www.elsevier.nlrlocateraqua-online
Demonstration of residual antibacterial activity in
plasma of vaccinated Penaeus
Õ
annamei
A.O. Alabi
), J.W. Latchford, D.A. Jones
School of Ocean Sciences, UniÕersity of Wales, Bangor, Menai Bridge, LL57 5EY, UK
Received 4 August 1998 ; received in revised form 11 December 1999 ; accepted 26 December 1999
Abstract
Plasma from naıve Penaeus¨ Õannamei juveniles lacked antibacterial factors and significantly enhanced the growth of Escherichia coli strain XL1-Blue MRFX compared with sea water
Ž .
complex SWC nutrient medium. In contrast, plasma from prawns previously injected with both live, non pathogenic Vibrio harÕeyi strain DPEX and formalin-killed cells of V. harÕeyi strains
Ž .
BP04, DPEX, E. coli and sterile shrimp salt solution SSS , inhibited bacterial growth. Antibacte-rial activity appeared within 6 h and was detectable until 7 days after ‘‘ vaccine’’ administration. Lysozyme activity was not detected in plasma from vaccinated prawns. Both in vitro and in vivo bacterial killing were facilitated by vaccine administration. Activation of the prophenoloxidase ŽproPO system was independent of bacterial virulence.. q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Immune system; Prophenoloxidase system; Antibacterial activity
1. Introduction
Most of the bacteria causing diseases in cultured prawns from farms and hatcheries
Ž
have been reported as part of the normal prawn microflora Vanderzant et al., 1971;
)Corresponding author. Current address: Island Scallops Ltd., 5552 West Island Highway, Qualicum Beach, BC, Canada, V9K 2C8. Tel.:q1-250-757-9811; fax:q1-250-757-8370.
Ž .
E-mail address: [email protected] A.O. Alabi .
0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
Yasuda and Kitao, 1980; Ruangpan, 1982; Lightner, 1985, 1988, 1992; Lavilla-Pitogo et al., 1990; Owens et al., 1992; Mohney et al., 1994; Prayitno, 1994; Prayitno and
.
Latchford, 1995 . Increased susceptibility to disease found in farmed prawns occurs as a consequence of impairment of the immune defense mechanisms resulting from the physical and environmental abuse characteristic of many aquaculture systems. With the growing importance of disease in prawn culture systems, a detailed knowledge of the functioning of the defense system of prawns is essential in disease control and treatment. Immunostimulation of prawns using products of microbial origin has been reported to
Ž
enhance resistance against disease causing pathogens Adams, 1991; Prayitno, 1994;
.
Latchford et al., 1995; Sung et al., 1996; Alabi et al., 1999 . Although both humoral and
Ž
cellular factors have been implicated in these resistance procedures Itami et al., 1989;
.
Adams, 1991; Vargas-Albores, 1995; Vargas-Albores et al., 1993b; Sung et al., 1996 , research has been primarily focused on processes of cellular immunity especially with
Ž
regard to generation of reactive oxygen species Song and Hsieh, 1994; Sung et al.,
. Ž .
1996 , phagocytosis Hose and Martin, 1989; Vargas-Albores, 1995 , encapsulation
ŽHose and Martin, 1989 , and multimeric systems such as the coagulation system. ŽOmori et al., 1989. and the prophenoloxidase proPOŽ . enzyme activation system ŽSoderhall, 1982; Soderhall and Unestam, 1979; Smith and Soderhall, 1983; Ashida and
¨
¨
¨
¨
¨
¨
Soderhall, 1984; Hose et al., 1987; Chisholm and Smith, 1992; Vargas-Albores, 1995;
¨
¨
.
Vargas-Albores et al., 1993a; Sung et al., 1996 .
Apart from these immediate responses to microbial infection, longer lasting antimi-crobial factors represent another component of the invertebrate immune response
ŽRatcliff et al., 1985 . Lysozyme-like activity has already been demonstrated in the. Ž
haemolymph of several insects Hultmark et al., 1980; Engstrom et al., 1984; Kaaya et
¨
. Ž
al., 1987 and bivalves Cheng and Rodrick, 1974; Foley and Cheng, 1977; Cheng et al., 1980 . Results of attempts to demonstrate lysozyme activity in penaeid prawns have so
Ž .
far been equivocal. Some authors Guzman et al., 1993 have reported haemolytic
´
Ž
activity in cell free haemolymph of PenaeusÕannamei while other researchers Noga et
.
al., 1996 have been unsuccessful in demonstrating such with P. setifeus.
Antibacterial factors with properties clearly different from those of lysozyme have
Ž
also been reported in insects Faye and Wyatt, 1980; Yoshida and Ashida, 1986; Boman
.
and Hultmark, 1987 and a few studies have described the acquisition of a longer lasting
Ž
‘‘immune’’ state following exposure of prawns to killed Vibrio sp. cells Itami et al.,
. Ž
1989; Adams, 1991; Prayitno, 1994; Alabi et al., 1999 and immunostimulants Sung et
.
al., 1996 . However, the significance of these long-term resistance factors to overall disease resistance has not been shown yet.
2. Materials and methods
2.1. Prawns used
P.Õannamei post-larvae were obtained from Centro de Investigacion in Alimentacion
Ž .
y desarrollo CIAD , Mazatlan, Mexico, and grown in the School of Ocean Sciences, University of Wales, Bangor, Menai Bridge, to a mean weight of 9.41 g in a recirculatory raceway at 28"28C with 31–33‰ salinity. To avoid inadvertent stimula-tion of prawn immune systems by additives in formulated diets, they were fed only cut squid and lugworms. Only apparently healthy, intermoult animals were used.
2.2. Bacterial species used andÕaccine preparation
In both in vitro and in vivo bacterial killing assays, and for stimulation of the proPO system of naıve P.
¨
Õannamei juveniles, non-pathogenic Vibrio harÕeyi strain DPEX, and pathogenic V. harÕeyi strains BP03, BP04, BP05, and IN7, isolated from bothŽ
healthy and diseased P. monodon in Indonesia Prayitno, 1994; Prayitno and Latchford,
.
1995 were obtained from bacterial collection of the School of Ocean Sciences. All
Ž .
Vibrio sp. used were maintained on thiosulphate citrate bile sucrose TCBS agar plates
Ž . Ž
at 48C, and cultured in modified sea water complex SWC Reichelt and Baumann,
. X
1973 at 288C for 24 h before use. In addition Escherichia coli strain XL1-Blue MRF
Ž . Ž . y1
Bullock et al., 1987 Stratagene , resistant to 15 mg ml tetracycline and with a recombinant plasmid providing resistance to 100 mg mly1 ampicillin, was maintained
Ž .
on Luria-Bertani LB slants and was grown in SWC at 378C for 24 h before use.
Ž .
Aeromonas hydrophila strain PM from diseased P. monodon Prayitno, 1994 were stored on SWC agar plates at 48C and grown in SWC medium. Micrococcus
lysodeikti-( ) Ž .
cus luteus obtained as a lyophilised isolate from Sigma , was stored at y208C and
similarly cultured in SWC medium at 288C for 24 h before use.
