Control of postharvest decay of citrus fruit with calcium
polysulfide
J.L. Smilanick
a,*, D. Sorenson
baUSDA-ARS,Horticultural Crops Research Laboratory,2021South Peach A
6enue,Fresno,CA93727, USA
bSunkist Growers,Technical Ser
6ices,222W.Lindmore Street,Lindsay,CA93247,USA
Received 3 April 2000; accepted 24 June 2000
Abstract
Incidence of green mold of citrus, caused byPenicillium digitatum, was reduced by 80% or more by the immersion of lemons or oranges for 1 – 4 min in warm (40.6 – 43.3°C) ‘liquid lime-sulfur’ (LLS) solution that contained 0.75% (wt vol−1) calcium polysulfide. The incidence of sour rot, caused byGeotrichum citri-aurantii, was reduced 35 – 70% by
this treatment. LLS was similar in effectiveness to other treatments employed to control postharvest decay. Effectiveness was higher on lemons than oranges, and on green compared to yellow lemons. LLS did not stop sporulation, a benefit now obtained with some fungicides. The sulfide content of oranges, lemons, and grapefruit after LLS treatment was 31.9, 33.1, and 36.3mg g−1, respectively. Rigorous cleaning of fruit with water applied at high
pressure after LLS treatment slightly improved LLS efficacy; conversely, similar cleaning reduces the efficacy of sodium carbonate or borax – boric acid solutions now in use. The risk of injury to fruit by LLS was low. Fruit of one lemon and five navel orange cultivars were not visibly injured after LLS treatment for 3 min at 40.6°C followed by storage for 7 weeks at 10°C. After LLS treatment at 48.9°C, 5°C higher than needed for effective LLS use, only Lisbon lemons and Bonanza navel oranges were slightly injured. Sulfide concentration in LLS solution declined at a rate of about 7% every 24 h, this rate was similar between 25 and 65°C, and it was accompanied by the appearance of resistant deposits on the equipment. Additional losses would occur when some LLS solution is carried on fruit out of the tank. Although H2S in the air above LLS solution in pilot tests was less than 1ml l−1and below the worker
safety threshold of 10ml l−1, LLS solution has an odor of H2S that can be a nuisance to workers. The disposal of
used LLS solutions is more readily accomplished than other tank treatments whose disposal can be difficult because they contain synthetic fungicides, are caustic, or have a high salt concentration. Because LLS improves water penetration in soils and is commonly used for this purpose, in many locations it can be disposed of by application to agricultural soils. Published by Elsevier Science B.V.
Keywords:Penicillium digitatum; Green mold;Geotrichum citri-auranti;Geotrichum candidum; Sour rot; Liquid lime sulfur www.elsevier.com/locate/postharvbio
1. Introduction
Green mold of citrus, caused by Penicillium digitatum (Pers.: Fr.) Sacc, and sour rot, caused * Corresponding author: Tel.: +1-559-4533084; fax: +
1-559-4533088.
E-mail address:jsmilanick@aol.com (J.L. Smilanick).
byGeotrichum citri-auranti(Ferraris) R. Cif. and F. Cif. (syn=Geotrichum candidumLink), are the most economically important postharvest diseases of citrus in arid growing regions of the world. The primary infection courts of both pathogens are wounds on fruit inflicted during harvest and sub-sequent handling, and these infections must be eradicated to achieve acceptable levels of control (Powell, 1908; Eckert and Brown, 1986). Cur-rently, measures employed to manage postharvest decay of citrus include treatments usually incor-porated into fruit waxes, such as the fungicides orthophenyl phenate (SOPP), imazalil, and thi-abendazole, or biological control formulations containingCandida oleophila or Pseudomonas sy
-ringae. Fruit are also immersed in tanks contain-ing sodium bicarbonate at ambient temperatures (Smilanick et al., 1999), or heated solutions of SOPP, sodium carbonate, or a mixture of borax and boric acid (Eckert and Eaks, 1989). Several issues make the development of new treatments important, including concerns about human health risks associated with fungicide residues, particularly in the diets of children (National Re-search Council, 1993), the widespread occurrence of fungicide-resistant isolates of P. digitatum
(Eckert et al., 1994), environmental problems as-sociated with the disposal of water used in pack-ing operations, and a lack of approved fungicides for the control of sour rot (Eckert and Eaks, 1989). To facilitate their regulatory approval and commercial acceptance, we evaluated compounds that have known environmental and animal toxi-cological properties and extensive precedents as additives or natural components in foods (Smilan-ick et al., 1995, 1997, 1999).
Calcium polysulfide, formulated as ‘liquid lime-sulfur’ (LLS) solution, was first described early in the nineteenth century and is one of the oldest fungicides (McCallan, 1967). LLS solution is a yellow-orange liquid, pH 11.5, with a density of 1.26 g ml−1. The formulation we used is typical (Tweedy, 1967), it contained a mixture of 29% (wt/vol) calcium polysulfide and small amount of calcium thiosulfate. It is prepared by combining hydrated lime (CaO·H2O) and elemental sulfur with water. When diluted to 3% (wt vol−1) LLS solution, a common rate recommended in foliar
fungicide applications, it has a pH of 10.0 and constantly releases small amount of hydrogen sulfide (H2S) gas. More H2S is released if the solution is acidified. At concentrations less than 1.5% (wt vol−1) or at low pH, yellow precipitates, which are primarily composed of elemental sulfur, form rapidly. In addition to its use as a fungicide, LLS solution is used to open sewer lines, immobi-lize metals in mine tailings, acidify soil, and im-prove water penetration into soil.
