Summary Possible causes of the genetic erosion that occurs during the fragmented phases of the tree-improvement delivery system are reviewed. The impacts of intentional and uninten-tional direcuninten-tional selection during phenotypic selection, seed production (with its associated reproductive-phenology asyn-chrony, fecundity differential and varying propensity to in-breeding), seed processing and storage, and seedling production are evaluated. In general, genetic diversity and heterozygosity parameters of seed orchards are higher or simi-lar to those observed in their natural-population counterparts. However, parental contribution to the resultant seed orchard seed crops is consistently asymmetrical, and this is a major cause of genetic erosion. In most cases, less than 20% of an orchard’s clones contribute 80% of the cone crop, thus reducing the effective population size. Because seed germination of coniferous tree species is under strong maternal genetic con-trol, the combined effects of differences in reproductive output and germination, as well as of management practices (e.g., simulated long-term storage of seed showed that loss of viabil-ity during storage is genotype specific), cause unintentional directional selection during seedling production. This review confirms the need for genetic monitoring of each phase of the tree-improvement delivery system, so that practical solutions can be developed to alleviate genetic erosion.
Keywords: closed populations, genetic erosion, genetic vari-ation, inbreeding, parental contribution, phenotypic selection, reproductive output, reproductive phenology, seed production, seed storage, seedling production.
Introduction
The reduction of genetic variation in populations with a rela-tively limited number of breeding individuals (closed popula-tions) is a well-established fact of modern population genetics theory. Thus, in closed populations, the number of breeding individuals and their fitness play a pivotal role in long-term survival and evolutionary adaptation to changing environ-ments. In domesticated species, the importance of maintaining and conserving genetic variation increases with the expected progressive reduction of breeding individuals. Loss of genetic diversity through domestication is well documented in agricul-tural crops (see Francis 1981); however, it is not clear to what
extent domestication (Figure 1) affects the genetic diversity of forest tree populations. The domestication of wild species has the potential to affect the rate of genetic change more than that expected under natural evolutionary processes. Because conif-erous trees have a long life span, the maintenance of high levels of genetic diversity in breeding and production populations is essential for their viability, survival and evolution (Hamrick and Godt 1989).
I have evaluated the three major steps of the domestication process in forest trees (i.e., tree-improvement delivery sys-tem), namely phenotypic selection, seed production and seed-ling production, to determine how they affect genetic diversity.
Phenotypic selection
The relationship between population size and genetic variabil-ity is critical to the domestication of wild, long-lived species. A reduction or a crash in population size is usually associated with a reduction in genetic variability (Frankel and Soulé
Evaluation of the tree-improvement delivery system: factors affecting
genetic potential
Y. A. EL-KASSABY
Pacific Forest Products Limited, Saanich Forestry Center, 8067 East Saanich Road, Saanichton, B.C. V8M 1K1, Canada
Received May 31, 1994
1981). In agricultural crops, it is well known that losses in genetic diversity are associated with genetic-improvement programs; however, no assessment of potential losses in forest tree species has been made. If isozyme genotypes (biochemi-cal finger-prints) are not associated with the phenotypic or quantitative attributes of individuals, then they present a unique set of neutral, random, gene markers for monitoring genetic variation through the process of ‘‘parent-tree selec-tion.’’