The concentrations of bacterial cells growing after 24 h was estimated for all the bacterial species used by spreading tenfold serial dilutions of 1 ml aliquots of bacterial suspensions on SWC agar plates. Natural seawater was adjusted to 25‰ salinity, with
Ž .
distilled water autoclaved in 9-ml volumes 1218C; 15 min; 15 psi and cooled to ambient temperatures before using as dilution medium. The number of colony forming
Ž .
units cfu of bacteria growing on three replicate agar plates per unit volume of added bacterial suspension was determined for each experimental run. Incubation periods used were 24 and 48 h for all species and temperatures used were 378C for E. coli and 288C for all other species used.
Vaccines were made by exposing log-phase cultures of the relevant bacterium to 0.5% formalin and incubating at 258C with shaking for 12 h. The formalin-killed bacteria were centrifuged at 7000=g for 2 min. The resulting pellet was rinsed three times and re-suspended in sterile 25‰ saline to the required concentrations before use.
2.3. Haemolymph extraction and separation
ŽSSS. ŽVargas-Albores et al., 1993a . The procedures were carried out on ice and.
subsequent manipulations were performed at 48C. The withdrawn blood was centrifuged in an Eppendorf microfuge at 13 000=g for 2 min. The supernatant fluid was passed
Ž .
through a 0.45-mm filter Gelman and stored at y1778C for no more than 4 weeks before use.
2.4. Assays of proPO actiÕity
To obtain the inactive proPO enzyme containing granules from the haemocytes, the haemocyte pellets obtained after decanting the plasma supernatant fluids were resus-pended in sterile SSS and washed again. The haemocytes were resusresus-pended in 3 ml of
Ž .
sodium cacodylate CAC buffer and homogenised on ice using a glass piston ho-mogeniser. The resulting suspension was centrifuged for 20 min at 30 000=g and 48C
Ž .
to pellet the cell debris. The supernatant haemocyte lysate HLS was the enzyme source and was stored aty1778C for no more than 4 weeks before use.
Incubation procedures were performed in 96 well microtitre plates with 50ml of HLS mixed with 50 ml of each of the gram negative bacteria listed above which had been
Ž y1.
formalin-killed, lyophilised, and resuspended 2 mg ml in CAC buffer. Blanks were
Ž . Ž
with CAC buffer alone and controls consisted of zymosan Sigma suspended 2 mg y1.
ml in CAC bufferq50 ml HLS. Additional controls against spontaneous oxidation of the substrate alone consisted of CAC buffer and zymosan. Plasma controls were included to ensure that no accidental activation of the HLS occurred during the assay procedure. Batches with plasma controls showing proPO activity were discarded. Each experimental treatment had three replicates. The samples were incubated at 208C for 65
Ž . Ž
min, after which 100 ml of L-3,4-dihydroxyphenyl-alanine L-Dopa dissolved 3 mg
y1.
ml in CAC buffer was added to each well and absorbance values at 490 nm were
Ž
taken at 5 min intervals over 20 min in a microplate reader Biokinetics Reader EL-340,
.
BIO-TEK Instruments . One unit of proPO activity was expressed as an increase in absorbance of 0.001 mg proteiny1 miny1.
2.5. Bacterial growth in plasma from naı
¨
Õe prawnsTo assess the bacterial growth in plasma from naıve juveniles, 25
¨
ml of E. coliw Ž . 5 x
2.6. Assays of antibacterial and lysozyme actiÕity in cell free haemolymph ofÕaccinated
P.Õannamei
2.6.1. Effects of addition of lysozyme to E. coli suspensions
Several methods of determining antibacterial activity in test samples may fail to reveal a biological effect because the concentration of active ingredients present is too
Ž .
low to operate with the minimum number of bacteria needed. Sung et al. 1996 detected antibacterial activity in stimulated P. monodon only after concentrating the plasma by a factor of three. In our present study, lysozyme was added to increase the sensitivity of the bacteria used in antibacterial assays to possible antibacterial factors present in the plasma. A lysozyme to sample ratio needed to be found which, while sensitizing the bacterial cells to antibacterial action, will not, by itself, cause a decline in bacterial numbers. Hence, preliminary tests were performed to check the effects of the addition of
y1 Ž .
1.24 mg ml of egg white lysozyme Sigma to E. coli suspensions. Twenty five
Ž 5 .
microlitres of E. coli suspension mean TVCs6.91=10 cfu was incubated with 25
Ž y1 .
ml of lysozyme 11.0 mg ml in sterile SSS , 25ml of SWC, and 125ml of SSS. The controls consisted of 25ml of E. coli suspension, 25ml of SWC, and 150ml of SSS. Each treatment had three replicates. The samples were incubated in sterile 1.5 ml Eppendorf tubes and incubated with shaking at 318C for 3 h, after which, the bacterial concentrations in each tube were estimated as described above.
2.7. Assays of antibacterial actiÕity
Forty eight-hour cultures of non-pathogenic V. harÕeyi strain DPEX were centrifuged
Ž .
at 11 000=g 5 min, 258C . The bacterial pellet was rinsed thrice and resuspended in
Ž . 5
sterile SSS pHs7.3 . A total of 100ml of the final suspension containing 2.22=10 – 3.00=105 cells at room temperature were injected into the ventral sinus of the first
abdominal segment of each of three replicate prawns. Positive controls were injected with 100ml of sterile SSS and negative controls were uninjected. Test prawns were kept
Ž
three each in net covered baskets with dimensions of 31.5=21.6=20.1 cm length= .
breadth=height suspended in a P. Õannamei rearing raceway as described above. Haemolymph was removed at 0, 6, 12, 24 and 48 h and 4, 7, 14 and 21 days and cell-free plasma was obtained as described.
Ž .
Log-phase E. coli and lyophilised M. luteus grown in SWC pHs6.4 were
Ž .
harvested by centrifugation 11 000=g; 2 min; 258C and washed once in sterile SSS in
Ž .
brought the final lysozyme concentration in the mixtures to 1.24 mg mly1. The incubation treatments were repeated in triplicate at each time interval. Initial total cell counts of the E. coli suspension was 6.91=105"1.90=104 cfu and antibacterial activity was regarded as a decrease in TVC compared to the initial TVC and ratio of
Ž .
change in TVC R was calculated as
AtyA0
Rs ,
A0
with A0 and A being the TVC at the beginning and the end of the reactions,t
respectively. One unit of antimicrobial activity was computed as a difference of 0.01
Ž . Ž .
between R control and R treatment .
2.8. Assays of lysozyme actiÕity
Lysozyme assays were similarly carried out with M. luteus replacing the E. coli used in the antibacterial assays and the egg white lysozyme was excluded. Initial total cell counts of the M. luteus suspension was 1.13=104"4.36=103 cfu and lysozyme activity was similarly regarded as a decrease in TVC compared to the initial TVC and
Ž .
where this occurred, ratio of change in TVC R was calculated as
AtyA0
Rs ,
A0
Ž . Ž . Ž .
and lysozyme activity units was computed as R control yR treatment .
2.9. InÕitro bacterial killing assays with plasma fromÕaccinated, placebo-injected, and
naı
¨
Õe prawnsŽ .
