SELECTIVE PROTEIN DEGRADATION has emerged as a regulatory mechanism for a wide variety of cellular processes. In eukaryotic cells, the ubiquitin system is a major pathway for regulated protein degradation. Ubiquitin is a highly con-served 76-amino-acid protein that cova-lently modifies target proteins, marking them for degradation by the 26S protea-some. This pathway regulates key bio-logical processes such as cell division, metabolism, immune response and apoptosis1.
Recent studies with Arabidopsis thalianahave revealed that the ubiquitin proteolytic system plays a central role in the auxin-response pathway. The plant hormone indole-3-acetic acid (IAA or auxin) controls many aspects of plant growth and development. Some of the best-characterized examples are tropic growth responses, stem elongation, lat-eral branching of roots and shoots, and vascular development2. These processes are controlled by auxin-mediated changes in cell division, cell expansion and cell differentiation. Given the promi-nent role of auxin in these basic cellular events, it is hardly surprising that plant biologists have long been intrigued by this hormone and have compiled an enor-mous amount of physiological data con-cerning the responses of plants to IAA. Nonetheless, fundamental aspects of auxin biology such as auxin biosynthesis, perception and response are still poorly
understood. This review will focus on recent advances that implicate the ubiquitin pathway in the auxin response.
The ubiquitin-conjugation pathway
Ubiquitin conjugation involves an en-zymatic cascade in which ubiquitin is first activated by the formation of a thiol-ester bond between its C terminus and a cysteine residue within the ubiquitin-activating enzyme (E1). Ubiquitin is then transesterified to a member of a family of ubiquitin-conjugating enzymes (E2). Finally, with the assistance of a ubiquitin ligase (E3), ubiquitin is covalently at-tached to an e-NH2 group of a lysine residue within the substrate protein3. Ubiquitin ligases have a crucial role in determining substrate specificity. E3 en-zymes are very diverse and the least understood components of the ubiquitin-conjugation pathway. In some cases, the E3 forms a catalytic intermediate with ubiquitin4, whereas in others, the major function of the E3 might be to bring the E2 and the substrate into close proxim-ity5. Reiteration of these reactions using a specific lysine residue within the con-jugated ubiquitin results in the gener-ation of a polyubiquitin chain. The 26S proteasome recognizes this chain and degrades the tagged protein, releasing free ubiquitin in the process.
In recent years, a number of ubiquitin-like proteins (Ubls) have been identified. These proteins are conjugated to a lysine residue of target proteins by a mecha-nism very similar to ubiquitin conju-gation6,7. However, in marked contrast to ubiquitination, Ubl conjugation does not generate a polyUbl chain and does not appear to affect target protein stability. The SUMO-1 protein (for small
ubiquitin-related modifier; also known as PIC1/ Ubl1/Sentrin, and Smt3p in yeast) is approximately 20% identical to ubiquitin and is conjugated to several proteins, in-cluding RanGAP1, PML and IkB in mam-mals and the septin proteins of budding yeast8–11. In several cases, modification by SUMO-1 appears to regulate subcellu-lar localization of the target protein. The RUB1 (related to ubiquitin 1) family (NEDD8 in mammals) of Ubls is 50–60% identical to ubiquitin. Less is known about the function of these proteins. Genetic analysis of the yeast cell cycle and the Arabidopsisauxin-response path-way suggests that RUB1/NEDD8 modifi-cation might regulate the activity of a subset of E3 ubiquitin ligases (see below).
Approaches to studying auxin action
Two strategies have been employed to identify genes involved in auxin-mediated growth and development. The first approach is the recovery of mu-tants that exhibit resistance or reduced response to applied auxin. The second strategy has been to identify genes that are rapidly induced by auxin and then link these to factors acting upstream in the response pathway. Both of these ap-proaches have been successful, and it appears that the two might be about to converge on the ubiquitin pathway.
