38 ELECTRODEPOSITION OF Cu ON THE SURFACE OF SILICA FREE RICE
HUSK ACTIVATED CARBON WITH ULTRASONIC IRRADIATION
Ryan Andhika1, Muhammad Zakir, Maming
Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Hasanuddin Jl. Perintis Kemerdekaan Km. 10 Makassar 90245
Abstrak. Elektrodeposisi logam Cu pada permukaan karbon aktif sekam padi bebas silika dengan iradiasi ultrasonik yang bertujuan untuk meningkatkan nilai kapasitansi spesifik telah dilakukan. Karbon aktif sekam padi bebas silika disintesis menggunakan aktivator H3PO4 dan ekstraksi silika menggunakan KOH. Luas permukaan karbon sekam padi yang diperoleh sebelum dan sesudah ekstraksi silika serta setelah aktivasi berturut-turut adalah 57,2833 m2/g, 180,5378 m2/g dan 184,6074 m2/g. Analisis menggunakan XRF menunjukkan bahwa logam Cu terdeposisi pada permukaan karbon aktif sekam padi bebas silika dan berdasarkan pengukuran CV menunjukkan bahwa elektrodeposisi logam Cu dengan iradiasi ultrasonik dapat meningkatkan nilai kapasitansi spesifik. Kapasitansi spesifik karbon aktif sekam padi bebas silika sebelum dan sesudah elektrodeposisi logam Cu dengan iradiasi ultrasonik adalah 657,75 nF/gdan721,08 nF/g.
Kata kunci: Cu, elektrodeposisi, kapasitansi spesifik, karbon aktif, ultrasonik.
Abstract. Electrodeposition of Cu on the surface of silica free rice husk activated carbon with ultrasonic irradiation aimed to increase the value of specific capacitance was carried out. Silica free rice husk activated carbon was synthesized using H3PO4 activator and extraction of silica using KOH. The surface area of rice husk carbon was obtained before and after the extraction of silica and after activation were 57.2833 m2/g, 180.5378 m2/g and 184.6074 m2/g, respectively. XRF analysis showed that Cu depositioned on the surface of silica free rice husk activated carbon and based of CV measurements showed that electrodeposition of Cu with ultrasonic irradiation can increased the value of specific capacitance. Specific capacitance of silica free rice husk activated carbon before and after electrodeposition of Cu with ultrasonic irradiation were 657.75 nF/g and 721.08 nF/g, respectively.
Key words: Cu, electrodeposition, specific capacitance, activated carbon, ultrasonic.
1
39 INTRODUCTION
Rapid technological progress requires more energy supply both for industry and vehicle propulsion (Suhada, 2001). Energy needs, especially in Indonesia parallel with increased economic and population growth it is estimated that average growth of energy needs amounted 4.7% per annum during 2011-2030. Usage coal increased considerably around 13.4% per annum from 2000 until 2011 led to high production of exhaust gas emissions such as CO2, SOx and NOx. The limited petroleum electrochemical capacitors. The material used for the electrode of the electrochemical capacitors was an activated carbon because it had a high internal surface area and good pore accessibility (Dell and Rand, 2001; Frackowiak and Beguin, 2001; Ariyanto et al., 2012).
Manufacture of activated carbon using various types of biomass wastes was increased the surface area of carbon (Wei et al., 2011). Another way that can be used to increased the surface area is to utilize ultrasonic waves (Suslick et al., 1996). The higher surface area then the higher value of the capacitance. The high capacitance value is an indicator of the ability of high energy accumulation (Zakir et al., 2013). One way to increased the capacitance value is to
utilize pseudocapacitance effect in the presence of the electroactive species such as transition metals (Frackowiak and Beguin, 2001).
This research was conducted electrodeposition of Cu on the surface of silica free rice husk activated carbon with ultrasonic irradiation treatment to create a superior carbon as electrode material electrochemical capacitors.
RESEARCH METHODS
Material
The materials that were used in this research are rice husk, commercial activated carbon, KOH (Merck), technical HCl, technical H3PO4 85%, CuSO4.5H2O
(Merck), H2SO4 (Merck), Argon gas,
methylene blue, paraffin wax, aquadest, copper wire, platinum wire, Ag/AgCl electode, Pt electrode, aluminum foil, universal pH indicator and Whatman filter paper number 42.