P. Õannamei juveniles mean weights9.41 g were injected with 100ml of thrice
washed vaccines of V. harÕeyi strains BPO4, DPEX and E. coli strain XL1-Blue MRFX. All vaccines were suspended in sterile SSS to give a wet weight concentration of about 10.88 mg mly1 which corresponded to total cfu of 7.46=106, 7.38=106, and
9.68=106 per 100ml, respectively. Each vaccine preparation was injected into each of three replicate prawns. Positive controls were injected with sterile SSS and negative controls were uninjected. Haemolymph was removed from the prawns’ hearts after 48 h and pooled for each group. The haemocytes were spun down in an Eppendorf mi-crofuge, and the supernatant fluids passed through a 0.45 mm filter and stored at y1778C for no more than 4 weeks until used.
Triplicate challenge tests were carried out in sterile 1.5 ml Eppendorf tubes. The test mixtures contained 200ml of plasma obtained from vaccinated, sterile SSS injected or unvaccinated controls, and were reacted with 100 ml of live E. coli suspension
Žcontaining a mean total cfus5.35=10 . A contamination control consisting of5.
Fifty microliter samples were removed at 0, 3, 6, 12 and 24 h from each of the replicates and plated after suitable dilution. Antibacterial activity was recorded as
Ž . Ž .
survival index SI values Wardlaw and Unkles, 1978 calculated as cfu at end
SIs =100,
cfu at start
with SI values greater than 100 indicating growth and lower than 100 indicating antibacterial activity.
2.10. In ÕiÕo bacterial killing assays in Õaccinated, sterile SSS injected and naiÕe
prawns
2.10.1. Measurement of haemolymphÕolume
To obtain the initial dilution of bacterial cells after injection into test prawns, the haemolymph volume of P. Õannamei juveniles was estimated using the inulin dilution
Ž . 14 Ž y1. Ž .
method of Levenbook 1958 . Inulin carboxyl- C, 1.4 mCi g Sigma was diluted
4 Ž .
with sterile SSS to give a solution containing 6.0=10 disintegrations per minute dpm
y1 Ž .
ml . P.Õannamei juveniles ns12; weight ranges2.10–13.90 g , were injected with
50 ml of this inulin solution and held at 288C. At 1, 2 and 3 h, 40 ml of haemolymph were removed from the pleopod base of the first abdominal segment of each prawn using a 20 ml auto-pipette equipped with an ultra thin microtip. A total of 100 ml of
Ž .
tissue solubilizer Soluene 350, Packard Canberra were added to the removed haemolymph and the mixture was made up to 4015.00ml in a high ionic strength liquid
Ž .
scintillation cocktail Hionic Flour, Packard Canberra . A set of standards were made up in SSS to reflect final dilutions in volumes of 1950–4950 ml. Blanks consisted of haemolymph from uninjected prawns and SSS for the samples and the standards, respectively. Radioactivity was measured using a Beckman LS7500 liquid scintillation counter.
2.11. Killing assays
Ž . Ž .
Lyophilised vaccines of V. harÕeyi strains BP04 virulent and DPEX avirulent , and
E. coli strain XL1-Blue MRFX were resuspended in SSS and the concentration of each
was adjusted to 7.26 mg mly1. A total of 100 ml of the final vaccine suspension
corresponding to total cfu of f7.38=106, 7.46=106, and 9.68=106 for V. harÕeyi strains DPEX and BP04 and E. coli strain XL 1-Blue MRFX, respectively, were injected into the ventral sinus of the first abdominal segment of each of the nine replicate P.
Ž .
Õannamei juveniles mean weights9.41"2.23 g . Vaccinated prawns were stocked
according to vaccines administered, with three prawns in 20 l of water in rectangular stacking boxes.
Bacterial challenge tests were carried out 48 h after vaccinations by injecting 100ml of each of the live bacteria from which the vaccines used were made into the ventral abdominal sinus of the target prawns. Each bacterial challenge treatment had three replicate prawns per vaccine treatment group. Concentrations of injected bacteria were f7.38=106, 7.46=106, and 9.68=106 total cfu for V. harÕeyi strains DPEX and
At 3, 8, 12, 24 and 48 h, 10ml of haemolymph were removed from the pleopod base
Ž
of the first abdominal segment of each prawn using a 10 ml auto-pipette Eppendorf
.
Reference equipped with an ultra thin microtip. The withdrawn haemolymph was
Ž .
immediately serially diluted 10-fold in sterile SSS, and spread on three replicate SWC agar plates. The resulting bacterial colonies were counted following 24-h incubation at 288C.
2.12. Statistical analysis
Statistical significance of differences obtained among measured parameters was
Ž .
computed using analysis of variance ANOVA . All data were tested for homogeneity of
Ž .
variance using Bartlett’s test Sokal and Rohlf, 1995 prior to further analysis. When
Ž .
ANOVA indicated statistical significance as0.05 between factors, Tukey’s all-pair-wise comparisons were applied to determine significant differences between individual treatment means.
All statistical calculations were performed with the minitab statistical software
Ž .
package Minitab .
3. Results
3.1. Bacterial growth in plasma from naı
¨
Õe prawnsTVCs for E. coli increased approximately threefold from 6.91=105"1.90=104 to
2.38=106"8.38=104cfu and about 2.3 times to 1.62=106"3.21=104 cfu after 3
h incubation in plasma from naıve prawns and SWC, respectively. Upper tailed
¨
two-sample t-tests revealed that the differences in the final concentrations weresignifi-Ž .
cantly different p-0.0001, ts y34.09 .
3.2. Effects of addition of lysozyme to E. coli suspensions
Final total TVCs obtained when E. coli suspensions were incubated with and without the addition of 1.24 mg mly1 of lysozyme were 1.28=106"2.41=105 and 1.50=
106"1.90=105 cfu, respectively. Lower tailed t-tests revealed that these differences
Ž .
were not statistically significant ps0.13, ts y1.30 indicating that the addition of egg white lysozyme to give a final concentration of 1.24 mg mly1 had no significant effect on bacterial growth.
3.3. Measurement of antibacterial actiÕity in plasma ofÕaccinated P.Õannamei
In assays for antibacterial activity, plasma obtained from naıve prawns increased
¨
Ž .
bacterial counts over 13-fold compared with initial bacterial counts. E. coli concentra-tions increased from 6.91=105"1.90=104 cfu at 0 h to 9.26=105"1.22=105cfu
for naıve control plasma samples used in the assay. Upper tailed two-sample t-tests
¨
Ž .
activity when analysed under present conditions. In contrast, a marked antibacterial
Ž
activity expressed as a decline in TVC values compared with initial bacterial
concentra-.
tions appears within 6 h in both vaccinated and sterile SSS injected juveniles and rises
Ž .
to maximum values at 12 h Fig. 1 . Antibacterial activity was detectable for 7 days after vaccination at which time ANOVA revealed no significant differences in antibacterial activities obtained in the vaccinated treatment and those of the sterile SSS injected and
Ž .
control groups fs0.407, ps0.683 . At all times, when antibacterial activity was detected, the levels were higher in the vaccinated treatments compared with the sterile
Ž .
SSS injected treatments and the untreated controls Fig. 1 , suggesting that antibacterial activity observed in prawn plasma was stimulated in the prawns not only by vaccination, but also by sterile SSS injection.