Studies of auxin-response mutants
In Arabidopsis, several mutants have been isolated that display diminished re-sponse to auxin12. Genetic analysis sug-gests that four of these genes (AXR1, TIR1, AXR4and SAR1) act in the same or over-lapping pathways. Loss-of-function mu-tations in AXR1, AXR4 and TIR1 confer diminished auxin response, and double mutant combinations between these genes display synergistic interactions13–15. Phenotypic analyses indicate that AXR1(auxin resistant 1) plays a central role in auxin signaling (Fig. 1a). axr1 mu-tants exhibit defects in essentially all processes thought to be mediated by auxin, including meristem function, tropic growth responses and cell elon-gation. Additionally, axr1 plants exhibit reduced expression of the Aux/IAAand SAUR (for small auxin up RNA) auxin-inducible genes. The AXR1 protein is re-lated to the N-terminal half of ubiquitin-activating enzyme16. AXR1 interacts with the ECR1 (E1 C-terminus-related 1) protein to form a bipartite enzyme that activates the RUB Ubl proteins17.
Arabidopsiscontains at least three mem-bers of the RUB family. RUB1 and RUB2
Function of the
ubiquitin–proteasome pathway
in auxin response
William M. Gray and Mark Estelle
The plant hormone auxin regulates many aspects of growth and develop-ment. Despite the importance of this hormone, the molecular basis for auxin action has remained elusive. Recent advances using molecular genetics in Arabidopsis have begun to elucidate the mechanisms involved in auxin signaling. These results suggest that protein degradation by the ubiquitin pathway has a central role in auxin response.
W.M. Gray and M. Estelleare at the Institute for Cellular and Molecular Biology, Section of Molecular, Cellular, and Developmental Biology, University of Texas at Austin, Austin, TX 78712, USA.
differ by only a single amino acid, whereas RUB3 is ~77% identical to the other two proteins18. In an in vitro acti-vation assay, the AXR1–ECR1 enzyme can form a thiolester adduct with all three RUB proteins but not ubiquitin. Like ubiquitin activation, RUB activation is a two-step process. The first step
is the formation of adenylated RUB. Analysis of RUB1 activation has re-vealed that the ECR1 protein is suffi-cient to generate AMP–RUB1, but AXR1 is required for the formation of the RUB1–ECR1 thiolester adduct19.
Once activated, RUB1 is transesteri-fied to the RUB1-conjugating enzyme (RCE1)19. In yeast and mammals, RUB1 (NEDD8 in mammals) is conjugated to members of the cullin protein fam-ily20,21. Cullins are components of a vari-ety of multisubunit E3 ubiquitin ligases, including SCF complexes (for Skp1p, Cdc53p/cullin and F-box protein), CBCVHL (for cullin, elonginB/C and the von-Hippel-Lindau tumor suppressor protein) and the APC (anaphase-pro-moting complex). An Arabidopsiscullin called AtCUL1 is also modified by RUB119,22 (Fig. 1b). When expressed and purified from a reticulocyte lysate, AtCUL1 can be RUB1-modified in a reac-tion mixture containing recombinant AXR1, ECR1, RCE1, RUB1 and ATP. RUB1 cannot be conjugated to a mutant derivative of AtCUL1 containing a K722M substitution. This residue is con-served in all cullins and is likely to be the site of RUB1 attachment. These re-sults suggest that at least in vitroRUB1 conjugation does not require E3 activ-ity. Alternatively, it is possible that factors derived from the reticulocyte
lysate copurify with AtCUL1 and pro-vide E3 activity in the conjugation assay.
