www.elsevier.com / locate / bres
Research report
Enhancement of whole cell calcium currents following transient
MCAO
a ,
*
b aClaus Bruehl
, Tobias Neumann-Haefelin , O.W. Witte
a
Heinrich-Heine-University, Department of Neurology, Geb.: 22.22 /TVA, 40225 Duesseldorf, Germany
b
Johann Wolfgang Goethe-University, Center for Neurology and Neurosurgery, Theodor-Stern-Kai 7, 60590 Frankfurt /Main, Germany
Accepted 29 August 2000
Abstract
Cerebral infarctions have been shown to cause widespread changes of neuronal excitability in non-infarcted tissue. Calcium currents are
21
major determinants of neuronal behavior, and pathological modulation of Ca -channels is known to lead to altered excitability states in a variety of paradigms. In the present study we addressed the question to what extent whole cell calcium currents are altered after middle cerebral artery occlusion (MCAO) in both the ipsi- and contralateral sensory cortex. Transient middle cerebral artery occlusion was induced for 1 h in rats using the intraluminal thread model. After 7 or 28 days survival, whole cell patch clamp studies were carried out on freshly isolated neurons of the ipsi- and contralateral sensory cortex, and high voltage activated (HVA) calcium currents were examined. In lesioned animals, we found a significant increase of calcium current amplitude and maximal conductance in the sensory cortex contralateral to the infarcts. This was paralleled by a prominent positive shift of the potential of half-maximal activation (V ) in theseh,a cells. Changes were long-lasting and at least stable for the following 28 days. These alterations were present in animals with lesions of moderate size, but not in those with massive infarction, and only in the cortex contralateral to the lesion. Following cortical infarctions, changes of calcium current properties are selectively observed in neurons contralateral to the lesion. At the behavioral level, compensatory mechanisms involving the unaffected hemisphere may induce this alteration of calcium current properties. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: Rat; Ischemia; Whole-cell patch-clamp; Ion currents
1. Introduction Most recently altered functional properties in remote brain areas have been demonstrated following the induc-Over the past few years several studies have reported a tion of ischemic brain lesions. In these studies a reduction variety of abnormalities in both peri-infarct and remote of GABAergic inhibition paralleled by increased firing brain regions following experimental stroke. These altera- rates were found ipsi- as well as contralateral to the lesion tions are of interest since they may have an impact on the site [3,30,31]. This finding has been coined ‘electrophysi-functional characteristics of the non-infarcted tissue, there- cal diaschisis’ though it is opposite to what has been by influencing neurological recovery. They include acute postulated by von Monakow in his classical description changes such as peri-infarct depolarizations [15] and [35], which describes diachitic phenomena merely as disturbances of calcium homeostasis [32], as well as long- decreases in activity.
lasting effects, e.g. inhibition of protein synthesis [24], Long-term excitability changes have been shown to be abnormal transmitter release, apoptosis [4,21,22,29,39] and associated with both an increase in NMDA-receptor-me-excitability changes of the neuronal network. diated responses and a reduction of GABAergic inhibition [22], leading to a net increase in neuronal excitability at the single neuron level. Presently, there are no reports *Corresponding author. Tel.:149-211-811-4437; fax: 1
49-211-811-available that focussed on whether changes of voltage-5007.
E-mail address: [email protected] (C. Bruehl). gated ion channels contribute to long-term excitability
changes following focal ischemia [26,27,29,31,39]. Cal- (n512) or 4 weeks (n53). The sham-procedure consisted cium influx into neurons, however, is known to occur of placing sutures around the right CCA and ECA without during ischemia through both voltage- and ligand-gated tightening them (sham group) while another group of
21 21
Ca -channels, and Ca -overload is believed to trigger animals (native group) received no treatment prior to the intracellular cascades that contribute to neuronal cell death. electrophysiological measurements.