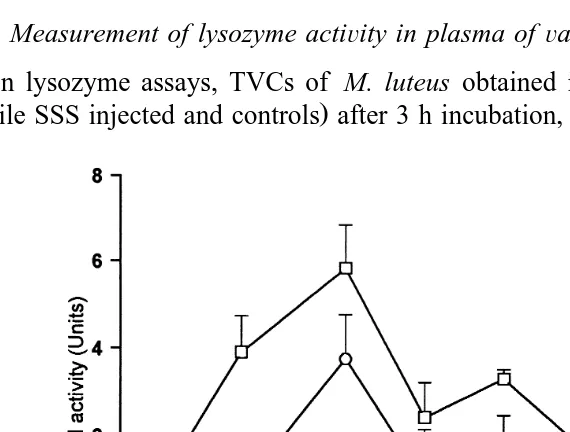
3.4. Measurement of lysozyme actiÕity in plasma ofÕaccinated P.Õannamei
Ž
In lysozyme assays, TVCs of M. luteus obtained in all the treatments vaccinated,
.
sterile SSS injected and controls after 3 h incubation, were consistently higher than the
Fig. 1. Effect of vaccination on antibacterial activity in plasma of P.Õannamei.
Haemolymph was removed from vaccinated, placebo injected, and naıve prawns at various times after¨
treatment and the haemocytes were spun down in an Eppendorf microfuge. A total of 150ml of plasma from each treatment group were then mixed with 25ml of sea water complex nutrient medium, 25ml of 11.24 mg
y1 Ž 4 .
ml lysozyme and 25ml of E. coli suspension mean TVCs2.76=10 cfu , and incubated for 3 h at 318C.
Ž .
Antibacterial activity is regarded as a decrease in TVC compared to the initial TVC and ratio of change R in
Ž .
TVC was calculated as Rs AtyA0rA . With A and A being the TVC at the beginning and the end of0 0 t
the reactions, respectively. One unit of antimicrobial activity was computed as a difference of 0.01 between R
Žcontrol and R treatment .. Ž . I: Antibacterial activity in plasma from prawns vaccinated by injection of 100ml of non pathogenic Õibrio harÕeyi strain DPEX containing , 2.61=105 cells; `: antibacterial activity in
Ž .
Ž .
initial TVCs at 0 h Fig. 2 , indicating a lack of lysozyme activity in all the treatments as defined for this study. Despite of this, ANOVA indicated that the increase in TVC obtained in plasma from vaccinated prawns 6 h after vaccination was not significant, suggesting a definite suppression of M. luteus growth when compared with plasma
Ž .
samples from sterile SSS injected and naıve prawns
¨
fs5.76, ps0.018 . After 6 h, TVCs obtained in all treatments were significantly higher than initial log TVCs up to 48Ž .
h Fig. 2 .
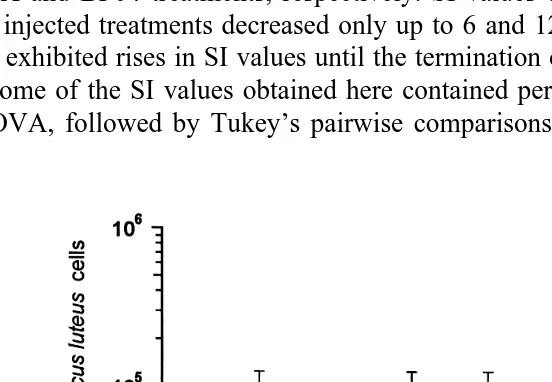
3.5. Measurement of inÕitro killing actiÕity in plasma ofÕaccinated P. Õannamei
Results of the in vitro E. coli killing assays in plasma from prawns injected with
Ž .
vaccines made from various bacterial strains Fig. 3 reveals that SI values fell consistently in all vaccinated treatments up to 24 h where SI values of 11.92"5.23%, 11.94"3.64%, and 27.25"7.78% were obtained for the E. coli and V. harÕeyi strains DPEX and BP04 treatments, respectively. SI values obtained in the control and sterile SSS injected treatments decreased only up to 6 and 12 h, respectively, after which they
Ž .
both exhibited rises in SI values until the termination of the experiment at 24 h Fig. 3 . As some of the SI values obtained here contained percentage values higher than 100%, ANOVA, followed by Tukey’s pairwise comparisons, were performed on TVC values
Fig. 2. TVC of M. luteus cells after 3 h incubation in plasma withdrawn at different times from vaccinated and naive prawns. Control prawns were injected with sterile SSS.
Haemolymph was removed from vaccinated, placebo injected, and naıve prawns at various times after¨
treatment and the haemocytes were spun down in an Eppendorf microfuge. A total of 150ml of plasma from each treatment group were then mixed with 25ml of SWC nutrient medium, 25ml of M. luteus suspension and incubated for 3 h at 318C. Lysozyme activity is regarded as a decrease in TVC compared to the initial
Ž . Ž .
TVC and ratio of change R in TVC was calculated as Rs AtyA0rA . With A and A being the TVC0 0 t
at the beginning and the end of the reactions, respectively. One unit of lysozyme activity was computed as a
Ž . Ž .
Fig. 3. Survival index values of E. coli strain XL1-Blue MRFX
suspensions’ various times after incubation in plasma from P.Õannamei juveniles which had been previously vaccinated by injection with 100ml of a 10.88
y1 Ž . Ž . Ž .
mg ml wet weight suspension of V. harÕeyi strains BPO4 virulent and DPEX avirulent and E. coli strain XL1-Blue MRFX in SSS. Injury controls were injected with 100ml of SSS and plasma controls were uninjected. Haemolymph was removed from prawns and plasma separated from the haemocytes 48 h after vaccination, and 200 ml of plasma from each treatment group were reacted with 100 ml of live E. coli suspension containing a mean total TVC of about 5.35=105 cfu and sensitized by addition of lysozyme to
give a final concentration of 1.24 mg mly1. A total of 50ml aliquots were removed from each treatment at 0,
3, 6, 12 and 24 h, serially diluted in sterile saline, and spread on seawater nutrient agar plates. Bacterial colonies growing after 24 h incubation at 318C were counted and antibacterial activity was recorded as SI
Ž .
values calculated as SIscfu at endrcfu at start=100. SI value greater than 100 indicates growth and one lower than 100 indicating antibacterial activity.I; plasma from prawns vaccinated with V. harÕeyi strain BP04:`; plasma from prawns vaccinated with V. harÕeyi strain DPEX:^; plasma from prawns vaccinated with E. coli strain XL-1 Blue MRFX:q; plasma from SSS injected prawns: N; plasma from unvaccinated
Žcontrol prawns..
obtained after 24 h. This revealed significant reductions in the survival of E. coli when incubated in plasma from all vaccinated prawns. In addition, bacteria incubated in all plasma samples from vaccinated prawns displayed significantly lower concentrations when compared with bacteria incubated in sterile SSS and naıve plasma controls,
¨
respectively. The concentrations of surviving bacteria in these two treatments were notŽ .
significantly different from each other ANOVA: fs22.65, p-0.001 .
3.6. In Õitro actiÕation of the proPO actiÕating systems of naiÕe P. Õannamei juÕeniles
by formalin-killedÕaccines from different bacterial species
Ž .
Scatterplot analyses of results of the kinetic assays of phenoloxidase PO activation in haemocytes of naıve P.