As a pesticide, LLS was first described in 1802 in England, by 1850, the present lime sulfur for-mula was standardized, and it was in common use by 1900 in California for apple scab, powdery mildew, San Jose scale, aphids, mites, brown rot of peaches, and other pests and diseases (Tweedy, 1967). It is certified as an acceptable pesticide by most ‘organic’ grower organizations and the USDA. Reports of its use to control postharvest diseases are few. Haller (1952) reviewed its use on stone fruit, where applications before harvest sub-stantially reduced the postharvest incidence of brown rot of peaches, caused by Monilinia fructi
-cola. Poulos (1949) reported postharvest applica-tions to peaches of LLS reduced postharvest brown rot incidence about 60% without injury to the fruit.
LLS has moderate acute toxicity (oral LD50for rats is 820 mg kg−1) but no chronic dietary toxicity hazard or carcinogenicity has been iden-tified (Anon, 1997). The primary safety hazards it poses are burns from skin or eye contact with the concentrated solution, or from exposure to H2S gas that can evolve from LLS. Occupational Health and Safety Administration workplace lim-its for H2S are 10ml l−1for 8 h and 15ml l−1for brief exposures of 15 min (Anon, 1997).
of postharvest decay has been well-investigated (Eckert and Eaks, 1989). We also quantified sul-fur residues in the LLS-treated fruit.
2. Materials and methods
2.1. Fruit
Commercially harvested lemons (Citrus lemon
[L.] N.L. Burm) or oranges (Citrus sinensis [L.] Osbeck) were randomized and inoculated within 2 days of harvest. Oranges were of typical commer-cial maturity. Lemons in most tests were light-green in color.
2.2. Liquid lime sulfur solution
The LLS solution was donated by Best Sulfur Products, 5427 E. Central Avenue, Fresno, CA 93725. It contained by weight 29% wt wt−1 cal-cium polysulfide. The treatment solutions were prepared by dispensing LLS solution, usually at 3%, by weight to water into stainless steel tanks. The active concentration of calcium polysulfide in a 3% LLS solution was 0.85% wt vol−1.
2.3. Culture of pathogens
P. digitatum isolate M6R (from J. W. Eckert, University of California, Riverside, CA) was cul-tured 1 – 2 wk on potato dextrose agar (PDA). Spores were harvested by adding 5 ml of water containing 0.05% Triton X-100 to the Petri dish, rubbing the surface with a sterile glass rod, and passing the suspension through two layers of cheese cloth. The suspension was diluted with water to an absorbance of 0.1 at 425 nm deter-mined with a spectrophotometer; this density con-tains about 106
spores per ml (Eckert and Brown, 1986). Geotrichum citri-aurantii isolate 99-3 (iso-lated from lemon) was similarly cultured and pre-pared, except the arthrospores from 2-week-old cultures were suspended in 10 mg l−1 cyclohex-amide to facilitate infection (Eckert and Brown, 1986) and 100 mg l−1 thiabendazole to minimize interference from P. digitatum.
2.4. In 6itro toxicity of LLS solution to spores
To determine the germinability of spores of P.
digitatum after immersion in LLS solution, 1 ml containing 1×106spores ofP.digitatum, cultured and prepared as previously described, was added to 30 ml of water alone or LLS solution (3% wt vol−1). The solutions were either 25 or 40.6°C. Initially, and after 0.5, 2, 3.5, 5, and 6 h, 3 ml aliquots of each solution were removed and placed on a 2.4 cm diameter glass fiber filter in a glass support in a vacuum apparatus. To remove the original solution and thoroughly rinse the spores, the solution was removed by vacuum fol-lowed by the addition of two, 30 ml volumes of deionized water, each of which was removed by vacuum. The filter was removed from the support, inverted, placed on PDA and removed, where most of the spores were deposited. After 18 h at 15°C, the proportion of germinated spores was determined by examination of 100 – 150 spores by light microscopy at 100×. The experiment was repeated twice.
2.5. Inoculation methods
Standard methods used to evaluate citrus postharvest fungicides were used. Lemons and oranges used in all experiments were selected by hand from field bins soon after harvest. The day before each experiment, the fruit were washed with water on commercial processing equipment, randomized, and inoculated with P. digitatum or
G. citri-aurantii by briefly immersing a stainless steel tool with a 1 mm wide and 2 mm long tip in the solution and wounding each fruit once.
2.6. Laboratory tests to control green mold and sour rot
lime-sulfur solution was compared to solutions now in commercial use (expressed as wt vol−1): 3% sodium carbonate (pH 11.5), 3% sodium bi-carbonate (pH 8.3), and a mixture of 4% borax and 2% boric acid (pH 10.5).