This pathway is of importance during ischemia and affects CA1 pyramidal neurons were isolated enzymatically mainly the ischemic region itself, rather than remote brain [16] as described in detail previously [36]. Brains were regions. During the subacute to chronic stages, on the other rapidly removed from the skull and stored for 2 min in hand, a number of changes occur in remote brain regions ACSF chilled with ice. 400 mm-thick slices from the
21
that could potentially influence voltage gated Ca -chan- whole brain were cut with a vibratome (Leica VT1000S) at nels, including inflammatory reactions and free radical around 48C. Tissue pieces (approx. 1 by 2 mm) were cut formation, which are associated with an upregulation of from the corresponding areas of the sensory cortex (main-Interleukine-1 beta (IL1-b) [28], tumor necrosis factor ly: Par1) of either side of the brain (Fig. 1), or in the case alpha (TNF-a) [9,33] and nitric oxide (NO) [5]. of a total loss of the infarcted brain hemisphere, only from In this study we focussed on calcium current characteris- the contralateral sensory cortex. These tissue pieces were tics and their possible modulation in peri-infarct and incubated for 21 min at 328C in oxygenated dissociation remote brain regions at relatively late time points (1 and 4 solution (in mM: NaCl 120, KCl 5, CaCl2 1, MgCl2 1, weeks) following focal ischemia. To this purpose we used PIPES 20, D-glucose 25; pH:7.0) containing 1 mg / ml
the whole cell voltage patch clamp technique on freshly protease (Type XIV), they were finally washed several isolated cortical neurons from animals which were subject- times and kept in protease-free dissociation solution (198C) ed to transient middle cerebral artery occlusion (MCAO). until used. A subslice was dispersed in bath solution by trituration through Pasteur pipettes of decreasing diameter and brought into the perfusion chamber shortly before the
2. Materials and methods measurements.
Bath solution contained in all experiments (in mM): A total of 21 adult male Wistar SPF strain rats (weighing NaCl 110, KCl 5, CaCl 5, MgCl 1, 4-AP 5, TEACl 25,2 2
270–330 g) were used for the experiments. The animals in HEPES 10,D-glucose 25, and tetrodotoxin (TTX) 1 mM;
the experimental group (n59), the controls (n56) and the pH was set at 7.4. All chemicals were obtained from native group (n56) were kept on a 12 h-light cycle. All Sigma (USA) or Merck (Germany).
experimental procedures were conducted according to Only neurons (n5116) with a bright and smooth appear-protocols approved by the Governmental Animal Care ance and no visible organelles were selected for recording.
Committee. From the various groups of cortical neurons only
pyrami-The animals in the experimental group were subjected to dal like cells of medium size were used for the measure-transient middle cerebral artery occlusion (MCAO) using ments. Current amplitudes and capacity were homoge-the slightly modified intraluminal thread model described
by Koizumi et al. [17]. The spontaneously breathing animals were anesthetized with enflurane (1.5%) in a mixture of N O / O (70 / 30%) and body temperature was2 2
kept constant using a heating pad (36.560.58C). The right cervical carotid bifurcation was exposed and both the proximal common carotid artery (CCA) as well as the external carotid artery (ECA; before the origin of the occipital artery) were ligated and a 4-0 silicone-coated filament was introduced into the distal common carotid artery. The filament was then advanced by 16–17.5 mm (origin: carotid bifurcation) into the internal carotid artery (ICA) until a weak resistance was felt. The filament was secured in this position using a tight ligature around the CCA. After 1 h of occlusion, the neurological status of the animals was assessed using the score introduced by Bederson [1], next the intraluminal thread was removed and the CCA was permanently occluded. Reperfusion was not directly assessed, but arterial backflow through the
neously distributed throughout the measured cells, indicat- ms voltage steps to voltage levels between 270 and 130 ing that there was no dichotomy of membrane properties mV (Fig. 2), while the steady-state inactivation of the due to different cell classes. Currents were measured under calcium current was determined using a test depolarization whole-cell voltage-clamp conditions at room temperature to110 mV following a period of 3 s at potentials between (20–228C) using patch pipettes of 2–4 MV resistance 2105 and 0 mV (Fig. 5). For analysis, peak amplitudes of when filled with (in mM): CsF 110, MgCl 2, CaCl 0.5,2 2 the evoked currents were plotted against the membrane TEACl 20, EGTA 10, phosphocreatine 20 mM, MgATP 2, potential to examine voltage dependence of activation. The NaGTP 0.1, leupeptin 0.1, HEPES 10, and phosphoc- IV-curve of the current evoked from 270 mV was fitted reatine kinase 50 units / ml; pH was set at 7.3. Run-down with a combination of a Boltzmann activation function and phenomena were prevented by the ATP regenerating the Goldman–Hodgkin–Katz current equation (GHK-fit) system, thus the time-dependent decrease in current am- (for details see Kortekaas and Wadman [18]):
plitude never exceeded 5% within the recording period
21
[Ca ]
(7–8 min). Calcium currents were recorded with an in
]]] 2exp(2aV )
21 21
Axopatch 200 B amplifier (Axon Instruments) and stored 2aF P0
f
Cag
out [Ca ]out]]]]] ]]]]]]]
I(V )5V ,
on an Atari ST computer (1 kHz sample frequency) using V2V 1 exp(2aV )
h
]]
11exp
a custom made data acquisition system and software
S
VD
C
(‘Neuron’ by W.J. Wadman). Holding potential was kept at
2F
280 mV. Series resistance was compensated for more than witha 5] (1)
RT 90% and any capacitive transient was removed on-line. All
current traces were off-line corrected for aspecific linear where: P is the maximal permeability, V is the membrane
0
leak currents (reversal potential 0 mV) specified at holding potential, V is the potential of half maximal activation, V
h c
potential by small voltage-steps (25 and15 mV). is proportional to the slope of the curve at V , T is the
h
From the two calcium current components present in absolute temperature and F;R are the Faraday and gas cortical neurons, only high voltage activated currents constant resp.