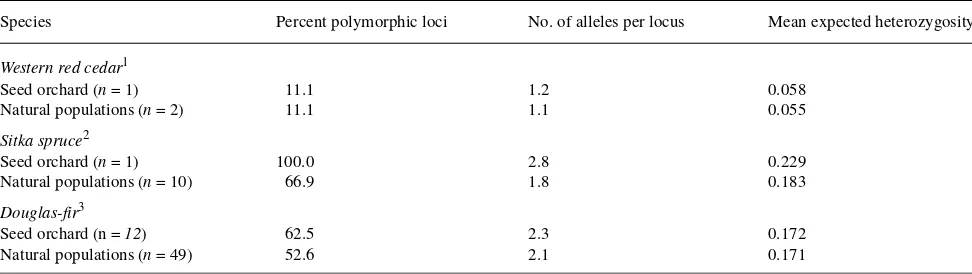
I used allozyme analysis to compare the heterozygosity of first-generation seed orchards of three conifers with their cor-responding natural populations. The species compared were western red cedar (Thuja plicata J. Donn ex D. Don), Sitka spruce (Picea sitchensis (Bong.) Carr.) and Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) (Table 1). A compari-son of the heterozygosity parameters (the number of alleles per locus, percentage of polymorphic loci and mean heterozygos-ity (Hartel 1980)) between the seed orchards and their corre-sponding natural populations’ pooled data indicated that the amount of genetic variation present in natural populations was retained or increased during the phenotypic-selection step (Ta-ble 1). This is because, although the sampling of natural popu-lations is extensive, it is usually limited to a relatively small area, such that trees sampled for breeding may include other alleles not originally sampled. This finding is also concordant with the observed clustering of similar genotypes within natu-ral forest tree populations. The reason why the frequently observed reduction of genetic diversity in agricultural crop plants was not observed in the three conifers studied is not known, but it may be related to the fact that conifers are still in the first stage of domestication and have a high genetic vari-ability (Hamrick and Godt 1989).
Seed production
Seed orchards represent the link between breeding programs (i.e., the product of phenotypic selection) and reforestation activities (i.e., the product of the breeding--testing--selection cycle) through the consistent delivery of genetically improved
seed. Because seed orchards normally consist of a restricted number of genotypes, there is potential for loss in genetic variability along this link. Attainment of genetic gain through selection and breeding is dependent on maintaining the fre-quency of desirable genes in the seed-orchard crops at the level present in the selected parent population comprising the seed orchard. This goal requires the attainment of the random-mat-ing assumption of the Hardy-Weinberg theorem. Panmictic equilibrium is achieved only when the seed orchard’s clones are in reproductive synchrony and contribute similarly to the gene pool (i.e., equal reproductive output). There are various biological factors affecting panmictic equilibrium in seed or-chards including the pattern of reproductive phenology, repro-ductive output and inbreeding.
Reproductive phenology
Synchrony of reproductive phenology among a seed orchard’s clones is a prerequisite to a successful seed-orchard crop. Failure to achieve synchrony of reproductive phenology af-fects the panmictic equilibrium of the seed orchard (Eriksson et al. 1973). It is well known that both time and temperature, summarized as heat sum, have a significant effect on reproduc-tive phenology (Worrall 1983). Because seed orchards consist of several genotypes that are usually adapted to different envi-ronmental conditions, with various heat-sum requirements for bud burst, time differences in reproductive bud phenology will occur among the orchard clones (Figure 2). When differences in the timing of reproductive phenology exist, the orchard clones form several temporally isolated breeding subpopula-tions thereby increasing the rate of inbreeding, reducing pan-mixia and increasing the likelihood that the early and late flowering classes will be exposed to nonorchard pollen (El-Kassaby and Ritland 1986a). Synchrony of reproductive phe-nology in several seed orchard species was investigated and, with the exception of western red cedar (El-Kassaby 1992), all displayed an extended pollination season and subdivision of the seed orchard population into several temporally isolated breeding subpopulations (El-Kassaby et al. 1984, Fashler and El-Kassaby 1987, El-Kassaby and Reynolds 1990). This
sub-Table 1. Comparison of heterozygosity parameters in seed orchards and natural populations.