Apparatus
The equipments were used in this research are glassware tool that commonly used in laboratories, furnace (Muffle furnace type 6000), a water bath (hot plate),
a sieve size of 100 mesh, desiccator, saucer porcelain, mortar porcelain, vacuum pump, solder fumes, pipette, oven (type SPNISOSFD), magnetic stirrer (CERAMAG Midi), analytical balance (Shimadzu AW220), Ultrasonic Cleaner
(Elmasonic S40H), FTIR (Shimadzu IR Prestige21), XRD (Shimadzu XRD-7000), XRF (ThermoFisherXRF), UV-Vis (Shimadzu UV-2600) and Cyclic voltammetry (Potentiostats EA161).
Procedure
1. Preparation and Carbonization
40 hour. After that, it washed with aquadest
repeatedly until the pH became neutral, then dried in an oven at 110 °C for 1 hour. Rice
husk was burn in furnace at 350 °
C for 1 hour. After that, the rice husk carbon is cooled, ground and sieved with a sieve size of 100 mesh (Zakir et al., 2012; Karyasa, 2014).
2. Extraction of Silica
Sample of rice husk carbon was added with 2, 4 and 8 M KOH with the ratio of the mass of carbon/volume of KOH 1:10. Samples were then heated to boil accompanied by stirring at the same pace for 1 hour and then filtered with Whatman filter paper number 42. The filtrate was removed and the results of filtration washed with aquadest, then dried in an oven at 110
°C for 1 hour. As a comparator, the carbon
was prepared without the addition of KOH. Furthermore, carbon characterized using XRF (Agung et al., 2013). Buchner funnel accompanied by washing with hot aquadest repeatedly until the pH became neutral, then dried in an oven at 110
°C for 1 hour. Furthermore, rice husk
activated carbon was cooled in a desiccator (Shofa, 2012).
4. Determination of The Surface Area 0.3 gram of activated carbon put into erlenmeyer, then added 25 mL of methylene blue 600 ppm solution, then stirred with a magnetic stirrer for 20 minutes, then filtered. The filtrate was measured absorbance at the maximum wavelength of
664.5 nm with a UV-Vis
spectrophotometer. The calibration curve or
a standard of methylene blue solution were made at a concentration of 0.5; 1, 2, 4, 8 and 16 ppm (Ramdja et al., 2008).
5. Electrodeposition of Cu
1 gram of silica free rice husk activated carbon and commercial activated carbon (as a comparator) dispersed in 50 mL of CuSO4.5H2O 200 ppm solution.
The mixture was stirred for 1 hour in a state saturated with argon gas. Activated carbon functionalised with CuSO4.5H2O and then
irradiated with ultrasonic waves for 6 hours. After that, filtered, washed with aquadest and dried at 110 °C for 1 hour. The result of
electrodeposition activated carbon with copper metals were characterized by XRD and XRF (Zakir et al., 2013).
6. Manufacture of Carbon Paste Electrodes
Body electrode was made by connecting copper and platinum wires using solder fumes. And then, it was inserted into the pipette and glued together by parafilm. Silica free rice husk activated carbon before and after electrodeposition of Cu mixed with paraffin wax with the ratio of mass of carbon/paraffin wax 1:1 and stirred until homogeneous using a spatula on a petri dish. After that, the carbon paste electrodes inserted into the body with pressed using a spatula in order to solidify (Vytras et al., 2009; Wachid and Setiarso, 2014).
7. Measurement of Specific Capacitance Specific capacitance of carbon paste electrodes measured by CV technique using Potentiostats EA161 with three electrodes, namely Pt electrode, Ag/AgCl electrode and carbon paste electrodes. Tests of electrode at the rate of scan 100 mV/s using an electrolyte solution of H2SO4 0.1 M thus
41 specific capacitance (Himmaty and
Endarko, 2013).
RESULTS AND DISCUSSION
Manufacture of Rice Husk Carbon
Preparation of raw material for made carbon was included washing and drying. Washing with water aims to remove impurities such as dust and sand attached to the hull as well as washing with technical HCl to lower levels of impurities such as metal oxides contained in rice husk. Drying under the sun and in the oven at 110 °C for 1
hour aims to remove moisture contained in rice husk (Andaka 2008; Mujiyanti et al., 2010).