(HVA) were evoked during this study, while low voltage Maximal conductance was calculated using: activated currents (LVA) were not investigated. The high
21
voltage activated calcium currents were activated by 200 gmax52aF P0
f
Cag
outThe kinetics of the calcium current were described with a from sham operated animals (n528). The maximal con-second order activation and a first order inactivation ductance in the native group was 213622 nS, which was
2
function (m h): not significantly different from the value derived from the
sham group (182629 nS). In both groups the calcium
2
t02t t02t
currents, evoked by an activation protocol (Fig. 2), had an
]] ]]
I(T )5Imax
F
12expS DG S D
t exp t (2)a i activation threshold around 240 mV and reached their
maximal amplitude between 0 and 10 mV (Fig. 3). The where: Imax is the current amplitude and the voltage
mean current amplitude at 10 mV, as evaluated by a fit with dependent activation (ta) and inactivation (ti) time
con-Eq. (1), was 21.3 nA in the native group and21.2 nA in stants following a voltage step at time t .0
cells from the sham operated animal group. Also the The voltage dependence of steady state inactivation can
potential of half-maximal activation (V ) and the slopeh,a
be estimated using the relation between the normalized
(V ) at the point Vc h,awere not different among both groups, peak amplitude of the current and the prepulse potential.
with V :h,a 2162 mV (sham), 261 mV (native) and V :c
This relation was well described by a Boltzmann function:
27.460.3 mV in the sham group and 27.560.2 mV in the
I(V ) 1 native group. Calcium currents in neurons (n510) from
]] ]]]]]
N(V )5 5 (3)
Imax V2Vh contralateral sensory cortex of infarcted animals (moderate
]]
11exp
S
D
Vc infarct) had a maximal current amplitude (around 15 mV)
which was by 10% larger (21.5 vs. 21.3 nA) than that where: N(V ) is the level of steady state inactivation measured in sham operated animals (Fig. 2). The current determined from the current amplitude I(V ) normalized to amplitude at a test potential of 10 mV was similar in both
Imax, V is the prepulse potential, V is the potential of halfh groups (1.4 vs. 1.3 nA). The increase in maximal
am-maximal inactivation and V is a factor proportional to thec plitude was paralleled by a significant increase in maximal
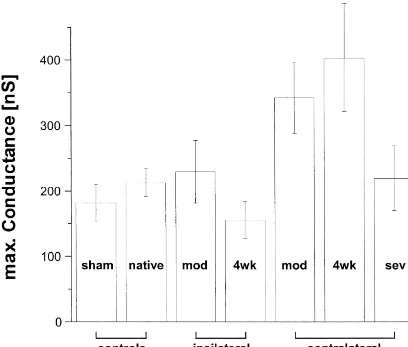
slope of the curve at V .h conductance (Fig. 4) to 342654 nS (P,0.05). Moreover,
Data are given as the mean6standard error of the mean the potential of half-maximal activation was shifted by 11 (S.E.M.). Statistical comparisons were made by two-way mV (Table 1) in positive direction to 1062 mV (P,0.01), ANOVA and Bonferroni test. Significance was achieved while the slope V was not different (27.660.3) when
c
with P,0.05. compared with the sham group (Fig. 3C). Similar changes
in calcium current properties were also found in neurons (n57) from animals, which were allowed to recover from
3. Results surgery for 4 weeks, which implies that these alterations were long-lasting. The maximal amplitude (21.5 nA) and Among six animals that were allowed to recover for 1 the maximal conductance (403682 nS; P,0.01) were still week, three animals had neocortical infarcts of moderate larger than in sham operated animals, and the half-maxi-size, i.e. at least half of the ipsilateral cortex was still mal potential of activation (V ) remained almost at theh,a
intact. The other three animals all had large cortical same positive value as in the group of animals with 1 week infarcts, where only relatively small neocortical regions of recovery (1364 mV, P,0.01; when compared with the (close to midline) remained intact. In the long-term group sham group). Again, the slope V was not altered and had ac
(n53), with a 4 week recovery period, all animals had (by value of 27.760.3 mV.