Genetic evidence in yeast suggests that Rub1p modification of the Cdc53p cullin affects the function of some SCF ubiquitin ligases20. The SCFs define a su-perfamily of E3s that employ combinato-rial control to mediate ubiquitin-ligase specificity. The core of the complex consists of Skp1p, Cdc53p, the recently identified Rbx1p/Roc1p/Hrt1p and the ubiquitin-conjugating enzyme Cdc34p (Fig. 2). F-box proteins bind to this core through an interaction between the F-box domain and Skp1p. The F-box pro-tein acts as an adaptor subunit that recruits specific substrates to the com-plex, targeting them for ubiquitination by Cdc34p. In S. cerevisiae, SCFCdc4is re-quired for ubiquitination and degrada-tion of the cyclin-dependent kinase (CDK) inhibitor Sic1p. Although muta-tions in the yeast Rub1p conjugation pathway confer no obvious phenotype, a synthetic lethal phenotype is observed with double mutants between genes in the pathway and conditional alleles of components of SCFCdc4. This suggests that Rub1p conjugation to Cdc53p has a role in SCFCdc4 function. Possible func-tions include facilitating SCF complex assembly, improving SCF catalytic efficiency or altering substrate speci-ficity. Unlike mutations in the yeast Rub1 pathway, mutations in the Arabidopsis RUB conjugation pathway have quite dramatic consequences, suggesting that the RUB pathway has a much more prominent role in higher eukaryotes.
Molecular characterization of the TIR1 (for auxin transport inhibitor re-sponse 1) auxin-rere-sponse gene has greatly strengthened the hypothesis that RUB1 conjugation regulates some aspect of SCF function. Mutations in TIR1confer an auxin-resistance pheno-type that is similar to that caused by mutations in AXR1, although less se-vere. TIR1 encodes an F-box protein with a series of leucine-rich repeats15. Recent findings have confirmed that TIR1 interacts with plant orthologs of Skp1p (called ASK1 and ASK2) and a cullin protein to form SCFTIR1 (Ref. 22). The cullin in SCFTIR1is the same AtCUL1 protein that is a substrate for AXR1-dependent RUB1 modification. This re-sult is consistent with earlier genetic studies, which suggested that TIR1 and AXR1 function in a common pathway15. In addition, TIR1 and AXR1exhibit over-lapping expression patterns, with highest levels in tissues actively undergoing cell
TiBS
C N O H
(b) (a)
ATP AMP + PPi
ECR1
AXR1 RCE1
S
AtCUL1 S
Figure 1
The AXR1 (for auxin resistant 1) auxin-response gene encodes a subunit of the RUB1 (for related to ubiquitin 1)-activating enzyme. (a)35-day-old wild-type (wt) and axr1-12 plants. Bar, 5 cm. (b) The heterodimeric AXR1–ECR1 (for E1 C-terminus-related 1) enzyme activates RUB1 (orange) for conjugation by forming a thiolester bond with RUB1. RUB1 is subsequently transesterified to the RUB1-conjugating enzyme 1 (RCE1), which covalently attaches RUB1 to the substrate AtCUL1, an Arabidopsis cullin.
TiBS
F
E2
Skp1
Rbx1
F F
Cul1
Figure 2
division or cell expansion17,22 (J.C. del Pozo and M. Estelle, unpublished).
Additional evidence implicating SCFTIR1in auxin response has been ob-tained by analysing ask1-1mutants and transgenic lines that overexpress TIR1. The ask1-1 mutation was identified in a screen for male sterile plants23. Mutant plants also exhibit several phenotypes consistent with reduced auxin response, including decreased lateral root devel-opment and resistance to applied auxin22. ASK1 is a member of a family of Skp1-like proteins in Arabidopsis that includes at least ten members. The iso-lation of ask1 mutants, along with the discovery that TIR1 does not interact with all of the Skp1-like proteins in a two-hybrid assay22, implies that individual family members have distinct functions. This could provide an additional layer of SCF combinatorial control in higher eukaryotes.
The auxin-regulated genes
The Aux/IAAgenes are rapidly (10–60 minutes) and specifically induced by auxin24. There are at least 20 mem-bers of the Aux/IAA gene family in Arabidopsis,and many members display distinct basal and induced expression levels, induction kinetics and patterns of expression. Because cycloheximide treatment also induces many of these genes, it has been proposed that a short-lived repressor could negatively regulate their transcription. The Aux/IAA pro-teins are 20–35 kDa and share four highly conserved domains (Fig. 3a). Domain III is related to the baa-fold in
b-ribbon DNA-binding domains of the Arc and MetJ prokaryotic transcriptional repressor proteins, suggesting that these factors might function as tran-scriptional regulators. Consistent with this possibility, several of these proteins have been localized to the nucleus.
