where appropriate.
4 Predict the properties of other elements in Group VII, given data where appropriate.
9.3 Transition elements
1 Describe the transition elements as a collection of metals having high densities, high melting points and forming coloured compounds, and which, as elements and compounds, often act as catalysts.
9.4 Noble gases
1 Describe the noble gases as being unreactive.
2 State the uses of the noble gases in providing an inert atmosphere, i.e. argon in lamps, helium for illing balloons.
C10. Metals
10.1 Properties of metals
1 Distinguish between metals and non-metals by their general physical and chemical properties.
3 Explain why metals are often used in the form of alloys.
2 Identify and interpret diagrams that represent the structure of an alloy.
10.2 Reactivity series
1 Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, hydrogen and copper, by reference to the reactions, if any, of the elements with:
• water or steam
• dilute hydrochloric acid (except for alkali metals).
2 Describe the reactivity series to the tendency of a metal to form its positive ion, illustrated by its reaction, if any, with:
• the aqueous ions of other listed metals
Syllabus content
31
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
10.3 Extraction of metals
1 Describe the use of carbon in the extraction of copper from copper oxide.
2 Describe the essential reactions in the extraction of iron in the blast furnace. 3 Relate the method of extraction of a metal
from its ore to its position in the reactivity series, limited to Group I and II metals, aluminium, iron and copper.
C11. air and water
1 State a chemical test for water. 2 Describe and explain, in outline, the
puriication of the water supply by iltration and chlorination.
3 Describe the composition of clean air as being a mixture of 78% nitrogen, 21% oxygen and small quantities of noble gases, water vapour and carbon dioxide.
5 State the formation of carbon dioxide:
• as a product of complete combustion of carbon-containing substances
• as a product of respiration
• as a product of the reaction between an acid and a carbonate
• as a product of thermal decomposition. 6 Describe the rusting of iron in terms of a
reaction involving oxygen and water, and simple methods of rust prevention, including paint and other coatings to exclude oxygen.
Syllabus content
Core Supplement
C12. Organic chemistry
12.1 Fuels
1 Recall coal, natural gas and petroleum as fossil fuels that produce carbon dioxide on combustion.
2 Name methane as the main constituent of natural gas.
3 Describe petroleum as a mixture of
hydrocarbons and its separation into useful fractions by fractional distillation.
5 State the use of:
• reinery gas for bottled gas for heating and cooking
• gasoline fraction for fuel (petrol) in cars
• diesel oil/gas oil for fuel in diesel engines.
4 Understand the essential principle of fractional distillation in terms of differing boiling points (ranges) of fractions related to molecular size and intermolecular attractive forces.
12.2 Hydrocarbons
1 Describe the properties of alkanes
(exempliied by methane) as being generally unreactive, except in terms of burning. 2 State that the products of complete
combustion of hydrocarbons, exempliied by methane, are carbon dioxide and water. 3 Name, identify and draw the structures of
methane, ethane, ethene and ethanol.
4 Recognise alkanes and alkenes from their chemical names or from molecular structures.
5 Describe the manufacture of alkenes by cracking.
Syllabus content
33
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
6.3 Physics
Core Supplement
P1. Motion
1 Deine speed and calculate average speed from: total distance
total time
2 Plot and interpret a speed-time graph and a distance-time graph.
3 Recognise from the shape of a speed-time graph when a body is:
• at rest
• moving with constant speed
• moving with changing speed.
7 Demonstrate a qualitative understanding that acceleration is related to changing speed.
4 Recognise linear motion for which the acceleration is constant, and calculate the acceleration.
5 Recognise motion for which the acceleration is not constant.
6 Calculate the area under a speed-time graph to work out the distance travelled for motion with constant acceleration.
P2. Matter and forces
2.1 Mass and weight
1 Be able to distinguish between the mass and weight of an object.
2 Know that the Earth is the source of a gravitational ield.
3 Describe, and use the concept of, weight as the effect of a gravitational ield on a mass.
2.2 Density
1 Describe an experiment to determine the density of a liquid and of a regularly shaped solid, and make the necessary calculation using the equation:
density = mass
/
volume or d = m
/
V
Syllabus content
Core Supplement
2.3 Effects of forces
1 Know that a force is measured in newtons (N).
2 Describe how forces may change the size, shape and motion of a body.
3 Plot and interpret extension-load graphs and describe the associated experimental procedure.
4 State Hooke’s Law and recall and use the expression:
force = constant × extension (F = kx). 5 Recognise the signiicance of the
term ‘limit of proportionality’ for an extension
/
load graph.
P3. Energy, work and power
3.1 Energy
1 Know that energy and work are measured in joules (J), and power in watts (W). 2 Demonstrate understanding that an object
may have energy due to its motion (kinetic energy, K.E.) or its position (potential energy, P.E.), and that energy may be transferred and stored.
4 Give and identify examples of energy in different forms, including kinetic, gravitational, chemical, nuclear, thermal (heat), electrical, light and sound. 5 Give and identify examples of the
conversion of energy from one form to another, and of its transfer from one place to another.