chance) cortical infarcts of moderate size. Infarction of the Calcium currents of neurons from the contralateral striatum was evident in all experimental animals. None of hemisphere (n513) in animals with severe infarction, the control animals (sham and native group; both n56) lacking wide parts of the ipsilateral sensory cortex, had showed signs of ischemic damage. current characteristics which equaled much more than Neurons were measured from both ipsi- and contralater- those from native or sham operated animals. The maximal al sensory cortex in the native and the sham group, as well conductance was not different (220650 nS) and the as in those animals with lesions of moderate size, while potential of half-maximal activation (262 mV) resembled only neurons from the contralateral sensory cortex were the values of the native or sham group. The same held true taken from the group with large infarcts. The groups will for the mean current amplitude measured at 10 mV (1.3 be referred to as: ipsi / mod (ipsilateral / moderate infarct); nA) and the slope V at the point of Vc h,a (28.360.4 mV). cont / mod (contralateral / moderate infarct); cont / sev (con- Alterations of calcium current characteristics in neurons tralateral / severe infarct). Neurons measured from animals from the ipsilateral sensory cortex of animals with moder-surviving 4 weeks will be referred to as: ipsi / 4wk ate infarction were only moderate, when compared to (ipsilateral / 4 weeks survival) and con / 4wk (contralateral / neurons from the contralateral side, and showed no
signifi-4 weeks survival). cant difference to the sham operated animal group. The
group, Vh,a was slightly more positive in this group (562 mV) and the slope V at the point of Vc h,a was in the same range with 27.060.2 mV. As described above, neurons from the contralateral hemisphere showed no additional, time-related changes when the groups with 1 week or with 4 weeks recovery were compared. In contrast to this finding neurons from the infarcted brain hemisphere, in animals with 4 week recovery, tended to show smaller maximal conductances and smaller mean current am-plitudes, than neurons from animals with 1 week recovery. The mean maximal conductance amounted to 156629 nS and the mean current amplitude, measured at 10 mV, was lowered to 21.0 nA. The potential of half-maximal activation Vh,a was similar to the sham or native group (062 mV), but the slope V was slightly steeper with ac
value of 26.760.3 mV.
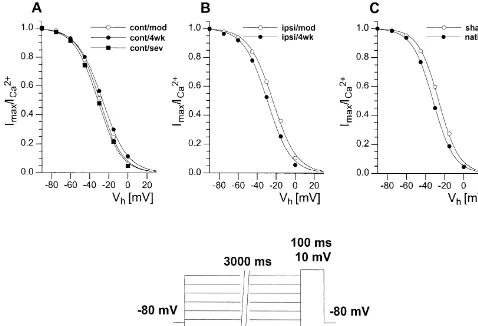
The time constants of activation and inactivation were fitted with Eq. (2) for five different voltages (220,210, 0, 10, 20 mV). This evaluation revealed no significant differences among all groups compared. Since time con-stants at the given voltages did not differ, they were lumped together for each group giving values for the time constant of activation of 21.2 and 70–80 ms for inactiva-tion (for details see Table 1). Only neurons from animals with a severe infarction showed a significantly faster activation (20.960.1 ms; P,0.05) and inactivation (26167 ms; n.s.). The steady state inactivation of calcium currents can be evaluated by holding the cell for 3 s at stepwise increased potentials, which are followed by a fixed test-pulse to 10 mV.