Carbonization was a burning process certain raw materials at a temperature of carbonization process in this research was done in a furnace at 350 °C for 1 hour. This
temperature is the optimum temperature for the carbonization of rice husk because a temperature below 350 °C the
carbonization process has not been perfect, while temperatures above 350 °C was
already happening ashing. Carbon produced and then crushed and sieved to 100 mesh size sieve to produce a homogeneous carbon sized and smaller particle size and then the surface area of the carbon becomes larger. 2011). According to the Agung et al. (2013) the higher the concentration of KOH was used, the more silica was extracted. The reaction that occurs in the extraction process silica with KOH is following:
SiO2+ 2KOH → K2SiO3 + H2O
Activation
Activation was a process for removed hydrocarbon coating the surface of the carbon thereby increasing the porosity of carbon. In this research used chemical activation because it is more profitable than the physical activation. Mineral elements activator entered among the sidelines of a hexagonal carbon layer and separates the surface initially covered. Thus, when the heating was carried out, contaminants were compounds in the pores to be more easily dislodged. This causes the active surface area becomes larger (Koleangan and Wuntu 2008; Ramdja et al., 2008).
One of the properties activators associated with the quality of activated carbon was covalently character. Covalent character associated with covalent interactions between carbon and carbon activator to open the pores. Therefore, the activator used in this research was H3PO4.
The elements that constituting H3PO4 were
covalently bonded polar. Composed of carbon atoms that are covalently C form flat hexagonal structure with one atom C at each corner, it would be better interact with substances that have covalent character than the character of ionic substances (Koleangan and Wuntu, 2008).
Determination of The Surface Area
42 directly proportional to the surface area of the adsorbent.
Table 1. The surface area of the carbon.
Samples Surface area (m2/g)
Siliceous rice husk carbon 57.2833 Silica free rice husk carbon 180.5378 Silica free rice husk activated carbon 184.6074
Table 1 showed the extraction of silica and activation can increase the surface area of the carbon. In addition, the use of KOH in extracting silica was very effective because it can increase the surface area of the carbon was three times greater.
Electrodeposition of Cu
Electrodeposition was one method of attachment of the metal on the surface of a material that can be used to increase the value of specific capacitance. An increase in specific capacitance was done by utilizing electroactive species such as transition metals, namely copper (Cu). Cu chosen because it was not easily oxidized and had a fairly high reduction potential was 0.340 V thus the estimated Cu can act as an electron trap (Rahmawati et al., 2008). Electrodeposition process in this research using silica free rice husk activated carbon and commercial activated carbon as a comparable mixed with a solution of
CuSO4.5H2O and stirred with a magnetic
stirrer in a state saturated with argon gas, then treated with ultrasonic irradiation.
XRF analysis results after the electrodeposition process showed the Cu metal deposited on the surface of silica free rice husk activated carbon by 75.98% and 12.24% for commercial activated carbon in the form of CuO. The concentration of Cu deposited on the surface of silica free rice husk activated carbon more than the commercial activated carbon because the surface area of silica free rice husk activated carbon was greater than the commercial activated carbon which still contains silica.
The reaction is thought to occur during the electrodeposition process is following (Kasuma, 2012; Triastuti and Purwanto, 2012):
Cu2+
(aq) + 2OH-(aq)→ Cu(OH)2 (s)
Cu(OH)2 (s)→ CuO(s) + H2O (l)
Table 2. The content of the compound Cu(I).
Content of Compound CuO8TbW2 Cu6Mo5O18 CuO8SmW2 Cu2O2Sr
CuLaO8W2 CuFeO6Pb2Sr2 CuO8W2Y CuO2Sc
XRD analysis results in Table 2 after being processed using software Match! indicates that during the process of electrodeposition occurs redox reaction
43 Reduction: 2Cu2+ + 2e-→ 2Cu+
Oxidation: H2O → ½O2 + 2H+ + 2e-
Measurement of Specific Capacitance Capacitance was the ability of a capacitor to hold the charge of electrons or electrochemical energy. Specific capacitance measurements in this research using a Potentiostats EA161 with three
electrodes, namely Pt electrode, Ag/AgCl electrode and carbon paste electrodes. Pt electrode as an comparison electrode, Ag/AgCl electrode as a reference electrode and carbon paste electrodes as the working electrode (Himmaty and Endarko, 2013). Tests carried out at the scan rate 100 mV/s using an electrolyte solution of H2SO4
0.1 M.
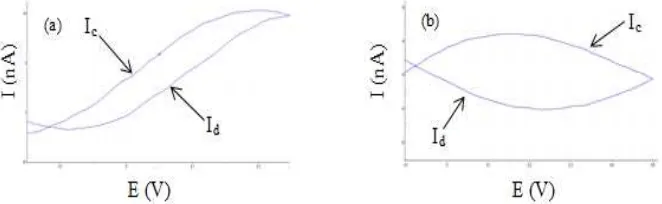
Figure 1. Voltammogram (a) before and (b) after electrodeposition.