2.7. Impact of lemon maturity on LLS and sodium bicarbonate effecti6eness
Fruit from field bins were segregated into two color classes: (1) green, where 50% or more of the lemon surface was green in color; or (2) yellow, where none of the fruit surface had any green color. These fruit were from coastal groves in California where fruit of all maturity stages occur simultaneously. Fruit surface color was quantifed by recording CIELab chroma values (McGuire, 1992) of a 20 fruit sample of each class with a tristimulus colorimeter with an 8 mm aperture (Minolta Model DP301). The soluble solids and titratable acid content of a 10 ml aliquot of the juice of each of four replicates (each replicate a composite of the juice of five fruit) from fruit of each maturity class was determined with a refrac-tometer and titration with 1.0 N NaOH. The fruit were inoculated 24 h before treatment in 3% (wt vol−1
) LLS or sodium bicarbonate at 40.6°C for 2 min, followed by a brief rinse in 2-ml diH2O per fruit, then the fruit were stored 1 week at 20°C before examination. The experiment was repeated three times.
2.8. Semi-commercial tests to control green mold and sour rot
Tests to assess the impact of temperature, LLS concentration, immersion period, and post-treat-ment fruit cleaning on LLS solution effectiveness and to compare it to other treatments were con-ducted with commercial fruit processing equip-ment at the University of California Lindcove Research and Extension Center (CREC) Fruit Quality Evaluation facility in Lindcove, CA. LLS solution was compared to treatments that are already in commercial use and their efficacy for the control of green mold have been well-charac-terized (Eckert and Eaks, 1989). These tests em-ployed commercial harvest crews, large numbers
of fruit per replicate, and processing equipment typical of commercial packing operations, such as a large tank with an overhead submerger/ ad-vancer that forced the fruit under the solution surface during immersion, a high-pressure water fruit washer, an overhead wax applicator with rotating brushes, and a dryer with high-velocity heated air.
In the first test to assess control of green mold, four replicates, each of 75 – 100 of oranges cv. Valencia or lemons cv. Eureka, were immersed for 90 s in a 2250 l tank containing LLS solution (3% wt vol−1
) at 26.5 or 43.3°C (90.5°C). After treatment, the fruit were rinsed briefly using a low-pressure overhead spray of 10 ml water alone per fruit, or washed for 45 s at high pressure (200 psi or 1350 kPa) with water containing 50 mg l−1 free chlorine applied at 2400 l min−1. The high-pressure water washer was 61 cm wide, 3 m long, and contained 15 rows of three 45° angle flat-fan nozzles per row. After rinsing or washing with water at high pressure, the fruit were waxed with a finishing wax (Britex 505, Brogdex) and dried at 50°C for 3 min. Other treatments included: (1) fruit that were inoculated but not treated or waxed; (2) fruit that were immersed for 3 min in 2250 l of sodium carbonate (3% wt vol−1
) at 43.3°C, washed with water at high pressure as previously described, waxed, and dried; and (3) fruit that were immersed for 3 min in 2250 l of sodium carbonate (3% wt vol−1) at 43.3°C, washed with water at high pressure as previously described, coated with wax that contained 2000 mg l−1 imazalil, and dried. The test was con-ducted once with Valencia oranges and once with Eureka lemons. After treatment, the fruit were stored at 10°C for 15 days and the incidence of green mold was determined.
40.6°C; (5) a mixture of borax and boric acid (4 and 2% wt vol−1, respectively, pH 8.4) at 40.6°C; or (6) SOPP (0.5% wt vol−1, pH 8.6, Freshgard® 20, 23% a.i.; FMC Corp., Riverside, CA) at 40.6°C. SOPP was also applied by passage of fruit for 15 s over eight rotating brushes where an overhead spray of SOPP (1.9% wt vol−1, pH 11.9) was constantly applied at 16.0°C.
2.9. Assessment of phytotoxicity of LLS to citrus fruit
Three replicates of 75 – 120 fruit each were im-mersed in LLS solution (3% wt vol−1
) at 40.6, 48.9, or 54.4°C for 3 min followed by pressure washing with water as previously described. Fruit were randomized, treated, waxed with a finishing wax (Britex 505, Brogdex Co., Pomona, CA), dried at 49°C, and stored at 10°C for 7 weeks. The treatments were applied to five navel orange cultivars (Atwood, Thomsom Improved, Fisher, New Hall, Bonanza, USA) and Lisbon lemons. After storage, fruit were sorted visually into four classes: (1) perfect; (2) slight but not significant rind blemish present; (3) modest rind blemish present; or (4) scald injury present. Fruit of the first two classes would typically be ranked as USDA No. 1, those in the last two classes would be of lower value and classified as choice, stan-dard, or juice-grade fruit.
Because lime-sulfur solution has a sulfide odor, H2S and sulfur dioxide (SO2) concentrations in air were monitored during semi-commercial tests to determine if these gases were present at levels hazardous to workers. The National Institute of Occupational Safety and Health workplace expo-sure limits for H2S and SO2 are 10 and 2 ml l−1, respectively, for 8 h (Anon, 1997). For brief expo-sures of 15 min, H2S and SO2 are limited to 15 and 5 ml l−1, respectively. Two models of colori-metric dosimeters for H2S (Drager
®
Model 10/ a-D and Synsidyne® Model 4D) and one for SO
2 (Synsidyne® Model 5D) were used. When acti-vated, each could detect a minimum of 1ml l−1of their respective gases for the next 8 h. They were placed at four locations 20 – 70 cm above LLS tank and along packingline at Lindcove CREC during 5 h of operation of the heated 2000-l tank.