When the amplitudes of the evoked calcium currents were normalized to the maximal current amplitude and plotted against the pre-potentials, the resulting current voltage relationship could well be described by Eq. (3). This Boltzmann fit delivers the half-maximal potential of inactivation Vh,i and the slope of the Boltzmann curve Vc,i
at this point. This evaluation showed a highly significant, but unique, increase in the slope of the Boltzmann curve for all groups with infarction (range of V :c,i
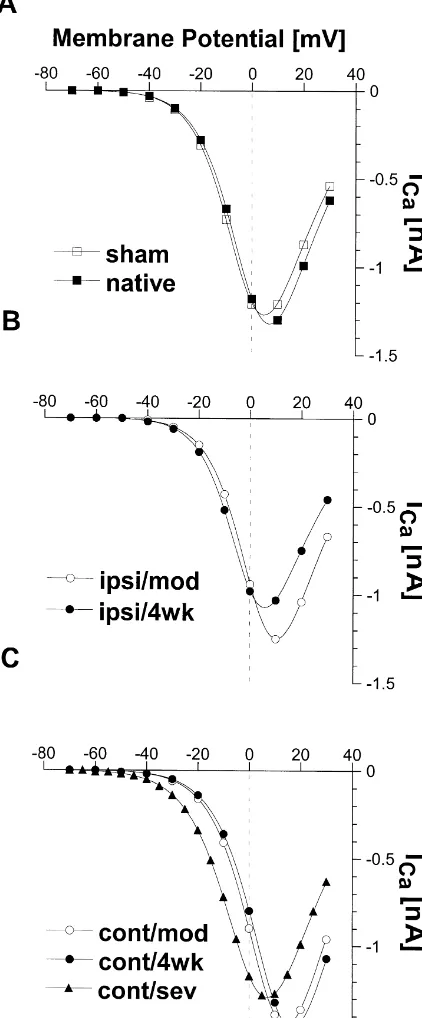
10.760.7 . . . 13.861.7 mV vs. 9.360.3 mV in sham; for detail see Table 1). Furthermore, there was a tendency for the potential of half-maximal inactivation Vh,i to be more positive (even in the sham group) than in native animals (range V :h,i 23063 . . .22663 mV vs. 23362 mV in Fig. 3. IV-curves from different animal groups and infarct characteristics.
native). The IV-curves were computed with Eq. (1) and multiplied with the
appropriate inactivation factor derived from Eq. (3) at holding potential (280 mV). (A) Neurons from sham operated and native (no operation)
animals. (B) Neurons from the ipsilateral side at P7 and P28 (moderate 4. Discussion
lesions) C: from the contralateral side at P7; P28 (moderate infarct) and with a severe infarction. Note the significant shift of the IV-curves in C
The present study demonstrates two distinct alterations for P7 and P28, while the IV-relationship in animals with a severe
of whole cell calcium current characteristics from neurons infarction is not different to sham operated animals.
Fig. 4. Maximal calcium conductances as were observed within the different animal groups. The conductance was significantly increased (as compared to sham operated animals) only in animals with a moderate infarct.
Fig. 5. Normalized current amplitudes were plotted against the selected pre-potentials (see voltage protocol), giving the Boltzmann relation for the steady state inactivation. No relevant differences in the halfmaximal potential of inactivation (V ) were found within the animal groups tested. Neverthelessh,i
infarcted animals had a somewhat steeper slope of the curve at the point of V .h,i
gating properties or by the formation of additional chan- rat hippocampus, additional calcium channels are formed. nels, thereby increasing the channel density of the neuronal This process was associated with an up-regulation of the membrane. It has been reported that during the establish- expression of mRNA encoding for different subunits of ment of a kindled focus — thus caused by repeated those channels [14]. It is conceivable that such a mecha-stimulation, i.e. in an activity-dependent manner — within nism also accounts for the increase in maximal
conduct-Table 1
Cont / mod Cont / 4wk Cont / sev Ipsi / mod Ipsi / 4wk Sham Native
Animals 3 2 3 3 3 6 6
Cells 10 7 13 12 13 28 28
Capacitance [pF] 8.660.6 6.460.7 6.660.7 9.260.6 9.360.8 7.960.6 6.560.4 Impedance [MV] 656693 758687 6716144 533685 7306161 698681 759679
Activation
ICa [nA] 21.4 21.3 21.3 21.2 21.0 21.2 21.3
Max. G [nS] 342654* 403682** 220650 229648 156629 182629 213622
Vh,a [mV] 1062** 1364** 262 562 062 2162 261
Vc,a [mV] 27.660.3 27.760.3 28.360.4 27.060.2 26.760.3 27.460.3 27.560.2
Tact. [ms] 21.160.1 21.460.1 20.960.1* 21.360.1 21.360.1 21.260.1 21.360.1
Inactivation
Vh,i [mV] 22862 22863 23063 22663 23063 22762 23362
Vc,i [mV] 1161 1462** 1161 1161 1261* 960.3 1060.4
Tina [ms] 27667 27067 26167 27566 27767 28167 28068
ance in neurons of the contralateral and not infarcted brain calcium channels in neurons from the peri-infarct region is hemisphere. Surprisingly, alterations of current characteris- prevented by those mechanisms, although an up-regulation tics were strictly dependent on the size of neocortical of mRNAs encoding for calcium channel subunits may infarcts. Thus, in neurons from animals with a severe occur within these cells like in contralateral neurons. To infarction no increase of calcium conductance and no prove this assumption further investigations are necessary, positive shift of the potential of half-maximal activation using experimental protocols which prevent spreading (V ) was found. This points towards a mediation of theh,a depression and reduce post-infarct edema formation. The calcium current changes by behavioral activity of the presumably use-dependent increase of the calcium currents animals. Animals with moderate infarct size show rela- in the hemisphere