Several members of the Aux/IAA fam-ily are capable of forming homo- and heterodimers through domains III and IV25. Additionally, some can interact with members of a second protein fam-ily, including the DNA-binding protein auxin-response factor 1 (ARF1)26,27. Members of the ARF protein family share domains III and IV with the Aux/IAA proteins (Fig. 3a). The ARFs bind to a 6-bp auxin-response element (AuxRE) found upstream of several auxin-inducible genes and apparently function to regulate transcription of these genes28,29. Ulmasov et al.27 have demonstrated by cotransfection of car-rot pcar-rotoplasts that some members of
the ARF family activate transcription of auxin-inducible reporters, whereas oth-ers repress expression of these reporter genes. Furthermore, overexpression of some Aux/IAA proteins represses tran-scription of an AuxRE-reporter26. Because the Aux/IAA proteins are not believed to bind directly to AuxREs, this negative regulation could occur through interaction with ARFs. Taken together, these results illustrate the complexity of auxin-mediated gene regulation. Literally hundreds of differ-ent Aux/IAA–Aux/IAA, ARF–ARF and Aux/ IAA–ARF combinations could modulate auxin-regulated gene expression in res-ponse to changes in auxin concentration and other developmental cues.
Recent genetic evidence has solidified the importance of the Aux/IAAgene family in auxin response. Mutations in the AXR3 gene were isolated in a screen for seedlings resistant to low concentrations
of applied auxin. The semi-dominant axr3 mutants exhibit elevated auxin responses, including increased apical dominance, ad-ventitious rooting and ectopic auxin-inducible gene expression30 (Fig. 3b). Cloning of AXR3revealed that it encodes the Aux/IAA family member IAA17 (Ref. 31). Dominant mutations in the SHY2 and AXR2genes also confer auxin-related growth defects. SHY2and AXR2were re-cently cloned and found to encode Aux/IAA proteins32(P. Nagpul, L. Walker, M. Estelle, J. Reed, unpublished). Curiously, mutations in AXR3, SHY2and AXR2all occur within a small, highly con-served region of domain II. Analysis of in-tragenic revertants of AXR3and SHY2 indi-cates that the original mutations in these genes confer a gain of function. This could be a dominant-negative effect resulting from the formation of non-functional dimers with ARF or Aux/IAA proteins. An alternative explanation favored by these
TiBS
ARF Aux/IAA
(b) (a)
DNA binding III IV
I II III IV
MR
Figure 3
authors is that the mutant proteins exhibit increased stability. Several wild-type Aux/IAA proteins have been shown to be short-lived with half-lives of 6–8 minutes33. Thus, dominant mutations that stabilize these proteins could affect downstream gene expression, thus altering auxin response.
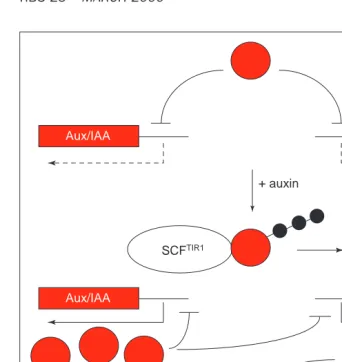
A model for auxin response
Figure 4 presents one possible model for the AXR1–TIR1 pathway in auxin sig-naling. In response to hormone, TIR1 re-cruits one or more repressors of auxin response to an SCF ubiquitin-ligase com-plex. Ubiquitination of this repressor is dependent upon the AXR1–ECR1-medi-ated RUB1 modification of AtCUL1. Subsequent degradation of the repres-sor via the 26S proteasome derepresses the auxin-response pathway resulting in the expression of auxin-regulated
genes and auxin-mediated growth and development.