Species Percent polymorphic loci No. of alleles per locus Mean expected heterozygosity
Western red cedar1
Seed orchard (n = 1) 11.1 1.2 0.058
Natural populations (n = 2) 11.1 1.1 0.055
Sitka spruce2
Seed orchard (n = 1) 100.0 2.8 0.229
Natural populations (n = 10) 66.9 1.8 0.183
Douglas-fir3
Seed orchard (n = 12) 62.5 2.3 0.172
Natural populations (n = 49) 52.6 2.1 0.171
division also affected seed yield. For example, El-Kassaby and Reynolds (1990) observed large reductions in seed set in a Sitka spruce seed orchard where maximum pollen release occurred 10 days after the commencement of seed cone recep-tivity. Based on the length of the pollination season, the pres-ence of a disproportionate number of pollen donors throughout the pollination season (El-Kassaby et al. 1984), and the short duration of seed cone receptivity and pollen-shedding in rela-tion to the reproductive-phenology profile, El-Kassaby et al. (1984) concluded that most orchard populations are in panmic-tic disequilibrium. Under this condition, the seed orchard’s population is a continuum of small breeding subpopulations throughout the pollination season. This reduces maximum exchange of genes among the different genotypes and leads to a decrease in the genetic diversity of the resultant seed crop.
Differences in the timing of reproductive phenology can affect the panmictic equilibrium of seed orchards in several ways. Under panmixia, the expected selfing rate is equal to n/n2, where n is the number of clones in the orchards. There-fore, in a hypothetical orchard that consists of 30 clones, the expected selfing rate is 3.33%. If this orchard is divided into three nonoverlapping breeding subpopulations with equal (1/1/1) or unequal proportions (1/4/1) of clones, the expected selfing rates will be 10 and 6.6%, respectively. This hypotheti-cal orchard will produce three- and twofold differences in selfing over that expected when a panmictic equilibrium exists, thus increasing the chance of reductions in genetic variation.
Reproductive-phenology differences also affect the gametic contribution of the clones to the resultant seed crop. A high seed- or pollen-producing clone in the early or late phenology classes could contribute the same as or less than the amount contributed by a low or medium reproductive-output clone during the peak of pollination (El-Kassaby and Askew 1991). The frequency and timing of receptive females and pollen-shedding males also affect the rate of pollen contamination. If background pollen is high when early or late phenology classes are receptive, most of the receptive trees will be polli-nated by background pollen (El-Kassaby et al. 1988). This will reduce the observed rate of inbreeding (El-Kassaby and
Rit-land 1986a), and consequently increase the effective popula-tion size (i.e., increasing the genetic variability). However, pollen contamination is often associated with reduction of the expected genetic gain (Askew 1986) and maladaptation, espe-cially if the orchard is located outside its natural range (Johnsen 1989a, 1989b, 1989c).
Reproductive output
The maintenance of similar allelic frequencies in a seed or-chard and its seed crop is dependent on the presence of bal-anced gametic production among the orchard’s clones and absence of effective selection involving the alleles studied. The parental balance of seed orchards is often based solely on seed-parent contributions. The so-called ‘‘20/80’’ rule (i.e., 20% of the clones produce 80% of the cone crop) was coined by the North Carolina State Tree Improvement Cooperative to describe the parental balance in seed orchards (Anonymous 1976). Parental-balance values based on seed-parent contribu-tions have become an accepted method to evaluate and even to provide genetic rating of seed-orchard crops. However, paren-tal-balance estimates of several studied seed orchards have revealed that the parental contribution to cone and seed crops varies substantially among a seed orchard’s clones (Griffin 1982, O’Reilly et al. 1982, Byram et al. 1986, El-Kassaby et al. 1989, Kassaby and Reynolds 1990, Reynolds and El-Kassaby 1990, Chaisurisri and El-El-Kassaby 1993) (Figure 3). In most cases, a small proportion of clones contribute the majority of cone/seed crops, thus reducing the expected ge-netic variation and producing crops with unpredictable allelic frequencies. Chaisurisri and El-Kassaby (1993) demonstrated that the concept of effective female population number (Crow and Kimura 1970) can be used to show deviation from the ideal case of equal contribution. In their study, cone counts were used to determine the effective female population size. The proportion of female effective and actual numbers (ne/n) was
also estimated, producing a value of 0.45 which is much smaller than that expected under the equal-contribution as-sumption. Male reproductive success was also investigated for Figure 2. Pattern of reproductive phenology in a western hemlock
(Tsuga heterophylla) seed orchard.