Figure 1a showed the storage process takes place unstable. This was because the magnitude of the current density tends to increase with increasing potential difference and at the time of the discharge process, charge out too unstable (Suwandana and Susanti, 2015) while Figure 1b showed the storage process tend to be stable because of the current density is almost fixed with increasing potential difference and at the time of discharge, a charge that came out was almost stable. Shape of the curve obtained from the measurement results with the technique of
cyclic voltammetry in Figure 1 showed the specific capacitance value generated. Specific capacitance values were influenced by the charge and discharge currents.
Calculation of the current charge and
discharge specific capacitance was taken at the midpoint of each curve (Herniyanti et al., 2014; Rizki et al., 2015). Charge and
discharge current value of the electrode can be known from the voltammogram obtained and the specific capacitance values can be calculated using the following equation:
Cs =
Cs is the specific capacitance (nF/g),
Ic and Id respectively charge and discharge
currents (nA), v is the scan rate (V/s) and m is the mass of carbon on the electrode.
Table 3.Cyclic voltammetry data of carbon paste electrodes.
Samples Scan rate
(V/s) Mass (g) Ic (nA) Id (nA) Cs (nF/g) Before electrodeposition 0,1 0,12 1,820 -6,073 657,75
44 Table 3 showed the larger charge and
discharge currents, the greater the value of specific capacitance generated. The resulting value was still very low compared with the results of research conducted by Gao et al. (2015) regarding the EDLC material from rice husk with a specific capacitance of 367 F/g. This was because the method of making electrodes used are different and the amount of carbon used was too little (Rizki et al., 2015; Suwandana and Susanti, 2015).
CONCLUSION
The surface area of rice husk carbon before and after the extraction of silica and after activation with an activator H3PO4 in a
row were 57.2833 m2/g, 180.5378 m2/g and
184.6074 m2/g. Cu electrodeposition with
ultrasonic irradiation can increase the value of specific capacitance of silica free rice husk activated carbon. The value of specific capacitance of silica free rice husk activated carbon before and after electrodeposition of Cu with ultrasonic irradiation were 657.75 nF/g and 721.08 nF/g.
Andaka, G., 2008, Penurunan Kadar Tembaga pada Limbah Cair Industri Kerajinan
Perak dengan Presipitasi
menggunakan Natrium Hidroksida, J. Teknol., 1 (2), 127-134.
Ariyanto, T., Prasetyo, I. dan Rochmadi, 2012, Pengaruh Struktur Pori terhadap Kapasitansi Elektroda Superkapasitor yang dibuat dari Karbon Nanopori,
Reaktor, 14 (1), 25-32.
Dell, R.M. and Rand, D.A.J., 2001, Energy Storage-A Key Technology for Global
Energy Sustainability, J. Power
Sources, 100, 2-7.
Frackowiak, E. and Beguin, F., 2001, Carbon Materials for The Electrochemical Storage of Energy in Capacitors,
Carbon, 39, 937-950.
Gao, Y., Li, L., Jin, Y., Wang, Y., Yuan, C., Wei, Y., Chen, G., Ge, J. and Lu, H., 2015, Porous Carbon Made from Rice Husk as Electrode Material for
Electrochemical Double Layer
Capacitor, Appl. Energy, 153, 41-47. Herniyanti, S., Taer, E. dan Sugianto, 2014,
Pengaruh Aktivasi Karbon Dioksida pada Produksi karbon Aktif Monolit dari Kayu Karet, JOM FMIPA, 1 (2), 205-210.
Himmaty, I. dan Endarko, 2013, Pembuatan Elektroda dan Perancangan Sistem
Capacitive Deionization untuk
Mengurangi Kadar Garam pada
Larutan Sodium Clorida (NaCl),
Berkala Fisika, 16 (3), 67-74.
Karyasa, I.W., 2014, Pembuatan Ultra Fine Amorphous Silica (UFAS) dari Jerami
Padi dan Sekam Padi, J. Sains
Teknol., 3 (1), 263-274.