¨
Õannamei juveniles over 20 min by the different bacterial2 Ž .
values were therefore calculated per milligram of protein per minute for each time
Ž .
interval Table 1 . Results revealed that for all the bacterial species tested, PO activation
Ž .
detected was higher in the initial 5 min after the addition of L-Dopa compared with
Ž .
subsequent times after which, the activities generally leveled out or declined Table 1 . The highest PO activation was recorded upon stimulation by V. harÕeyi strain IN7, followed by A. hydrophila, V. harÕeyi strain BP04, zymosan, and V. harÕeyi strains DPEX, BP03, and BP05 with mean values of 26.57"3.20, 22.75"5.30, 20.76"3.32, 15.28"9.84, 12.63"2.01, 11.79"0.29, and 11.29"4.20 units, respectively. E. coli
Ž .
gave the lowest mean PO activity value of 5.65"1.60 units over 5 min Table 1 .
3.7. Estimation of the initial concentration of injected bacteria
Total dpm obtained in the standards gave a linear correlation with a prepared set of
Ž 2 . Ž .
standard volumes r s92.6 . Variance ratios f and p-values obtained were fs25.05 and ps0.038 and normal plots of the residuals and the fits gave a straight line. Haemolymph volumes in the sample prawns were estimated from the total. Some error may have been introduced into the estimation of haemolymph volumes by the use of the
Ž
inulin dilution method, which may measure both intra and intercellular fluid Levenbook,
.
1958 and give correspondingly higher values. Despite this, regression of estimated haemolymph volume against body weight of sample prawns indicated a linear equation with r2s71.9, and f-statistic and p-values of 15.33 and 0.008, respectively. In
addition, normal plots of the residuals and the fits gave a straight line. The estimated
Ž .
total fluid volumes inulin haemolymph were therefore utilised in the calculations of initial bacterial dilutions.
Ž .
The volume "SD of haemolymph corresponding to the mean weight of the prawns used in in vivo killing assays was estimated from this regression line and this was used
Table 1
Ž .
PO activity mean"SD exhibited by haemocyte lysates of P. Õannamei elicited on exposure to A. hydrophila, E. coli, various strains of V. harÕeyi and zymosan
A total of 50ml of haemocyte lysate from naıve P.¨ Õannamei was reacted for 1 h with 50ml of 2 mg mly1of each of the listed bacteria suspended in sodium cacodylate buffer and 100ml ofL-Dopa was added. proPO activation was measured as dopa chrome formation by absorbance at 490 nm at 5 min intervals over 20 min. One unit of proPO activity is expressed as an increase in absorbance of 0.001 mg proteiny1 miny1.
Elicitor N 5 min 10 min 15 min 20 min
to estimate the initial mean concentrations of bacteria in the haemolymph following injection.
Ž .
The mean weights of prawns used 9.41"2.23 g , corresponded to an estimated volume of 5.63"0.63 ml. Initial concentration of injected bacteria after dilution in haemolymph was therefore 1.31=103, 1.32=103 and 1.72=103cfumly1 for prawns
injected with V. harÕeyi strains DPEX and BP04 and E. coli strain XL1-Blue MRFX, respectively.
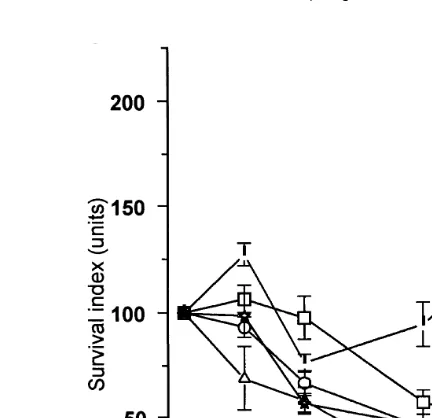
3.8. In-ÕiÕo killing assays
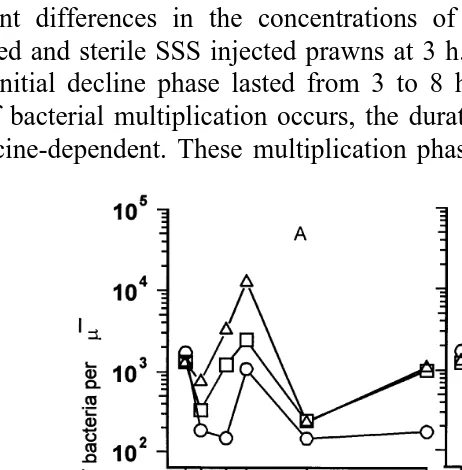
In all the treatments, injection of bacteria into the haemolymph was followed by a reduction in the mean TVC of bacteria within 3 h, although these reductions were not
Ž .
always significant Fig. 4 . Prior immunostimulation with vaccines was not implicated in these initial decreases, as for each challenge treatment group, ANOVA revealed no significant differences in the concentrations of surviving bacteria obtained in both vaccinated and sterile SSS injected prawns at 3 h.
Ž .
The initial decline phase lasted from 3 to 8 h Table 2 . Following this, a second
Ž .
phase of bacterial multiplication occurs, the duration 8–12 h of which is both strain-and vaccine-dependent. These multiplication phases generally lasted only up to 8 h in
Ž . X
Fig. 4. Survival mean"SD of cells of V. harÕeyi strains BP04 and DPEX and E. coli strain XL1-Blue MRF at various times after injection into P.Õannamei juveniles which had been vaccinated 48 h prior to challenge, with formalin-killed cells of V. harÕeyi strains BP04 or DPEX or E. coli strain XL-1 Blue MRFX. Controls
Ž .
were injected with sterile SSS.I: challenged with V. harÕeyi strain BP04 virulent ;`: challenged with V.
Ž . X
Table 2
Lower tailed one-sample t-tests on the mean survival in bacterial numbers of cells of V. harÕeyi strains BP04 and DPEX and E. coli strain XL1-Blue MRFX
per microlitre, 3 h after injection into P.Õannamei juveniles which had been previously vaccinated with formalin-killed cells of V. harÕeyi strains BP04 or DPEX or E. coli strain XL1-Blue MRFX
. Controls were injected with SSS. Bacterial challenge was carried out 48 h after vaccination
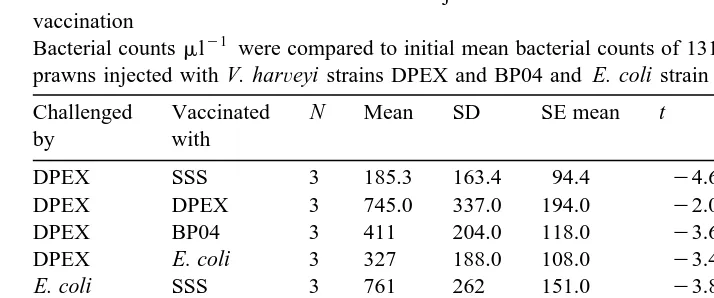
Bacterial countsmly1 were compared to initial mean bacterial counts of 1310, 1320, and 1720 cellsmly1 for
prawns injected with V. harÕeyi strains DPEX and BP04 and E. coli strain XL1-Blue MRFX, respectively.
Challenged Vaccinated N Mean SD SE mean t p-Value Significant
by with reduction?