Douglas-fir and loblolly pine (Pinus taeda L.), and distorted contributions to the gametic pollen pool were found, further reducing the genetic diversity (Roberds et al. 1991, El-Kass-aby and Ritland 1992, Nakamura and Wheeler 1992).
Inbreeding
Although estimates of natural outcrossing in conifers are high (t > 0.9, Adams and Birkes 1990), even a low level of inbreed-ing in seed orchards is important because most forestry pro-grams rely on noncompetitive plantings in both nursery-production and plantation-establishment phases.
In general, estimates of outcrossing rates for seed orchards are higher than those reported for natural stands of the same species, suggesting that population structure (i.e., the physical arrangement of related individuals within a population) affects the rate of outcrossing. If the arrangement of an orchard popu-lation can increase the rate of outcrossing, then additional manipulation of pollen dynamics could further reduce inbreed-ing, and consequently increase heterozygosity in the seed crop. The use of an overhead-cooling treatment in coastal seed orchards has been successful as a method for preventing pollen contamination (El-Kassaby and Ritland 1986b) and has pro-duced additional benefits, including a large reduction in repro-ductive-phenology differences (Fashler and El-Kassaby 1987). The duration of flowering and pollination in cooled seed or-chards is shorter and outcrossing rates are higher than in uncooled seed orchards (El-Kassaby and Davidson 1990, 1991).
In summary, the extent of genetic variability in seed orchard crops is greatly affected by the interplay among reproductive phenology, reproductive output and inbreeding. The mainte-nance of high levels of genetic variability during seedling production is dependent on a detailed understanding of seed biology.
Seed biology
Seed-orchard seed crops represent the vehicle by which the genetic gain of tree-improvement programs is transmitted to new generations. In most cases, seed-orchard crops are col-lected, extracted and stored in bulked seedlots. Bulked seedlots are usually composed of undetermined proportions of seed from each of several seed parents. Treating a collection of seed produced from different genotypes uniformly during extrac-tion, germination and storage assumes that all seeds will re-spond to the treatments in a uniform fashion. However, recent studies on the genetics of conifer seed size, dormancy and germination have demonstrated that genotypic differences rep-resent a major and important component of the total variation observed for these attributes (Chaisurisri et al. 1992, El-Kass-aby et al. 1992, 1993b), indicating that a knowledge of the genetic differences in seed traits would be beneficial in the maintenance of the genetic diversity of seed crops. Broad-sense heritability estimates of germination parameters for western red cedar, Sitka spruce and Douglas-fir are presented in Table 2. Because all germination parameters of these species produced high heritability estimates, which are indicative of
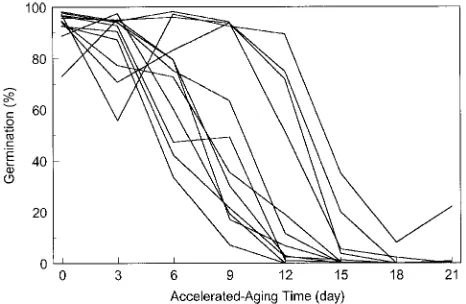
the degree and extent of genetic control over these attributes, it is expected that any uniform dormancy-breaking treatment applied to bulked seedlots will produce different responses because of the different genetic backgrounds of the seedlots (Figure 4). Furthermore, because germination rate is under genetic control, the different genotypes will germinate at dif-ferent rates, resulting in a large variation during emergence (Figure 5).