2.10. Impact of temperature on life of lime-sulfur solution sulfide content
Lime-sulfur solution (3% wt vol−1) was dis-tributed in 22 l aliquots to temperature-controlled stainless-steel tanks at 25, 40, 45, 50, 55, 60, or 65°C and the sulfide concentration was deter-mined initially and after 24 and 48 h. Sulfide content was determined by the addition of stan-dardized I2 in excess of the sulfide content, fol-lowed by titration of the solution with stardardized thiosulfate to determine the I2 re-maining (Skoog and West, 1980). To a 10 ml aliquot of lime sulfur solution, 20 ml of 0.1 N I2 was added, followed by additions of 1 ml of starch indicator and 50 ml of distilled water. The solution was titrated with 0.1 N calcium thiosul-fate until it was colorless. The molarity of sulfide was calculated stochiometrically from the moles of 0.1 N thiosulfate titrant consumed.
2.11. Sulfur residues
Sulfur residues were determined by distillation of a macerated sample of the whole fruit tissue followed by titration or liquid chromatography of the distillate to determine the content of sulfite or sulfide. Ten lemons, oranges, and grapefruit were immersed for 2 min in 3% wt vol−1 LLS at 40.6°C, then rinsed for 5 s in deionized water applied in a low pressure spray at 50 ml s−1, then stored for 5 days at 5°C before analysis. A distil-lation method was employed to determine the total volatile sulfite/sulfide content of the fruit, the aeration – oxidation procedure, that is typically employed to estimate the total sulfur dioxide con-tent in foods (‘Modified Monier Williams proce-dure’; Zoecklein et al., 1990). A 1 l capacity distillation flask containing 400 ml of water and 5 ml of ethanol was purged with nitrogen at a flow rate of 20 ml min−1
ethyl-ene glycol. The receiver contained 30 ml of 2.0 mM Na2CO3 plus 15 mM NaHCO3.
To determine the sulfide/sulfite content, the re-ceiver contained 10 ml of 0.3% H2O2 and after distillation its contents were titrated with 0.01 N NaOH with methyl red as an indicator. Recovery efficiency was determined by spiking the fruit sample with 10 mg sulfite per gram fresh sample weight. In a second test, the receiver contents were not oxidized with H2O2and ion chromatog-raphy was applied to determine the nature of the sulfur content of the distillate. A 7.5ml-aliquot of the distillate was injected into a Dionex model DX- 120 with a Ionpac Fast Ion column (10 – 32) with a 2.0 mM Na2CO3+0.15 mM NaHCO3 eluent at a flow rate of 2 ml min−1. A conductiv-ity detector was employed. Sodium sulfite stan-dards of 5, 10, 20, 50, and 100 mg l−1 were injected using the same conditions. The detection limit was 0.73 mg l−1. Recovery efficiency of both the distillation and ion chromatography proce-dures together was determined by spiking the fruit sample with 30 mg sulfite/g fresh sample weight.
2.12. Statistical analysis
A one- to three-way analysis of variance was applied to the square root of the arcsin of the proportion of infected or injured fruit, followed by Fisher’s Protected LSD to separate means. Orthogonal analysis and paired-t tests were ap-plied in some tests. Actual values are shown.
3. Results
3.1. In 6itro toxicity of LLS solution to spores
The germinability of spores ofP.digitatum was only slightly diminished after exposures as long as 6 h (Fig. 1) in either water or lime-sulfur solution at 25°C. At 40.6°C, however, spore germinability declined rapidly, particularly in LLS solution, where it decreased from 80 to 14% after 30 min exposure, and none could germinate after 2 h exposure. In water at 40.6°C, spore germinability declined from 80 to 59% after 30 min exposure, to 2.5% after 2 h exposure, and none survived 3.5 h exposure.
3.2. Impact of concentration and duration of treatment on LLS effecti6eness
The incidence of green mold was equally re-duced by the immersion of inoculated lemons in 2, 3, 4, or 6% wt vol−1LLS solution at 40.6°C for 1.5 min (Fig. 2A). No injury to the lemons was observed after storage of the lemons after any treatment. The incidence of green mold was pro-gressively reduced by the immersion of inoculated lemons in 3% wt vol−1
LLS at 40.6°C for 0.25, 1, 2, or 4 min (Fig. 2B). injury to the lemons was observed after storage of the lemons after any of the treatments.
3.3. Comparison of LLS to sodium carbonate and borax–boric acid
The effectiveness of LLS treatment for the con-trol of citrus green mold was not significantly different from sodium carbonate or borax – boric treatments (Table 1). Water alone at 40.6°C did not reduce green mold significantly. Lime-sulfur treatment reduced green mold incidence by 93, 88, and 81%, respectively, in tests one, two, and three. No injury to the lemons was observed after stor-age of the lemons after any treatment.