Kasuma, N.Y., 2012, Penggunaan Komposit ZnO-CuO yang disintesis secara
Sonochemistry yang digunakan
sebagai Katalis untuk Fotodegradasi
Metil Orange dan Zat Antibakteri,
Tesis diterbitkan, Program Studi
Kimia, Pascasarjana, Universitas
Andalas, Padang.
Koleangan, H.S.J. dan Wuntu, A.D., 2008, Kajian Stabilitas Termal dan Karakter Kovalen Zat Pengaktif pada Arang Aktif Limbah Gergajian Kayu Meranti (Shorea spp), Chem. Prog., 1 (1), 43-46.
Mahvi, A.H., Maleki, A. and Eslami, A., 2004, Potential of Rice Husk Ash for Phenol Removal in Aqueous Systems,
Am. J. Appl. Sci., 1 (4), 321-326. Mujiyanti, D.R., Nuryono dan Kunarti, E.S.,
45
diimobilisasi dengan
3-(Trimetoksisilil)-1-Propantiol, Sains Ter. Kim., 4 (2), 150-167.
Rahmawati, F., Wahyuningsih, S. dan Handayani, N., 2008, Modifikasi
Permukaan Lapis Tipis
Semikonduktor TiO2 Bersubstrat
Grafit dengan Elektrodeposisi Cu,
Indo. J. Chem., 8 (3), 331-336.
Ramdja, A.F., Halim, M. dan Handi, J., 2008, Pembuatan Karbon Aktif dari Pelepah Kelapa (Cocus nucifera), J. Tek. Kim.,
15 (2), 1-8.
Rizki, A., Taer, E. dan Rika, 2015,
Kebolehulangan (Reproducibility)
dalam Pembuatan Sel Superkapasitor dari Kayu Karet, JOM FMIPA, 2 (1), 93-101.
Shofa, 2012, Pembuatan Karbon Aktif
Berbahan Baku Ampas Tebu dengan
Aktivasi Kalium Hidroksida, Skirpsi
diterbitkan, Program Studi Teknik Kimia, Fakultas Teknik, Universitas Indonesia, Depok.
Sugiyono, A., Permana, A.D., Boedoyo, M.S. dan Adiarso, 2013, Outlook Energi
Indonesia 2013: Pengembangan
Energi dalam Mendukung Sektor Transportasi dan Industri Pengolahan
Mineral, Pusat Teknologi
Pengembangan Sumber Daya Energi, Jakarta.
Suhada, H., 2001, Fuel Cell sebagai Penghasil Energi Abad 21, J. Tek. Mesin, 3 (2), 92-100.
Surest, A.H., Kasih, J.A.F. dan Wisanti, A., 2008, Pengaruh Suhu, Konsentrasi Zat
Aktivator dan Waktu Aktivasi
terhadap Daya Serap Karbon Aktif dari Tempurung Kemiri, J. Tek. Kim.,
15 (2), 17-21.
Suslick, K.S., Hyeon, T. and Fang, M., 1996, Nanostructured Materials Generated
by High-Intensity Ultrasound:
Sonochemical Synthesis and Catalytic Studies, Chem. Mater., 8, 2172-2179. Suwandana, R.F. dan Susanti, D., 2015,
Analisis Pengaruh Massa Reduktor
Zinc terhadap Sifat Kapasitif
Superkapasitor Material Graphene, J. Tek. ITS, 4 (1), 95-100.
Triastuti, W.E. dan Purwanto, D.B., 2012,
Efek Penambahan Ion Tartrate
terhadap Elektrodeposisi Mn-Cu pada Pipa Baja Karbon, Kapal, 9 (3), 167-170.
Vytras, K., Svancara, I. and Metelka, R., 2009, Carbon Paste Electrodes in Electroanalytical Chemistry, J. Serb.
Chem. Soc., 74 (10), 1021-1033.
Wachid, M.R. dan Setiarso, P., 2014,
Pembuatan Elektroda Pasta Karbon Termodifikasi Bentonit untuk Analisis Ion Logam Tembaga(II) secara Cyclic
Voltammetry Stripping, Prosiding
Seminar Nasional Kimia, Universitas
Negeri Surabaya, Surabaya, 20
September.
Wei, X., Xiao, L., Jin, Z. and Ping, Z.S., 2011, Nanoporous Carbon Derived from Rice Husk for Electrochemical Capacitor Application, Adv. Mater. Res., 239-242. Santi, 2012, Pemanfaatan Energi
Gelombang Ultrasonik dalam