Seed viability is highest at the time of physiological matur-ity and declines with age. The time courses for seed deteriora-tion range from days to years. In agricultural crops, it has been demonstrated that genotypes differ in the degree of seed viabil-ity loss during storage (Delouche and Baskin 1973). If conifer seed crops are collected, extracted and stored on a bulk basis, then seed-viability losses by a specific genotype cannot be traced, and the reduction in total genetic diversity passes unde-tected (El-Kassaby 1992). The effects of viability loss in Sitka spruce, Douglas-fir, western hemlock (Tsuga heterophylla (Raf.) Sarg.), mountain hemlock (Tsuga mertensiana (Bong.) Carr.) and lodgepole pine (Pinus contorta Dougl. ex Loud.) seed were assessed by a simulated-aging technique known as accelerated aging (Delouche and Baskin 1973). In this test, seeds are exposed to a range of temperatures and humidities for varying times. In all the species investigated, germination rate declined steadily with time of exposure; however, the rate of decline varied among the genotypes (El-Kassaby 1992,
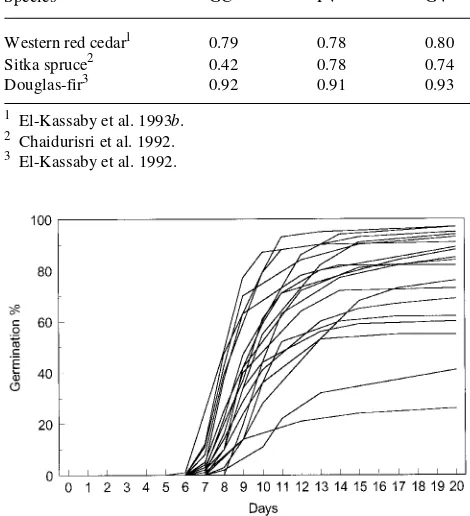
Table 2. Estimates of broad-sense heritabilities (h2) for germination capacity (GC), peak value (PV) and germination value (GV) for western red cedar, Sitka spruce and Douglas-fir.
Species GC PV GV
Western red cedar1 0.79 0.78 0.80
Sitka spruce2 0.42 0.78 0.74
Douglas-fir3 0.92 0.91 0.93
1 El-Kassaby et al. 1993b. 2 Chaidurisri et al. 1992.
3 El-Kassaby et al. 1992.
Chaisurisri et al. 1993) (Figure 6). These differences in the rate of viability loss indicate that the genetic makeup of bulked seedlots changes during storage. If seedlots were harvested, extracted and stored on a clonal basis, then reductions in genetic variability caused by viability losses could be identi-fied by periodic tests.
Seedling production
Maximizing diversity in orchard crops is at odds with the uniformity required for large-scale commercial production of seedlings. Maximizing the number of plantable seedlings per unit area is paramount if container nurseries are to reach their maximum economic potential. If the seed sources used are bulked seedlots, then biological factors such as differences in parental contribution and germination parameters may affect the genetic diversity of the crop. In a bulked seedlot, seed viability is the weighted average of the viabilities of the indi-vidual clones contributing to the seedlot. In addition, if clones vary in their rates of germination, the practice of thinning multiple germinants per cavity (i.e., leaving the largest
germi-nant) will result in unintentional selection pressure in favor of the faster germinating clones (El-Kassaby and Thomson 1990).
Conclusions
Systematic monitoring of genetic variation through the frag-mented processes of some conifer tree-improvement delivery systems has identified several sources of genetic erosion re-sulting in a reduction of the genetic potential of these pro-grams. The causes of genetic erosion were identified and the importance of understanding the underlying genetic control and the biological peculiarities of these processes were empha-sized. The fragmented, horizontal organization of the tree-im-provement delivery system requires evaluation and modifi-cation so that genetic diversity is not affected during the process.
Acknowledgments
Thanks are extended to all my colleagues who collaborated on the various studies cited in this paper.
References
Adams, W.T. and D.S. Birkes. 1990. Estimating mating patterns in forest tree populations. In Proc. Int. Workshop on Plant Biol., Biochemical Markers in Population Genetics of Forest Trees. Eds. S. Fineschi, M.E. Malvolti, F. Cannata and H.H. Hattemer. Institute for Agroforestry of the National Research Council of Italy, Prono-Orvieto, Italy, pp 157--172.
Askew, G.R. 1986. Implications of non-synchronous flowering in clonal conifer seed orchards. In IUFRO Conference: a Joint Meet-ing of WorkMeet-ing Parties on BreedMeet-ing Theory, Progeny TestMeet-ing and Seed Orchards. Williamsburg, Virginia, pp 182--191.