In a laboratory test, LLS treatment reduced sour rot of lemons was significantly, from 64% incidence among inoculated control fruit to a mean of 35% among treated fruit (data not shown). However, increasing the LLS concentra-tion (from 2 to 4.5% wt vol−1) or treatment time (from 1.5 to 3 min) did not significantly improve
Fig. 2. Influence of (A) lime-sulfur solution concentration during 1.5 min of treatment, and (B) the period of fruit immersion in 3% (wt vol−1) lime-sulfur solution on the subsequent incidence of green mold on lemons. The fruit were inoculated 24 h before treatment, the solution temperature during treatment was 40.6°C, and after treatment the fruit were stored for 2 weeks at 20°C before examination.
control of sour rot. Treatment at 43.3°C was slightly but significantly superior to treatment at 21.1 or 32.2°C. These results are of one successful test, two other trials were inconclusive because sour rot did not develop among the inoculated control fruit. In all three tests, no injury to the lemons was observed after any treatment immedi-ately or after 2 wk storage at 20°C.
3.4. Impact of fruit maturity
Green lemons had lower chromicity values and higher soluble solid and acid contents than yellow lemons. Among the green lemons, theL,a, andb
chromicity values were 67.4, −14.8, and 47.6, respectively, and the soluble solids content was 7.5% and 5.7 g acid per 100 ml of juice. Among the yellow lemons, the L, a, and b chromicity values were 75.7, −3.3, and 58.4, respectively, and the soluble solids content was 6.8% and 4.9 g acid per 100 ml of juice. The differences in chromicity, soluble solids, and acid content were all significant (P50.05). Lemon maturity signifi-cantly influenced LLS efficacy. Control of green mold by LLS was superior to sodium bicarbonate on green lemons (Fig. 3). Conversely, the control of green mold on yellow lemons by LLS was significantly inferior to its control on green lemons, and the efficacy of LLS and sodium bicarbonate was not significantly different.
3.5. Semi-commercial tests
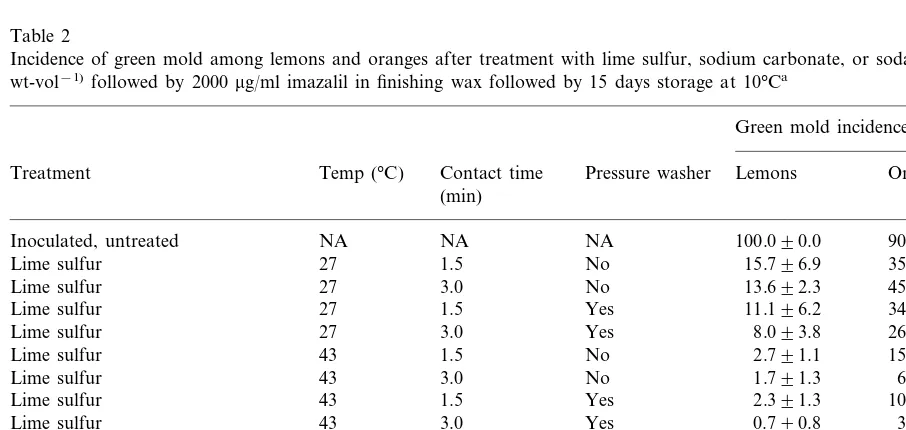
In the first semi-commercial test, LLS signifi-cantly reduced the incidence of green mold on both oranges and lemons (Table 2). Heating the LLS solution, increasing the length of time the fruit were in the LLS solution, and pressure wash-ing the fruit after LLS treatment all significantly (P50.05) improved its effectiveness. On oranges, this LLS treatment was significantly superior to the sodium carbonate and imazalil combination (P=0.0138, paired t-test). On lemons, the most
Table 1
Incidence of green mold (%) after the treatment of lemons inoculated 24 h before treatment with spores ofP.digitatuma
Test numberb
Treatment 1 2 3
99 a
Inoculated, untreated control 99 a 97 a 99 a 97 a Inoculated, 40.6°C water- treated 91 b
12 b 19 b Lime-sulfur solution 3%c 7 c
14 b 14 b Sodium carbonate 3% 20 c
9 b Borax 4%–boric acid 2% NDd 13 b
aThe temperature of all solutions was 40.6°C. The fruit were immersed for 1.5–2 min, rinsed briefly with 2 ml per fruit of deionized water, and stored 2 wk.
bMeans in columns followed by the same letter are not significantly different (Fisher’s Protected LSD;P50.05).
Fig. 3. Influence of lemon maturity on the effectiveness of LLS and sodium bicarbonate. The fruit were inoculated, 24 h later immersed in 3% wt vol−1of each solution at 40.6°C for 2 min duration, then stored for 1 week at 20°C before examination.
or sodium ortho-phenyl phenate applied over ro-tating brushes. LLS was inferior only to immer-sion in sodium ortho-phenyl phenate for green mold control. For the control of sour rot, LLS was not significantly different than immersion in borax/boric acid, sodium carbonate, or sodium
ortho-phenyl phenate. It was superior to sodium
ortho-phenyl phenate applied over rotating brushes. No visible injury to the lemons occurred in this test.