contralateral to the lesion suggests that tively small behavioral deficits [1], and have the ability to an enhancement of the use of the affected limb e.g. by compensate for the deficit of the affected forepaw. Infarc- physical therapy might favor processes of plasticity and ted animals rely primarily on the unaffected ipsilateral thus increase the recovery from the lesion. It should be forepaw resulting in a preferential use of this limb. This noted, however, that a forced use of the affected limb e.g. was paralleled by an overgrowth of dendrites of layer V due to casting of the healthy limb might exert a detrimental pyramidal neurons in the non-infarcted hemisphere. The effect if started too early following lesion induction [19]. sprouting of the dendrites was use-dependent and could be Calcium plays a major role in processes of brain plasticity, prevented by an immobilization of the unimpaired limb and it is conceivable that the alteration of the calcium [19]. It is therefore conceivable that the modulation of currents is not only a consequence of adaptation, but may calcium currents may be initiated by qualitatively similar also enhance such processes of brain plasticity [6]. There-mechanisms. The signaling cascade responsible for these fore, the use of substances which modulate calcium alterations of calcium currents is not known. The liberation currents like calcium antagonists as given to many patients of trophic factors is modulated by activity, and trophic might also impair the brain plasticity. The criteria used in factors have been described to alter neuronal calcium this study discriminating the neurons taken for the mea-current [2,20]. They are therefore possible candidates surements surely covers the risk that different neuron which may induce the observed calcium current changes. classes with different membrane properties are pooled Rats, suffering from severe infarction, not only have a together leading to inaccurate results and false differences higher mortality (up to 30%) but show, in contrast, between the animal groups compared. Moreover neuronal strongly reduced spontaneous activity and clumsy move- necrosis occurring within the peri-infarct region [10,23,25] ments (unpublished observation). It is therefore conceiv- may have induced an unintended pre-selection of cells, able that the use-dependent modulation, as is to be with different properties in whole cell calcium currents. assumed in animals with a moderate infarction, is absent in Nevertheless, parameters describing the properties of the animals with severe brain damage due to their reduced membrane of the concerned neurons, like capacitance and mobility. Interestingly, neurons of the ipsilateral infarcted impedance were uniformly distributed and showed no sign cortex displayed calcium conductances that were close to for a dichotomy between the selected neurons. Therefore, normal. This finding is in line with a recent study it is unlikely that an incorrect selection of neurons may demonstrating reduced depolarization induced calcium have contributed to the observed differences in calcium accumulations in hippocampal neurons after transient current properties. We used freshly isolated neurons, since vessel occlusion [7]. This reduction was not accompanied the experimental conditions can be tightly controlled and by an enhanced intracellular calcium buffering and was currents can be measured without space-clamp problems therefore attributed to a reduction in calcium permeability due to the compact appearance of cells. Nevertheless, the through voltage gated calcium currents. In a recent report information collected with this configuration is clearly concerning the expression of mRNA encoding for thea1- focussed on the single cell level, especially the somatic anda2-subunits of GABA -receptors following the induc-A part of the cells, since most of the synaptic mass has been tion of a focal cortical lesions [26,27], it was found that a disrupted by the dissociation process. Despite this limita-partial translation block of that mRNA occurs. It was tion the parameters measured under these conditions are a speculated that spreading depression or edema formation, good indication of what will be seen in cells within their both frequently observed after cortical insults, might natural network environment [8,36,38].
clamping from adult mammalian central nervous systems, J.
Neuro-Acknowledgements
sci. Methods 16 (1986) 227–238.