DPEX SSS 3 185.3 163.4 94.4 y4.66 0.022 yes
DPEX DPEX 3 745.0 337.0 194.0 y2.07 0.087 no
DPEX BP04 3 411 204.0 118.0 y3.66 0.034 yes
DPEX E. coli 3 327 188.0 108.0 y3.42 0.038 yes
BP04 DPEX 3 507.0 77.4 44.7 y11.33 0.0038 yes
BP04 BP04 3 449.3 153.2 88.5 y4.97 0.019 yes
BP04 E. coli 3 456.0 154.2 89.0 y5.14 0.018 yes
prawns vaccinated with bacterin from V. harÕeyi strains BP04 and DPEX, in contrast to prawns vaccinated with the sterile SSS and E. coli bacterin where these multiplication phases generally lasted up to 12 h. Bacterial concentrations then declined in all vaccinated treatments up to 24 h, after which this decline either continued or bacterial multiplication occurred.
When prawns were vaccinated with V. harÕeyi strain BP04 bacterin, and challenged with non-virulent V. harÕeyi strain DPEX or E. coli, the second phase of bacterial multiplication was absent as the initial decline continued up to 24 h, after which it either
Ž . Ž . Ž
continued DPEX bacterin or bacterial multiplication occurred E. coli bacterin Fig.
.
4 .
4. Discussion
Results obtained in the assays of growth of E. coli incubated in naıve plasma reveal
¨
that P. Õannamei plasma constitutes a rich nutritive medium for bacterial growth inŽ .
vitro. This contrasts with the results obtained by Noga et al. 1996 , who obtained antibacterial activity in haemolymph of apparently unstimulated P. setiferus. This discrepancy may indicate a difference in the inherent toxicities of naıve P.
¨
Õannamei and P. setiferus plasma to bacteria. It could also be due to procedural differences. The previous researchers used whole haemolymph, which was allowed to clot before crushing, centrifugation and subsequent use in antibacterial assays. Clotting incrus-Ž
.
Omori et al., 1989 the exocytosis of which provides mechanisms for the simultaneous release of other bioactive molecules and the triggering of the proPO activating system
ŽSmith and Chisholm, 1992; Soderhall and Cerenius, 1992 . In addition, homogenisation
¨
¨
.of clot would break the haemocytes, also releasing the cellular antibacterial factors that may have led to the observed antibacterial activity.
No apparent effects of incubating E. coli suspensions with 1.24 mg mly1 of
lysozyme on growth were observed after incubation for 3 h. Lysozyme cleaves theb1,4 glycosidic bonds in the cell walls of gram positive bacteria. With gram negative bacteria, it weakens, but does not rupture the cell wall, thereby making the bacteria more
Ž .
sensitive to antimicrobial action Vaara, 1992 . The difficulty of detecting antibacterial activity in prawn haemolymph may be due to the concentration of the active agent being
Ž .
too low to be detected by the method used. Sung et al. 1996 detected antibacterial activity in stimulated P. monodon only after concentrating the plasma by a factor of three. However, our study demonstrates that by pre-conditioning the target bacteria with lysozyme treatment, its sensitivity to the antimicrobial substances present is enhanced and direct evidence of antimicrobial activity can be obtained.
The antibacterial activity observed in sterile SSS injected animals indicates that not only vaccination but also wounding has the ability to induce antibacterial activity in
Ž .
prawns. Similar observations have been made by Adams 1991 who detected bacteri-cidins in P. monodon controls injected with saline and suggested that it may have been a non-specific response to injection stress. However, in contrast to results obtained by the previous author, the amount of antibacterial activity detected in our current work was lower in sterile SSS treated animals compared with vaccinated animals while the antibacterial activity was detectable. The duration of antibacterial activity suggests that the immune response in P.Õannamei juveniles is limited, being evident only for 7 days.
Ž .
Though lysozyme activity as defined here was not detected in any of the treatments tested, there was a brief period of significant growth stasis for M. luteus observed in vaccinated plasma 6 h after administration. Having confirmed the requirement of egg white lysozyme in sensitisation of gram negative bacteria to activity by antibacterial
Ž .
factors, it is possible that the responsible factor lysozyme-like factor? , works in synergy with other antibacterial factors, making a brief appearance only to condition the bacteria for subsequent activity by antibacterial factors.
The anti E. coli activity observed in P.Õannamei plasma was not due to inherent
Ž .
toxicity or other factors in the plasma since this study has demonstrated that this bacterium grows well in cell-free haemolymph of naıve juveniles. The bacterial killing
¨
obtained, is thus, attributed to antibacterial factors produced following vaccination. In vitro killing assays reveal partial specificity by these antibacterial factors with the lowestŽ .
initial 3 h SI value being obtained in the plasma from prawns vaccinated with E. coli compared to the V. harÕeyi strains DPEX and BPO4 as well as the sterile SSS vaccinated plasma, and this difference persisted up to 12 h. This type of partial
Ž .
specificity has been reported for P. monodon bacterins Adams, 1991 .
Ž .
results were obtained by Mori and Stewart 1978 who reported loss of bactericidal activity in plasma of vaccinated Homarus americanus following adsorption with
Ž .
Pseudomonas perolens and AerococcusÕiridans var homari.
In the current study, proPO activity was measured in vitro in plasma from naıve
¨
Ž .
prawns and the activation values given by zymosan 15.28 units are not greatly different from the lowest values obtained with the pathogenic V. harÕeyi strain BP05
Ž11.29 units . Highest stimulation was observed for the non-pathogenic V. har. Õeyi strain
Ž .
IN7 26.57 units . E. coli vaccines gave the lowest activation of proPO in P.Õannamei
Ž . Ž .
HLS 5.65 units . This contrasts with results reported by Sung et al., 1996 , who obtained PO activity values of 5.04 and 2.30 units in naıve P. monodon plasma when
¨
stimulated with Vibrio sp. antigen and zymosan, respectively. The lower proPO activity values obtained in naıve P. monodon HLS by these researchers as compared to our¨
study may, however, be attributed to species or procedural differences: the higherŽ . Ž .
volumes of reaction mixtures used by Sung et al. 1996 200ml as opposed to 100ml
Ž y1.
used in this study, the lower concentration ofL-Dopa substrate used 1.60 mg ml as opposed to 3.00 mg mly1 used in our work, different dilution factors, as well as the different reaction times used. In our study, elicitors were incubated with the P.
Õannamei HLS at 208C for 65 min and then reacted withL-Dopa at 5-min intervals for
Ž .
20 min, while Sung et al., 1996 incubated the elicitors with the P. monodon HLS for 15 min at 378C and then reacted withL-Dopa for 1 min.
The observed higher stimulatory effect on the proPO system by the non-pathogenic
V. harÕeyi strain IN7 as opposed to the more pathogenic strains BP03, BP04, and BP05,
suggests that the higher protection conferred on P. indicus larvae due to vaccination
ŽAlabi, 1997; Alabi et al., 1999 by vaccines from a more virulent Vibrio sp. strain as.
opposed to a less virulent one, is not entirely due to the stimulation of the proPO system. Since the more pathogenic strains did not give proPO activation values as high as those given by the less pathogenic strain IN7, it suggests the presence of some other recognition and antimicrobial factors which must be present in the plasma. This view is further supported by the observation that despite the very low stimulation of the PO
Ž .
system given by E. coli PO activation unitss5.65 in in vitro killing assays, plasma from prawns vaccinated with this E. coli strain exhibited SI values which showed no
Ž
significant difference from those exhibited by V. harÕeyi strain DPEX PO activation
. Ž .
unitss12.62 and V. harÕeyi strain BP04 PO activation unitss20.76 .