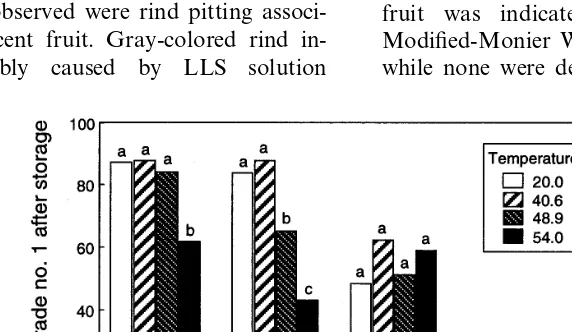
3.6. Assessment of phytotoxicity of LLS to citrus fruit
The percentage of fruit marketable as USDA No. 1 of six citrus fruit cultivars was not influ-enced by 3% wt vol−1 LLS treatment at 40.6°C, and only two cultivars had a reduction in USDA No. 1 ratings at 48.9°C (Fig. 4). Even at 54.4°C, the quality of two cultivars (Fisher and Atwood navel oranges) was not reduced. The 7 week stor-age period used in this test was long and a normal and high incidence of rind breakdown occurred, particularly among navel oranges. Most of the effective LLS treatment was not significantly
dif-ferent (P=0.2152, orthogonal comparison) in ef-fectiveness than sodium carbonate followed by imazalil. No visible injury to the lemons or or-anges occurred in this test.
In a second semi-commercial test, LLS signifi-cantly reduced the incidence of green mold and sour rot on lemons (Table 3). For the control of green mold, LLS was significantly superior to immersion in borax/boric acid, sodium carbonate,
Table 2
Incidence of green mold among lemons and oranges after treatment with lime sulfur, sodium carbonate, or soda ash (each at 3% wt-vol−1)followed by 2000
mg/ml imazalil in finishing wax followed by 15 days storage at 10°Ca
Green mold incidence (% 9S.D.)
Oranges Contact time
Temp (°C) Pressure washer Lemons Treatment
(min)
90.097.2
NA NA
Inoculated, untreated NA 100.090.0
35.592.5 15.796.9
No
Lime sulfur 27 1.5
45.496.3
3.0 No
Lime sulfur 27 13.692.3
27 1.5
Lime sulfur Yes 11.196.2 34.697.7
26.393.4 8.093.8
Yes
Lime sulfur 27 3.0
43 1.5
Lime sulfur No 2.791.1 15.592.9
6.792.4
3.0 No
Lime sulfur 43 1.791.3
43 1.5
Lime sulfur Yes 2.391.3 10.892.2
Lime sulfur 43 3.0 Yes 0.790.8 3.391.4
Yes 3.0
43
Sodium carbonate 2.791.1 10.496.0
10.894.0 1.791.3
Yes
43 3.0
Sodium carbonate+2000 ppm imazalil
Statistical comparisons
Did pressure washing improve LLS effectiveness? Yes (P=0.0247) Yes (P=0.0001) Yes (P=0.0001)
Did heating improve LLS effectiveness? Yes (P=0.0001)
Was control of green mold after 3 min contact in LLS superior to contact for 1.5 min? Yes (P=0.0343) Yes (P=0.0008)
Table 3
Incidence of green mold and sour rot among lemons inocu-lated with spores of P. digitatum or G. citri-auranttii 24 h before treatmenta
Sour rot
Treatment Green moldb
Water immersion, 16.0°C 99.6 a 58.1 a 92.4 b
Water immersion, 40.6°C 39.7 b 85.1 c 26.9 c SOPPc(1.9%)dover rotating
brushes, 16.0°C, 15 s
34.0 d
Borax (4%) plus boric acid 13.8 d (2%) immersion, 40.6°C
Sodium carbonate (3%) 30.6 d 10.1 d immersion 40.6°C
17.8 e 17.8 d Lime-sulfur solution (3%)
immersion 40.6°C
9.2 f 15.2 d SOPP (0.5%) immersion,
40.6°C
aThe fruit were treated by immersion in the solutions for 90 s or by an overhead spray for 15 s, then stored at 13°C for 3 or 4 weeks when the incidence of green mold or sour rot was determined, respectively.
bMeans followed by unlike letters differ significantly by Fisher’s Protected LSD (P50.05) applied after a one-way ANOVA of the arcsin transformed incidence data. Actual percentages shown.
cSOPP=sodiumortho-phenyl phenate. dConcentrations in wt vol−1.
scalding, were observed on a few fruit treated at 54.4°C.
H2S and SO2 were not detectable by the dosimeters in the air above the treatment tank during a 5 h-period of continuous operation of the semi-commercial tests, and therefore was be-low the 1 ml l−1detection limit of the dosimeters.
3.7. Impact of temperature on life of LLS solution sulfide content
Freshly prepared LLS solution (3% wt vol−1 ) contained 101.0 (92) mM sulfide. After 24 and 48 h, the mean sulfide contents in the tanks at 25, 40, 45, 50, 55, 60, or 65°C were similar and not significantly different (P50.05). The sulfide con-tent (9S.D.) among all the tanks after 24 and 48 h was 92.8 (90.9) mM and 87.6 (91.5) mM, respectively, representing losses of 93.1 and 84.9% from the original sulfide content. Yellow-colored scale deposits accumulated within the tanks dur-ing this period.