[17] J. Koizumi, Y. Yoshida, T. Nakazawa, G. Ooneda, Experimental The authors thank W.J. Wadman for helpful discussion studies of ischemic brain edema, I: a new experimental model of on this paper, and D. Steinhoff and M. Srejic for perfect cerebral embolism in rats in which recirculation can be introduced in technical assistance. The investigations were supported by the ischemic area, Jpn. J. Stroke 8 (1986) 1–8.
[18] P. Kortekaas, W.J. Wadman, Development of HVA and LVA calcium SFB 194 B2.
currents in pyramidal CA1 neurons in the hippocampus of the rat, Brain Res. Dev. Brain Res. 101 (1997) 139–147.
[19] D.A. Kozlowski, D.C. James, T. Schallert, Use-dependent exaggera-tion of neuronal injury after unilateral sensorimotor cortex lesions, J.
References
Neurosci. 16 (1996) 4776–4786.
[20] E.S. Levine, C.F. Dreyfus, I.B. Black, M.R. Plummer, Differential [1] J.B. Bederson, L.H. Pitts, M. Tsuji, M.C. Nishimura, R.L. Davis, H. effects of NGF and BDNF on voltage-gated calcium currents in Bartkowski, Rat middle cerebral artery occlusion: evaluation of the embryonic basal forebrain neurons, J. Neurosci. 15 (1995) 3084– model and development of a neurologic examination, Stroke 17 3091.
(1986) 472–476. [21] Y. Li, M. Chopp, N. Jiang, F. Yao, C. Zaloga, Temporal profile of in [2] A. Bouron, C. Becker, H. Porzig, Functional expression of voltage- situ DNA fragmentation after transient middle cerebral artery gated Na1 and Ca21 channels during neuronal differentiation of occlusion in the rat, J. Cereb. Blood Flow Metab. 15 (1995) PC12 cells with nerve growth factor or forskolin, Naunyn 389–397.
Schmiedebergs Arch. Pharmacol. 359 (1999) 370–377. [22] H.J. Luhmann, Ischemia and lesion induced imbalances in cortical [3] I. Buchkremer-Ratzmann, M. August, G. Hagemann, O.W. Witte, function, Prog. Neurobiol. 48 (1996) 131–166.
Epileptiform discharges to extracellular stimuli in rat neocortical [23] G. Mies, L.M. Auer, G. Ebhardt, H. Traupe, W.D. Heiss, Flow and slices after photothrombotic infarction, J. Neurol. Sci. 156 (1998) neuronal density in tissue surrounding chronic infarction, Stroke 14
133–137. (1983) 22–27.
[4] C. Charriaut-Marlangue, I. Margaill, A. Represa, T. Popovici, M. [24] G. Mies, S. Ishimaru, Y. Xie, K. Seo, K.A. Hossmann, Ischemic Plotkine, Y. Ben-Ari, Apoptosis and necrosis after reversible focal thresholds of cerebral protein synthesis and energy state following ischemia: an in situ DNA fragmentation analysis, J. Cereb. Blood middle cerebral artery occlusion in rat, J. Cereb. Blood Flow Metab.
Flow Metab. 16 (1996) 186–194. 11 (1991) 753–761.
21
[5] C. Chen, G.G. Schofield, Nitric oxide donors enhanced Ca [25] M. Nedergaard, Mechanisms of brain damage in focal cerebral
21
currents and blocked noradrenaline-induced Ca current inhibition ischemia, Acta Neurol. Scand. 77 (1988) 81–101.
in rat sympathetic neurons, J. Physiol. (Lond.) 482 (1995) 521–531. [26] T. Neumann-Haefelin, F. Bosse, C. Redecker, M.I. HW, O.W. Witte, [6] J.A. Connor, J. Petrozzino, L.D. Pozzo-Miller, S. Otani, Calcium Upregulation of GABAA-receptor alpha1- and alpha2-subunit signals in long-term potentiation and long-term depression, Can. J. mRNAs following ischemic cortical lesions in rats, Brain Res. 816 Physiol Pharmacol. 77 (1999) 722–734. (1999) 234–237 [In Process Citation].
[7] J.A. Connor, S. Razani-Boroujerdi, A.C. Greenwood, R.J. Cormier, [27] T. Neumann-Haefelin, J.F. Staiger, C. Redecker, K. Zilles, J.M.