Anonymous. 1976. Twentieth annual report on cooperative tree im-provement and hardwood research program. North Carolina State University, Raleigh, North Carolina.
Byram, T.D., W.J. Lowe and J.A. McGriff. 1986. Clonal and annual variation in cone production in loblolly pine seed orchards. For. Sci. 32:1067--1073.
Chaisurisri, K., D.G.W. Edwards and Y.A. El-Kassaby. 1992. Genetic control of seed size and germination in Sitka spruce. Silvae Genet. 41:348--355.
Chaisurisri, K., D.G.W. Edwards and Y.A. El-Kassaby. 1993. Acceler-ating aging in Sitka spruce seed. Silvae Genet. 42:303--308. Chaisurisri, K. and Y.A. El-Kassaby. 1993. Estimation of clonal
con-tribution to cone and seed crops in a Sitka spruce seed orchard. Ann. Sci. For. 50:461--467.
Chaisurisri, K. and Y.A. El-Kassaby. 1994. Genetic diversity in a seed production population vs. natural populations of Sitka spruce. Bio-diversity Conserv. 3:512--523.
Crow, J.F. and M. Kimura. 1970. An introduction to population genetic theory. Harper and Row Publishers, New York.
Delouche, J.C. and C.C. Baskin. 1973. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci. Technol. 1:427--452.
El-Kassaby, Y.A. 1992. Domestication and genetic diversity----should we be concerned? For. Chron. 68:687--700.
El-Kassaby, Y.A. and G.R. Askew. 1991. The relation between repro-ductive phenology and output in determining the gametic pool profile in a Douglas-fir seed orchard. For. Sci. 37:827--835. Figure 5. Germination curves for the 19 Douglas-fir families of
Fig-ure 4, based on the method of Thomson and El-Kassaby (1993) for demonstrating variation in germination rate.
El-Kassaby, Y.A. and R. Davidson. 1990. Impact of crop management practices on the seed crop genetic quality in a Douglas-fir seed orchard. Silvae Genet. 39:230--237.
El-Kassaby, Y.A. and R. Davidson. 1991. Impact of pollination envi-ronment manipulation on the apparent outcrossing rate in a Douglas-fir seed orchard. Heredity 66:55--59.
El-Kassaby, Y.A. and S. Reynolds. 1990. Reproductive phenology, parental balance and supplemental mass pollination in a Sitka spruce seed orchard. For. Ecol. Manage. 31:45--54.
El-Kassaby, Y.A. and K. Ritland. 1986a. The relation of outcrossing and contamination to reproductive phenology and supplemental mass pollination in a Douglas-fir seed orchard. Silvae Genet. 35:240--244.
El-Kassaby, Y.A. and K. Ritland. 1986b. Low level pollen contamina-tion in a Douglas-fir seed orchard as detected by allozyme markers. Silvae Genet. 35:225--229.
El-Kassaby, Y.A. and K. Ritland. 1992. Frequency-dependent male reproductive success in a polycross of Douglas-fir. Theor. Appl. Genet. 83:752--758.
El-Kassaby, Y.A. and K. Ritland. 1995. Impact of selection and breed-ing on the genetic diversity in Douglas-fir. Biodiversity Conserv. In press.
El-Kassaby, Y.A. and A.J. Thomson. 1990. Continued reliance on bulked seed orchard crops: is it reasonable? Joint Meeting of West-ern For. Genet. Assoc. and IUFRO Work Parties. 4:56--65. El-Kassaby, Y.A., D.G.W. Edwards and D.W. Taylor. 1992. Genetic
control of germination parameters in Douglas-fir and its importance for domestication. Silvae Genet. 41:48--54.