3 Recall and use the expressions K.E. = 2
1 mv2
and P.E. = mgh
Syllabus content
35
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
3.2 Energy resources
1 Distinguish between renewable and non-renewable sources of energy.
2 Know that the Sun is the source of energy for all our energy resources except geothermal and nuclear.
3 Describe how electricity or other useful forms of energy may be obtained from:
• chemical energy stored in fuel
• water, including the energy stored in waves, in tides, and in water behind hydroelectric dams
• geothermal resources
• heat and light from the Sun (solar cells and panels)
• wind.
4 Give advantages and disadvantages of each method in terms of reliability, scale and environmental impact.
5 Demonstrate a qualitative understanding of eficiency.
6 Recall and use the equation: eficiency = useful energy output
energy input × 100%
3.3 Work
1 Relate (without calculation) work done to the magnitude of a force and the distance moved.
2 Describe energy changes in terms of work done.
3 Recall and use W = F×d
3.4 Power
1 Relate (without calculation) power to work done and time taken, using appropriate examples.
2 Recall and use the equation P = E /
Syllabus content
Core Supplement
P4. Simple kinetic molecular model of matter
4.1 States of matter
1 State the distinguishing properties of solids, liquids and gases.
4.2 Molecular model
1 Describe qualitatively the molecular structure of solids, liquids and gases.
2 Relate the properties of solids, liquids and gases to the forces and distances between molecules and to the motion of the
molecules.
3 Interpret the temperature of a gas in terms of the motion of its molecules.
4.3 Evaporation
1 Describe evaporation in terms of the escape of more energetic molecules from the surface of a liquid.
2 Relate evaporation to the consequent cooling.
P5. Matter and thermal properties
1 Describe qualitatively the thermal expansion of solids, liquids and gases. 2 Identify and explain some of the everyday
applications and consequences of thermal expansion.
3 State the meaning of melting point and
boiling point.
P6. Transfer of thermal energy
6.1 Conduction
1 Describe experiments to demonstrate the properties of good and bad conductors of heat.
2 Explain heat transfer in solids in terms of molecular motion.
Syllabus content
37
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
6.3 Radiation
1 Recognise radiation as the method of heat transfer that does not require a medium to travel through.
2 Identify infra-red radiation as the part of the electromagnetic spectrum often involved in heat transfer by radiation.
3 Describe experiments to show the properties of good and bad emitters and good and bad absorbers of infra-red radiation.
6.4 Consequences of energy transfer
1 Identify and explain some of the everyday applications and consequences of
conduction, convection and radiation.
P7. Waves
7.1 General wave properties
1 Describe what is meant by wave motion as illustrated by vibration in ropes and springs and by experiments using water waves. 3 State the meaning of and use the terms
speed, frequency, wavelength and
amplitude.
2 Distinguish between transverse and longitudinal waves and give suitable examples.
4 Recall and use the equation v = fλ
5 Identify how a wave can be relected off a plane barrier and can change direction as its speed changes.
P8. light
8.1 Relection of light
1 Describe the formation and give the characteristics of an optical image formed by a plane mirror.
3 Use the law:
angle of incidence, i = angle of relection, r.
2 Perform simple constructions,
Syllabus content
Core Supplement
8.2 Refraction of light
1 Describe an experimental demonstration of the refraction of light.
2 Identify and describe internal and total internal relection using ray diagrams. 3 Describe, using ray diagrams, the passage
of light through parallel-sided transparent material, indicating the angle of incidence i
and angle of refraction r.
4 State the meaning of critical angle. 5 Describe the action of optical ibres, particularly in medicine and
communications technology.
8.3 Thin converging lens
1 Describe the action of a thin converging lens on a beam of light, using ray diagrams. 2 Use the terms principal focus and focal
length.
P9. Electromagnetic spectrum
1 Describe the main features of the electromagnetic spectrum.
3 Describe the role of electromagnetic waves in:
• radio and television communications (radio waves)
• satellite television and telephones (microwaves)
• electrical appliances, remote controllers for televisions and intruder alarms (infra-red)
• medicine and security (X-rays). 4 Demonstrate an awareness of safety
issues regarding the use of microwaves and X-rays.
Syllabus content
39
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
Core Supplement
P10. Sound
1 Describe the production of sound by vibrating sources.
3 State the approximate human range of audible frequencies.
4 Demonstrate understanding that a medium is needed to transmit sound waves.
5 Describe an experiment to determine the speed of sound in air.
7 Relate the loudness and pitch of sound waves to amplitude and frequency. 8 Describe how the relection of sound may
produce an echo.
2 Describe transmission of sound in air in terms of compressions and rarefactions.
6 State the order of magnitude of the speed of sound in air, liquids and solids.
P11. Electricity
11.1 Electrical quantities
1 Demonstrate understanding of current,
potential difference and resistance, and use
with their appropriate units.