Curiously, injection of P.Õannamei juveniles with formalin-killed E. coli cells and subsequent challenge by non-pathogenic E. coli produced total mortalities in in vivo tests after 24 h. Toxic shock or bacterial septicaemia are unlikely to be the reasons for this, as in preliminary tests of virulence this strain of E. coli had proven to be
Ž .
non-pathogenic to prawn larvae data not shown . In addition, the other prawns in the same treatment, challenged with live E. coli exhibiting similar levels of mean TVC, did not succumb to bacterial septicaemia. It may be that the animals were overwhelmed by injection, stress or physical damage during the injection process.
In vivo killing assays reveal an initial significant reduction in the mean TVC of all injected strains of bacteria over 3 h, which sometimes lasted up to 8 h. Since naıve
¨
haemolymph has been demonstrated to be non-bactericidal and the magnitude of theŽ .
-vaccinated prawns in all the challenge treatment groups, the initial rapid reduction in the concentrations of injected bacteria is not attributed to vaccination and may be due to the primary cellular mechanisms of immune defence: phagocytosis, nodule formation, and agglutination. This also implies an active role for haemocytes in the induction of the longer lasting humoral immune response.
This initial reduction was followed by a second phase of bacterial multiplication, the length of which was strain- and vaccine-dependent, generally lasting up to 12 h when vaccination was carried out using SSS and E. coli bacterin, and up to 8 h when vaccination was with bacterin from V. harÕeyi strain DPEX and also when prawns vaccinated with bacterin from the more virulent V. harÕeyi strain BP04 were challenged by live V. harÕeyi strain BPO4.
Ž
Antibacterial activity appears at 8 h usually in prawns vaccinated with bacterin from
.
V. harÕeyi strains BP04 and DPEX , leading to a slight reduction in bacterial numbers.
This antibacterial activity is more evident in all treatments at 12 h when a more discernible decline is evident, reflecting differences in the efficacies of the various vaccines. This period is in agreement with the period taken to attain maximal anti-bacterial activity as time course experiments in this study have revealed.
After the initial decline in bacterial numbers, subsequent more effective bacterial
Ž
killing exhibited by the treatments vaccinated with V. harÕeyi strains BPO4, DPEX and
.
E. coli in that order , as compared to the sterile SSS injected prawns, results from pre-existing antibacterial factors present in immune haemolymph.
It seems likely that initial cellular responses serve to maintain the bacterial
concentra-Ž
tions at low levels while de novo synthesis of antibacterial factors possibly mediated by
.
a factor or factors released from the haemocytes is proceeding.
The absence of the second phase of bacterial multiplication, observed when prawns vaccinated with V. harÕeyi strain BP04 were subsequently injected with non-pathogenic
V. harÕeyi strain DPEX or E. coli, confirms the advantages of using more virulent
strains in vaccine production.
In contrast to reports on other prawn species by researchers working on P. monodon
ŽSung and Song, 1996; Sung et al., 1996 , total clearance of viable bacteria was not.
observed from the haemolymph of P. Õannamei. The clearance of bacteria from haemolymph in our experiments may not be as efficient as reported by these authors and this may be due to species or methodological differences. For instance, the previous authors subjected the tiger prawns to infection by immersion, while in our study, infection was carried out by injection
In addition to previous work by various authors, results obtained in this study have profound implications for disease control in prawn culture systems. Knowledge of the dynamics of bacterial activity in vivo and in vitro, differences in the efficacies of vaccines made from different species of bacteria, differential responses of various aspects of the immune systems to such vaccines, and the limited duration of the antibacterial activity generated should lead to increased manipulative advantages in prawn culture systems. However, given the limited duration of the antibacterial activity
Ž .
Acknowledgements
This study was funded by a Commonwealth scholarship awarded to the first author.
References
Adams, A., 1991. Response of penaeid shrimp to exposure to Vibrio species. Fish and Shellfish Immunology 1, 59–70.
Alabi, A.O., 1997, Aspects of bacterial disease prevention and control in penaeid prawns, PhD Thesis, University of Wales, Bangor, Menai Bridge, 206 pp.
Alabi, A.O., Latchford, J.W., Jones, D.A., 1999. The efficacy of immersion as opposed to oral vaccination of Penaeus inducus. Aquaculture 178, 1–11.
Ashida, M., Soderhall, K., 1984. The prophenoloxidase activating system in crayfish. Comparative Biochem-¨ ¨ Ž .
istry and Physiology 77B 1 , 21–26.
Boman, H.G., Hultmark, D., 1987. Cell-free immunity in insects. Annual Review of Microbiology 41, 103–126.
Bullock, W.O., Fernandez, J.M., Short, J.M., 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques 5, 376–379.
Cheng, T.C., Huang, J.W., Karadogan, H., Renwrantz, L.R., Yoshino, T.P., 1980. Separation of oyster hemocytes by density gradient centrifugation and identification of their surface receptors. Journal of Invertebrate Pathology 36, 35–40.
Cheng, T.C., Rodrick, G.E., 1974. Identification and characterization of lysozyme from the hemolymph of the soft-shelled clam Mya arenaria. Biological Bulletin 147, 311–320.
Chisholm, J.R.S., Smith, V.J., 1992. Antibacterial activity in the haemocytes of the shore crab, Carcinus maenas. Journal of the Marine Biological Association of the United Kingdom 72, 529–542.
Durliat, M., Vranck, R., 1981. Action of various anticoagulants on hemolymphs of lobsters and spiny lobsters. Biological Bulletin 160, 55–68.
˚
Engstrom, P., Carlsson, A., Engstrom, A., Tao, Z., Bennich, H., 1984. The antibacterial effect of attacins from¨ ¨
the silk moth Hyalophora cecropia is directed against the outer membrane of Escherichia coli. EMBO
Ž .
Journal 3 13 , 3347–3351.
Faye, I., Wyatt, G.R., 1980. The synthesis of antibacterial proteins in isolated fat body from Cecropia silkmoth pupae. Experientia 36, 1325–1326.
Foley, D.A., Cheng, T.C., 1977. Degranulation and other changes of molluscan granulocytes associated phagocytosis. Journal of Invertebrate Pathology 29, 321–325.
Ž
Guzman, M.A., Ochoa, J.L., Vargas-Albores, F., 1993. Haemolytic activity in the brown shrimp Penaeus´
. Ž .
californiensis Holmes haemolymph. Comparative Biochemistry and Physiology 106A 2 , 271–275. Hose, J.E., Martin, G.G., 1989. Defence functions of granulocytes in the ridgeback prawn Sicyonia ingentis.
Journal of Invertebrate Pathology 53, 335–346.
Hose, J.E., Martin, G.G., Nguyen, V.A., Lucas, J., Rosenstein, T., 1987. Cytochemical features of shrimp haemocytes. Biological Bulletin 173, 178–187.
Hultmark, D., Steiner, H., Rasmuson, T., Boman, H.G., 1980. Purification and properties of three inducible bactericidal proteins from haemolymph of immunized pupae of Hyalophora cecropia. European Journal of Biochemistry 106, 7–16.
Itami, T., Takahashi, Y., Nakamura, Y., 1989. Efficacy of vaccination against vibriosis in cultured kuruma prawns Penaeus japonicus. Journal of Aquatic Animal Health 1, 238–242.