3.8. Sulfur residues in LLS-treated fruit
The presence of sulfur residues in LLS-treated fruit was indicated after application of the Modified-Monier Williams distillation procedure, while none were detected in untreated fruit. The quality defects observed were rind pitting
associ-ated with senescent fruit. Gray-colored rind in-juries, presumably caused by LLS solution
recovery of a 10.2 mg g−1 sodium sulfite spike added to untreated oranges, lemons, and grape-fruit was 93.7, 87.8, and 108.0%, respectively. The volatile sulfur content, calculated either as sulfite or sulfide, was 31.9, 33.1, and 36.3 mg g−1 of oranges, lemons, and grapefruit. In the second experiment, the distillation was repeated and the receiver contents were not oxidized by H2O2 but analyzed by ion chromatography. The recovery of a 30mg g−1 sodium sulfite added to orange and lemon samples followed by distillation and by ion chromatography was 87.3 and 23.3% respectively. No sulfite was detected when untreated fruit were analyzed. When LLS-treated oranges and lemons were analyzed, no sulfite was detected by ion chromatography, suggesting the volatile sulfur present was sulfide and not sulfite. Therefore, the sulfur content of the LLS-treated fruit from the initial distillation procedure was not sulfite and should be calculated as sulfide.
4. Discussion
Used LLS can be safely disposed of by many routes, and this is its most compelling advantage over other methods to control postharvest decay. Because LLS is frequently applied to sewers or agricultural soils, it can be used in locations where the disposal of other fungicides, particularly those used in large tanks (whose capacity can exceed 10 000 l) has become difficult due to environmental concerns. LLS can be an effective and inexpensive treatment for the control of postharvest green mold and provide some control of sour rot. There was little risk of injury to citrus fruit. The treat-ment was more effective on lemons than oranges, and on less mature lemons than on more mature lemons. Therefore, LLS is a good candidate for applications to fruit soon after harvest, rather than to fruit that are reprocessed after long storage. LSS effectiveness was similar when it was applied be-fore or after fruit cleaning operations, therebe-fore it can be used in processing lines either before or after the fruit are cleaned. Cleaning alone, even when done with water at high pressure, does not reduce postharvest decay after the inoculation of wounds with pathogens (Smilanick et al., 1999).
Like other tank treatments such as borax – boric acid or sodium carbonate, it is very important that attention be paid to sanitation of the fruit after treatment, because LLS treatment probably does not leave residues capable of providing persistent protection of the fruit. Therefore, spores from the air or surfaces of packing equipment could inocu-late the fruit through wounds made during han-dling after LLS treatment. Opportunities for re-contamination are minimized if LLS treatment is applied at the end of the packing process, after sorting and grading, and just before waxing. This way, exposure of the treated fruit to contamination is minimized.
In this work we quantified sulfur residues in the LLS-treated fruit and showed they were probably sulfides and not sulfites. This is important because sulfite residues are under regulatory control and typically cannot exceed 10 mg g−1 without a consumer warning label attached (Zoecklein et al., 1990).
The mode of action of LLS is incompletely known. Two toxic components of LLS are H2S, produced by the decomposition of LLS, and ele-mental sulfur, which comprises part of the yellow scale deposits on equipment that accumulated after prolong use of LLS. Elemental sulfur oxidizes cytochromebto cytochromec, with a concomitant production of H2S, itself an inhibitor of cy-tochrome oxidase (Smith et al., 1977), and a fungistatic, not fungicidal, inhibition results (Tweedy, 1967). LLS did not kill P. digitatum
spores immediately, but caused a persistent inhibi-tion of their growth. P. digitatum spores germi-nated very slowly after LLS treatment, even when they have been rinsed repeatedly with fresh water, which suggests the LLS deposits a very persistent residue on them. Gadoury et al. (1994) reported 1 h of exposure to LLS was required before the viability of ascospores within cleistothe-cia of Uncinula necator was significantly reduced, while formaldehyde killed most all of them within 5 min.
to citrus trees in many parts of the world. In some areas, residuals of these metals from field applica-tions represent water quality problems when treated fruit are cleaned in packinghouses, be-cause the wash water zinc and copper content can exceed water quality standards when it is dis-charged into sewers or ponds. Insoluble zinc and copper sulfides would accumulate in the bottom of LLS tanks, and disposal of these solids would occur when the tank scale is removed.
An important practical consideration is that the sulfide content of LLS solution declined slowly as the solution aged. Additional losses would occur when some LLS is carried away as fruit pass out of commercial processing tanks. Because the vol-ume of fruit processed and the size of the tanks differs considerably among packinghouses, the life of the LLS solution and the intervals at which it must be recharged in a commercial packingline needs to be determined empirically at each facil-ity. Sulfide concentration can be determined by titration or with a sulfide selective electrode (Papp, 1971). If the LLS concentration falls below 1.5% wt vol−1 it will decompose rapidly to ele-mental sulfur, which precipitates in the tank. The solution concentration can be routinely approxi-mated with a salt refractometer or a hydrometer calibrated for LLS solution. The odor of H2S was present and could conceivably be a significant nuisance, although it was below safety thresholds. Placement of the tank in an isolated and well-ven-tilated area is recommended. Another issue of concern is the corrosiveness of the solution. The tank, heat exchanger, rollers, and other compo-nents should be plastic or stainless steel; mild steel, iron, and non-ferrous metals are not com-patible with LLS. Scale is also deposited on equipment, and the capability to dismantle and mechanically remove scale should be accommo-dated in the design of this equipment. We pre-sume most of the scale is calcium carbonate and elemental sulfur. In other tests, we evaluated the effectiveness and stability of potassium polysulfide solution. Unlike calcium polysulfide, the potas-sium polysulfide solution deposited little scale on equipment, and its effectiveness for the control of green mold was equal to LLS (data not shown). However, the amount of potassium polysulfide
available for commercial use was small and its price was higher than LLS, so we did not evaluate its use further.