21
J.J. Petrozzino, R.C. Lin, Reduced voltage-dependent Ca signaling Fritschy, H. Mohler, O.W. Witte, Immunohistochemical evidence for in CA1 neurons after brief ischemia in gerbils, J. Neurophysiol. 81 dysregulation of the GABAergic system ipsilateral to photochemi-(1999) 299–306. cally induced cortical infarcts in rats, Neuroscience 87 (1998) [8] G.C. Faas, M. Vreugdenhil, W.J. Wadman, Calcium currents in 871–879.
pyramidal CA1 neurons in vitro after kindling epileptogenesis in the [28] C.R. Plata-Salaman, J.M. French-Mullen, Interleukin-1 beta inhibits
21
hippocampus of the rat, Neuroscience 75 (1996) 57–67. Ca channel currents in hippocampal neurons through protein [9] K. Furukawa, M.P. Mattson, The transcription factor NF-kappaB kinase C, Eur. J. Pharmacol. 266 (1994) 1–10.
mediates increases in calcium currents and decreases in NMDA- and [29] M. Qu, T. Mittmann, H.J. Luhmann, A. Schleicher, K. Zilles, AMPA / kainate-induced currents induced by tumor necrosis factor- Long-term changes of ionotropic glutamate and GABA receptors alpha in hippocampal neurons, J. Neurochem. 70 (1998) 1876– after unilateral permanent focal cerebral ischemia in the mouse
1886. brain, Neuroscience 85 (1998) 29–43.
[10] J.H. Garcia, N.A. Lassen, C. Weiller, B. Sperling, J. Nakagawara, [30] S. Reinecke, M. Lutzenburg, G. Hagemann, C. Bruehl, T. Neumann-Ischemic stroke and incomplete infarction, Stroke 27 (1996) 761– Haefelin, O.W. Witte, Electrophysiological transcortical diaschisis
765. after middle cerebral artery occlusion (MCAO) in rats, Neurosci.
[11] A. Ghosh, M.E. Greenberg, Calcium signaling in neurons: molecular Lett. 261 (1999) 85–88.
mechanisms and cellular consequences, Science 268 (1995) 239– [31] K. Schiene, C. Bruehl, K. Zilles, M. Qu, G. Hagemann, M.
247. Kraemer, O.W. Witte, Neuronal hyperexcitability and reduction of
[12] U.F. Greber, L. Gerace, Depletion of calcium from the lumen of GABAA-receptor expression in the surround of cerebral photo-endoplasmic reticulum reversibly inhibits passive diffusion and thrombosis, J. Cereb. Blood Flow Metab. 16 (1996) 906–914. signal-mediated transport into the nucleus, J. Cell Biol. 128 (1995) [32] B.K. Siesjo, F. Bengtsson, Calcium fluxes, calcium antagonists, and
5–14. calcium-related pathology in brain ischemia, hypoglycemia, and
[13] G.E. Hardingham, S. Chawla, C.M. Johnson, H. Bading, Distinct spreading depression: a unifying hypothesis, J. Cereb. Blood Flow functions of nuclear and cytoplasmic calcium in the control of gene Metab. 9 (1989) 127–140.
21 expression, Nature 385 (1997) 260–265. [33] B. Soliven, J. Albert, Tumor necrosis factor modulates Ca [14] H. Hendriksen, W. Kamphuis, and d. S. F. Lopes, Changes in currents in cultured sympathetic neurons, J. Neurosci. 12 (1992)
voltage-dependent calcium channel alpha1-subunit mRNA levels in 2665–2671.
the kindling model of epileptogenesis, Brain Res. Mol. Brain Res. [34] L. Stehno-Bittel, A. Luckhoff, D.E. Clapham, Calcium release from 50 (1997) 257–266. the nucleus by InsP3 receptor channels, Neuron 14 (1995) 163–167. [15] K.A. Hossmann, Periinfarct depolarizations, Cerebrovasc. Brain [35] C. von Monakow, Die Lokalisation im Großhirn und der Abbau der
rat hippocampal CA1 neurons induced by kindling epileptogenesis, [38] T.R. Werkman, S. Van der Linden, M. Joels, Corticosteroid effects Neuroscience 49 (1992) 373–381. on sodium and calcium currents in acutely dissociated rat CA1 [37] M. Vreugdenhil, W.J. Wadman, Kindling-induced long-lasting en- hippocampal neurons, Neuroscience 78 (1997) 663–672.
hancement of calcium current in hippocampal CA1 area of the rat: [39] O.W. Witte, G. Stoll, Delayed and remote effects of focal cortical relation to calcium-dependent inactivation, Neuroscience 59 (1994) infarctions: secondary damage and reactive plasticity, Adv. Neurol.