Kaaya, G.P., Flyg, C., Boman, H.G., 1987. Insect immunity: induction of cecropin and attacin-like
anti-Ž .
bacterial factors in the haemolymph of Glossina morsitans morsitans. Insect Biochemistry 17 2 , 309–315.
Latchford, J.W., Prayitno, S.B., Alabi, A.O., 1995. The use of vaccines and immunostimulants in the culture
Ž .
Lavilla-Pitogo, C.R., Baticados, M.C.L., Cruz-Lacierda, E.R., de la Pena, L.D., 1990. Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture 91, 1–13.
Levenbook, L., 1958. Intracellular water of larval tissues of the southern armyworm as determined by the use of C14-carboxyl-inulin. Journal of Cellular and Comparative Physiology 52, 329–339.
Lightner, D.V., 1985. A review of the diseases of cultured penaeid shrimps and prawns with emphasis on the
Ž .
recent discoveries and developments. In: Taki, Y., Primavera, J.H., Llobrera, J.A. Eds. , Proceedings of the First International Conference on the Culture of Penaeid PrawnsrShrimps. SEAFDEC Aquaculture Department, Illoilo City, Philippines, pp. 79–103.
Ž .
Lightner, D.V., 1988. Vibrio disease. In: Sindermann, C.J., Lightner, D.V. Eds. , Disease Diagnosis and Control in North American Marine Aquaculture. Elsevier, Amsterdam, pp. 42–47.
Lightner, D.V., 1992. Shrimp pathology: major diseases of concern to the farming industry in the Americas. Memorias l Congreso Ecuatoriano de Acuicultura, 177–195.
Mohney, L.L., Lightner, D.V., Bell, T.A., 1994. An epizootic of vibriosis in Ecuadorian pond-reared Penaeus
Ž . Ž .
Õannamei Boone Crustacea: Decapoda . Journal of the World Aquaculture Society 25 1 , 116–125.
Ž .
Mori, K., Stewart, J.E., 1978. The hemolymph bactercidin of American lobster Homarus americanus : adsorption and activation. Journal of the Fisheries Research Board of Canada 35, 504–1507.
Noga, E.J., Arroll, T.A., Bullis, R.A., Khoo, L., 1996. Antibacterial activity in hemolymph of white shrimp Penaeus setiferus. Journal of Marine Biotechnology 4, 181–184.
Omori, S.A., Martin, G.G., Hose, E.J., 1989. Morphology of hemocyte lysis and clotting in the ridgeback prawn Sicyonia ingentis. Cell and Tissue Research 255, 117–123.
Owens, L., Muir, P., Sutton, D., Wingfield, M., 1992. The pathology of microbial diseases in tropical
Ž .
crustacea. In: Shariff, M., Subasinghe, R.P., Arthur, J.R. Eds. , Disease in Asian Aquaculture. Fish Health Section, Asian Fisheries Society, Philippines, pp. 165–172.
Prayitno, S.B., 1994. Studies of bacteria causing prawn disease in Indonesia with special emphasis on luminous bacterial disease, PhD thesis, University of Wales, Bangor, 250 pp.
Prayitno, S.B., Latchford, J.W., 1995. Experimental infections of crustaceans with luminous bacteria related to Photobacterium and Vibrio: effect of salinity and pH on infectiosity. Aquaculture 132, 105–112. Ratcliff, N.A., Rowley, A.F., Fitzgerald, S.W., Rhodes, C.P., 1985. Invertebrate immunity: basic concepts and
recent advances. International Review of Cytology 97, 183–350.
Reichelt, J.L., Baumann, L., 1973. Taxonomy of the marine luminous bacteria. Archives Microbiology 94, 283–330.
Ž .
Ruangpan, L., 1982. Diseases and parasites of P. monodon Fabricus. In: Arthur, J.R. Ed. , Asian Fish Health Bibliography and Abstracts 1: South East Asia. Fish Health Section, Asian Fisheries Society, Philippines, p. 188.
Smith, V.J., Chisholm, J.R.S., 1992. Non-cellular immunity in crustaceans. Fish and Shellfish Immunology 2, 1–31.
Smith, V.J., Soderhall, K., 1983.¨ ¨ b-1,3 glucan activation of crustacean hemocytes in vitro and in vivo. Biological Bulletin 164, 299–314.
Soderhall, K., 1982. Prophenoloxidase activating system and melanization — a recognition mechanism of¨ ¨
arthropods? A review. Developmental and Comparative Immunology 6, 601–611.
Soderhall, K., Cerenius, L., 1992. Crustacean immunity. Annual Review of Fish Diseases, 3–23.¨ ¨
Soderhall, K., Unestam, T., 1979. Activation of serum prophenoloxidase in arthropod immunity. The¨ ¨
specificity of cell wall glucan activation and activation by purified fungal glycoproteins of crayfish phenoloxidase. Canadian Journal of Microbiology 25, 406–414.
Sokal, R.R., Rohlf, F.J., 1995. Biometry, The Principles and Practice of Statistics in Biological Research. 3rd edn. Freeman, New York, 887 pp.
Ž .
Song, Y., Hsieh, Y., 1994. Immunostimulation of tiger shrimp Penaeus monodon hemocytes for generation of microbicidal substances. Analysis of reactive oxygen species. Developmental and Comparative
Im-Ž .
munology 18 3 , 201–209.
Sung, H.-H., Song, Y.-L., 1996. Tissue location of Vibrio antigen delivered by immersion to tiger shrimp
ŽPenaeus monodon . Aquaculture 145, 41–54..
Sung, H.H., Yang, Y.L., Song, Y.L., 1996. Enhancement of microbicidal activity in the tiger shrimp Penaeus
Ž .
Vaara, M., 1992. Agents that increase the permeability of the outer membrane. Microbiological Review 56, 395–411.
Vanderzant, C., Nickelson, R., Judkins, P.W., 1971. Microbial flora of pond-reared shrimp Penaeus aztecus. Applied Microbiology 21, 916–921.
Ž .
Vargas-Albores, F., 1995. The defence system of brown shrimp Penaeus californiensis : humoral recognition and cellular responses. Journal of Marine Biotechnology, 153–156.
Vargas-Albores, F., Guzman, M.A., Ochoa, J.L., 1993a. An anticoagulant solution for haemolymph collection´
Ž .
and prophenoloxidase studies of penaeid shrimp Penaeus californiensis . Comparative Biochemistry and Physiology 16A, 299–303.
Vargas-Albores, F., Guzman, M.A., Ochoa, J.L., 1993b. A lippolysaccharide-binding agglutinin isolated from´
Ž .
brown shrimp Penaeus californiensis Holmes haemolymph. Comparative Biochemistry and Physiology
Ž .
104B 2 , 407–413.
Wardlaw, A.C., Unkles, S.E., 1978. Bactericidal activity of coelomic fluid from the sea urchin Echinus esculentus. Journal of Invertebrate Pathology 32, 25–34.
Yasuda, K., Kitao, T., 1980. Bacterial flora in the digestive tract of prawns Penaeus japonicus Bate. Aquaculture 19, 229–234.
Yoshida, H., Ashida, M., 1986. Microbial activation of two serine enzymes and propenoloxidase in the plasma
Ž .