The putative repressor targeted for degradation by SCFTIR1is unknown. One possible candidate is the SAR1(for sup-pressor of auxin resistance 1) gene product. Recessive mutations in SAR1 were isolated in a screen for suppres-sors of axr1(Ref. 34). In addition to sup-pressing nearly every aspect of the axr1 phenotype, sar1mutations also confer a distinct phenotype that is epistatic to axr1, suggesting that SAR1 acts down-stream of AXR1 in the auxin-response pathway. Given that loss of SAR1 largely alleviates the need for AXR1 in auxin re-sponse, one attractive possibility is that SAR1 is the target of the AXR1–TIR1 pathway. This seems somewhat unlikely, however, given that sar1mutants do not display any constitutive auxin-response phenotypes, and mutations in sar1 do
not suppress mutations in tir1 (A. Cernac and M. Estelle, unpublished). Thus, SAR1 could act at a point between AXR1 and TIR1. One possibility is that SAR1 encodes a protein that removes RUB modifiers from their substrates. Li and Hochstrasser35recently identified a yeast protease that is required for cell-cycle progression. This protease specifi-cally cleaves the Smt3p/SUMO-1 Ubl from substrates, thus demonstrating that Ubl removal is an important cellular function.
Members of the Aux/IAA family are also potential substrates of SCFTIR1. As dis-cussed above, these proteins have short half-lives, suggesting that they might be regulated by ubiquitin-mediated degradation. Furthermore, certain mem-bers of this family negatively regulate auxin-inducible gene expression. Thus, it seems possible that a regu-latory loop exists where the basal ex-pression of specific Aux/IAA proteins re-presses the auxin response pathway, and auxin relieves inhibition by promot-ing the ubiquitin-mediated degradation of these factors. Removal of the repres-sors causes a transient auxin response, including the increased expression of Aux/IAA genes, which downregulate the response pathway (Fig. 5). The direct measurement of Aux/IAA protein stabil-ity in axr1and tir1mutants is an impor-tant test of this model.
Where is the regulation?
Despite these advances in our under-standing of auxin action, the mecha-nisms of auxin perception and regu-lation of the AXR1–TIR1 pathway are still largely a mystery. Although several auxin-binding proteins have been iden-tified, convincing evidence for recep-tor function has remained elusive. Nonetheless, recent findings with the Arabidopsis auxin-binding protein 1 (ABP1) are encouraging. Using an in-ducible expression system, Alan Jones and colleagues have demonstrated that ABP1 promotes auxin-dependent cell ex-pansion when overexpressed in tobacco and maize leaf cells36. It will be interest-ing to determine whether this effect is dependent on the AXR1–TIR1 pathway.
If ABP1 does indeed function as an auxin receptor in the AXR1–TIR1 path-way, it is still unclear how the signal is transduced. The sequence of ABP1 re-sembles no other proteins in the data base, thus providing no information on what types of signaling mechanism might be employed. The identification of an SCF complex in the auxin response suggests
TiBS
ECR1
AXR1 E2
ASK1
Rbx1
AtCul1
TIR1
SCFTIR1 complex
Auxin
RCE1
Ubiquitin
RUB1
E1
Proteasome Regulation of RUB1
conjugation? Auxin-dependent
kinase?
Repressor
Repressor
Repressor
Auxin-mediated growth and development
Early-auxin-response genes
Figure 4
that phosphorylation-based signaling pathways might be involved. All known substrates of SCF ubiquitin ligases must be phosphorylated to trigger their associ-ation with the SCF (Refs 5,37). Perhaps, an auxin-activated kinase phosphorylates substrates of SCFTIR1. Auxin-stimulated kinases have been identified38, but ge-netic evidence implicating them in auxin response is lacking.
Another possible avenue for auxin input is through regulation of E3 activ-ity. Auxin could potentially regulate the assembly, localization, specificity or ac-tivity of SCFTIR1. One possible means by which this could occur is by regulated RUB1 modification of AtCUL1.
Concluding remarks
The complexity of auxin physiology has been appreciated for many years.