Acknowledgements
We are thankful for the financial support of the California Citrus Research Board, the formula-tion and donaformula-tion of lime-sulfur soluformula-tion by Best Sulfur Products and the assistance of Faith Pot-ter, Don Holbrook, and Alex Holman of that company, John Maze, Walter Stutzman, and Louis Whitendale of University of California Lindcove Citrus Research and Extension Center are gratefully acknowledged, we thank D.A. Mar-gosan, D.J. Henson, Jim Sievert for technical support and Paul Nelson of FMC regarding com-mercial tests in Yuma AZ. We appreciate the donation by Sunkist Growers of much of the labor to conduct these tests, and we thank Husain Ajwa, Monir Monsour, and Dennis Margosan for review of the manuscript.
References
Anonymous, 1997. National Institute for Occupational Safety and Health Pocket Guide to Chemical Hazards. US De-partment of Human Health Services (NIOSH) Publication No. 97-140. US Government Printing Office, Washington, DC.
Eckert, J.W., Brown, G.E., 1986. Evaluation of postharvest treatments for citrus fruits. In: Hickey, K.D. (Ed.), Meth-ods for Evaluating Pesticides for Control of Plant Patho-gens. American Phytopathological Society, St Paul, MN, pp. 93 – 97.
Eckert, J.W., Eaks, I.L., 1989. Postharvest disorders and diseases of citrus fruits. In: Reuther, W., Calavan, E.C., Carman, G.E. (Eds.), The Citrus Industry, vol. 4. Univer-sity of California Press, Berkeley, CA, pp. 179 – 260. Eckert, J.W., Sievert, J.R., Ratnayake, M., 1994. Reduction of
imazalil effectiveness against citrus green mold in Califor-nia packinghouses by resistant biotypes ofPenicillium digi
-tatum. Plant Dis. 78, 971 – 974.
McCallan, S.E.A., 1967. History of fungicides. In: Torgeson, D.C. (Ed.), Fungicides: An Advanced Treatise, vol. 1. Academic Press, New York, pp. 1 – 37.
McGuire, R.G., 1992. Reporting of objective color measure-ments. HortScience 27, 1254 – 1255.
National Research Council, 1993. Pesticides in the Diets of Infants and Children. National Academy Press, Washing-ton, DC.
Papp, J., 1971. Potentiometric determination of sulphur com-pounds in white, green and black liquors with sulphide ion-selective electrode. Cellul. Chem. Technol. 5, 147 – 159. Poulos, P.L., 1949. The use of sodium hypochlorite for the control of the brown rot disease of peach in Delaware. Plant Dis. Rep. 33, 413 – 415.
Powell, G.H., 1908. The decay of oranges while in transit from California. Bulletin No. 123, Bureau of Plant Industry. United States Department of Agriculture, Washington, DC.
Salvato, J.A., 1992. Environmental Engineering and Sanita-tion, 4th edn. Wiley-Interscience, New York.
Skoog, D.A., West, D.M., 1980. Analytical Chemistry, 3rd edn. Saunders College, Philadelphia, PA.
Smilanick, J.L., Mackey, B.E., Reese, R., Usall, J., Margosan, D.A., 1997. Influence of the concentration of soda ash, temperature, and immersion period on the control of postharvest green mold of oranges. Plant Dis. 81, 379 – 382. Smilanick, J.L., Margosan, D.A., Henson, D.J., 1995. Evalua-tion of heated soluEvalua-tions of sulfur dioxide, ethanol, and hydrogen peroxide to control postharvest green mold of lemons. Plant Dis. 79, 742 – 747.
Smilanick, J.L., Margosan, D.A., Mlikota, F., Usall, J., Michael, I.F., 1999. Control of citrus green mold by car-bonate and bicarcar-bonate salts and the influence of commer-cial postharvest practices on their efficacy. Plant Dis. 83, 139 – 145.
Smith, L., Kruszyna, H., Smith, R.P., 1977. The effect of methemoglobin on the inhibition of cytochrome c oxidase by cyanide, sulfide and azide. Biochem. Pharmacol. 26, 2247 – 2250.
Tweedy, B.G., 1967. Elemental sulfur. In: Torgeson, D.C. (Ed.), Fungicides: An Advanced Treatise, vol. 2. Academic Press, New York, pp. 119 – 145.
Zoecklein, B.W., Fugelsang, K.C., Gump, B.H., Nury, F.S., 1990. Production Wine Analysis. Van Nostrand Reinhold, New York.