2 Use and describe the use of an ammeter and a voltmeter.
11.2 Electric charge
1 Describe simple experiments to show the production and detection of electrostatic charges.
2 State that there are positive and negative charges.
3 State that unlike charges attract and that like charges repel.
5 Distinguish between electrical conductors and insulators and give typical examples.
4 Describe an electric ield as a region in which an electric charge experiences a force.
11.3 Current and potential difference
1 State that current is related to the low of charge.
2 State that the current in metals is due to a low of electrons.
Syllabus content
Core Supplement
11.4 Resistance
1 State that resistance = p.d. / current and understand qualitatively how changes in p.d. or resistance affect current.
2 Recall and use the equation R = V
/ I. 4 Describe an experiment to determine
resistance using a voltmeter and an ammeter.
3 Relate (without calculation) the resistance of a wire to its length and to its diameter.
11.5 Electrical energy
1 Recall and use the equations
P = IV and E = IVt
11.6 Dangers of electricity
1 Identify electrical hazards including:
• damaged insulation
• overheating of cables
• damp conditions.
2 Demonstrate understanding of the use of fuses.
P12. Electric circuits
12.1 Circuit diagrams
1 Draw and interpret circuit diagrams containing sources, switches, resistors (ixed and variable), lamps, ammeters, voltmeters and fuses.
12.2 Series and parallel circuits
1 Demonstrate understanding that the current at every point in a series circuit is the same.
3 Calculate the combined resistance of two or more resistors in series.
4 State that, for a parallel circuit, the current from the source is larger than the current in each branch.
2 Recall and use the fact that the sum of the p.d.s across the components in a series circuit is equal to the total p.d. across the supply.
Practical assessment
41
Cambridge IGCSE Combined Science 0653. Syllabus for examination in 2017 and 2018.
7.
Practical assessment
Scientiic subjects are, by their nature, experimental. It is therefore important that an assessment of a candidate’s knowledge and understanding of science should contain a practical component (see assessment objective AO3).
Schools’ circumstances (e.g. the availability of resources) differ greatly, so two alternative ways of examining the practical component are provided. The alternatives are:
• Paper 5: Practical Test
• Paper 6: Alternative to Practical (written paper).
Whichever practical assessment route is chosen, the following points should be noted:
• the same assessment objectives apply
• the same practical skills are to be learned and developed
• the same sequence of practical activities is appropriate.
Candidates may not use textbooks in the practical component, nor any of their own records of laboratory work carried out during their course.
Calculators may be used in all parts of the assessment.
7.1 Teaching experimental skills
The best preparation for these papers is for learners to pursue a course in which practical work is fully integrated so that it is a normal and natural part of the teaching.
Teachers are expected to identify suitable opportunities to embed practical techniques and investigative work throughout the course, rather than as an isolated aspect of preparation for examination. This approach will not only provide opportunities for developing experimental skills but will increase the appeal of the course, and the enjoyment of the subject. Practical work helps learners to acquire a secure understanding of the syllabus topics and to appreciate how scientiic theories are developed and tested. It also promotes important scientiic attitudes such as objectivity, integrity, co-operation, enquiry and inventiveness.
Experimental work
Experimental work is an essential component of all science and should form a key part of teachers’ delivery plans for this syllabus.
Experimental work within science education:
• gives candidates irst-hand experience of phenomena
• enables candidates to acquire practical skills
Practical assessment
Note on taking readings
When approximate volumes are used, e.g. about 2 cm3
, it is expected that candidates will estimate this and not use measuring devices.
A measuring instrument should be used to its full precision. Thermometers may be marked in 1 °C intervals but it is often appropriate to interpolate between scale divisions and record a temperature to the nearest 0.0 °C or 0.5 °C. Measurements using a rule require suitable accuracy of recording, such as 15.0 cm rather than 15 cm; the use of millimetres when appropriate should be encouraged. Similarly, when measuring current, it is often more appropriate to use milliamperes rather than amperes.
Apparatus list
The list below details the apparatus expected to be generally available for both the teaching and the examination of Paper 5. The list is not exhaustive: in particular, some items that are commonly regarded as standard equipment in a science laboratory are not included.
The Conidential Instructions, provided to Centres prior to the examination of Paper 5, will give the detailed
requirements for the examination.
• rulers capable of measuring to 1 mm
• metre rule
• mounted needles or seekers or long pins with large heads
• means of cutting biological materials, such as scalpels, solid edged razor blades or knives
• scissors
• forceps
• means of writing on glassware
• beakers, 100 cm3
, 250 cm3
• polystyrene or other plastic beakers of approximate capacity 150 cm3
• test-tubes (Pyrex or hard glass), approximately 125 mm × 16 mm
• test-tubes, approximately 125 mm × 16 mm
• boiling tubes, approximately 150 mm × 25 mm
• delivery tubes
• conical lasks, within the range 150 cm3
to 250 cm3
• means of measuring small volumes of liquids, such as syringes (with needles removed)
• measuring cylinders, 100 cm3
, 50 cm3, 25 cm3, 10 cm3
• dropping pipettes
• white tiles
• spotting tiles
• water-bath
• large containers (e.g. plastic bowl) to hold cold water