El-Kassaby, Y.A., A.M.K. Fashler and O. Sziklai. 1984. Reproductive phenology and its impact on genetically improved seed production in a Douglas-fir seed orchard. Silvae Genet. 33:120--125. El-Kassaby, Y.A., A.M.K. Fashler and M. Crown. 1989. Variation in
fruitfulness in a Douglas-fir seed orchard and its effect on crop management decisions. Silvae Genet. 38:113--121.
El-Kassaby, Y.A., J. Russell and K. Ritland. 1993a. Mixed-mating in an experimental population of western red cedar, Thuja plicata. J. Hered. 85:227--231.
El-Kassaby, Y.A., K. Chaisurisri, D.G.W. Edwards and D.W. Taylor. 1993b. Genetic control of germination parameters of Douglas-fir, Sitka spruce, western red cedar and yellow-cedar, and its impact on container nursery production. In Dormancy and Barriers to Germi-nation: Proc. Int. Symp., Victoria, B.C. Ed. D.G.W. Edwards. For-estry Canada, Pacific ForFor-estry Center, Victoria, B.C., Canada, pp 37--42.
El-Kassaby, Y.A., K. Ritland, A.M.K. Fashler and W.J.B. Devitt. 1988. The role of reproductive phenology upon the mating structure of a Douglas-fir seed orchard. Silvae Genet. 37:76--82.
Eriksson, G., A. Jonsson and D. Lindgren. 1973. Flowering in a clonal trial of Picea abies (L.) Karst. Stud. For. Suec. 110:5--45. Fashler, A.M.K. and Y.A. El-Kassaby. 1987. The effect of water spray
cooling treatment on reproductive phenology in a Douglas-fir seed orchard. Silvae Genet. 36:245--249.
Francis, C.A. 1981. Development of plant genotypes for multiple cropping systems. In Plant Breeding II. Ed. K.J. Frey. The Iowa State University Press, Ames, Iowa, pp 179--231.
Frankel, O.H. and M.E. Soulé. 1981. Conservation and evolution. Cambridge University Press, New York.
Griffin, A.R. 1982. Clonal variation in radiata pine seed orchards. I. Some flowering, cone and seed production traits. Aust. J. For. Res. 12:295--302.
O’Reilly, C., W.H. Parker and J.E. Barker. 1982. Effect of pollination period and strobili number on random mating in a clonal seed orchard of Picea mariana. Silvae Genet. 31:90--94.
Hamrick, J.L. and M.J.W. Godt. 1989. Allozyme diversity in plant species. In Differentiation Patterns in Higher Plants. Ed. K. Urban-ska. Academic Press, New York, pp 53--67.
Hartel, D.L. 1980. Principles of population genetics. Sinauer Associ-ates Inc., Sunderland, MS, 488 p.
Johnsen, Ø. 1989a. Phenotypic changes in progenies of northern clones of Pices abies (L.) Karst. grown in southern seed orchard. I. Frost hardiness in a phytotron experiment. Scand. J. For. Res. 4:317--330.
Johnsen, Ø. 1989b. Phenotypic changes in progenies of northern clones of Picea abies (L.) Karst. grown in southern seed orchard. II. Seasonal growth rhythm and height in field trials. Scand. J. For. Res. 4:331--341.
Johnsen, Ø. 1989c. Phenotypic changes in progenies of northern clones of Picea abies (L.) Karst. grown in southern seed orchard. III. Climatic damage and growth rhythm in a progeny trial. Scand. J. For. Res. 4:343--350.
Nakamura, R.R. and N.C. Wheeler. 1992. Pollen competition and parental success in Douglas-fir. Evolution 46:846--851.
Reynolds, S. and Y.A. El-Kassaby. 1990. Parental balance in a Douglas-fir seed orchard: cone vs. seed production. Silvae Genet. 39:40--42.
Roberds, J.H., S.T. Friedman and Y.A. El-Kassaby. 1991. Effective number of pollen parents in clonal seed orchards. Theor. Appl. Genet. 82:313--320.
Thomson, A.J. and Y.A. El-Kassaby. 1993. Interpretation of seed-ger-mination parameters. New For. 7:123--132.