Auxin is involved in virtually every aspect of plant growth and development, evokes a diversity of rapid biochemical changes, and has disparate effects on cell growth, depending on the context. It should there-fore come as no surprise that the machin-ery of auxin signal transduction and auxin response is also complex. In addi-tion to the large Aux/IAA and ARF families of transcriptional regulators, there are likely to be multiple SCFs that function in auxin response. So far, three close rela-tives of TIR1, called Leucine-Rich Repeat F-box (LRF), have been identified in the Arabidopsis genome. The proteins en-coded by these genes are likely to inter-act with specific members of the ASK and cullin protein families to form specialized SCFs. A major challenge for future studies is to determine how this molecular complexity relates to the physiological
complexity observed in the developing plant.
Acknowledgements
The research in the authors’ labora-tory is supported by NIH GM 43644 and DOE DE-FG02-98-ER20313 to M.E. and NIH GM 18680 to W.M.G.
References
1Peters, J-M. et al. (1998) Ubiquitin and the Biology of the Cell, Plenum Press
2Davies, P.J., ed. (1995) Plant Hormones: Physiology, Biochemistry, and Molecular Biology, Kluwer Academic Publishers
3Haas A.L. and Siepmann T.J. (1998) Pathways of ubiquitin conjugation. FASEB J. 11, 1257–1268
4Scheffner, M. et al. (1995) Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thiolester cascade. Nature 373, 81–83
5Patton, E.E. et al. (1998) Combinatorial control in ubiquitin-dependent proteolysis: don’t Skp the F-box hypothesis. Trends Genet. 14, 236–243
6Johnson, E.S. et al. (1997) The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519
7Liakopoulos, D. et al. (1998) A novel protein modification pathway related to the ubiquitin system. EMBO J. 17, 2208–2214
8Mahajan, R. et al. (1998) Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J. Cell Biol. 140, 259–270
9 Muller, S. et al. (1998) Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17, 61–70
10 Desterro, J.M. et al. (1998) SUMO-1 modification of IkBainhibits NF-kB activation. Mol. Cell 2, 233–239
11 Takahashi, Y. et al. (1999) Smt3, a SUMO-1 homolog, is conjugated to Cdc3, a component of septin rings at the mother-bud neck in budding yeast. Biochem. Biophys. Res. Commun. 259, 582–587
12 Hobbie, L. and Estelle, M.A. (1995) Genetic approaches to auxin action. Plant Cell Environ. 17, 525–540
13 Lincoln, C. et al. (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2, 1071–1080
14 Hobbie, L. and Estelle, M. (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 7, 211–220
15 Ruegger, M. et al. (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev. 12, 198–207
16 Leyser, H.M.O. et al. (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364, 161–164
17 del Pozo, J.C. et al. (1998) The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 280, 1760–1763
18 Rao-Naik, C. et al. (1998) The Rub family of ubiquitin-like proteins. J. Biol. Chem. 273, 34976–34982
19 del Pozo, J.C. and Estelle, M. The Arabidopsis cullin AtCUL1 is modified by the ubiquitin-related protein RUB1. Proc. Natl. Acad. Sci. U. S. A. 96, 15342–15347
20 Lammer, D. et al. (1998) Modification of yeast Cdc53p by the ubiquitin-related protein Rub1p affects function of the SCFCdc4complex. Genes Dev. 12, 914–926
21 Osaka et al. (1998) A new NEDD8-ligating system for cullin-4A. Genes Dev. 12, 2263–2268
22 Gray et al. (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev. 13, 1678–1691
23 Yang, M., et al. (1999) The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc. Natl. Acad. Sci. U. S. A. 96, 11416–11421
24 Abel, S. and Theologis, A. (1996) Early genes and auxin action. Plant Physiol. 111, 9–17
25 Kim, J. et al. (1997) Protein-protein interactions among Aux/IAA proteins. Proc. Natl. Acad. Sci. U. S. A. 94, 11786–11791
26 Ulmasov, T. et al. (1999) Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. U. S. A. 96, 5844–5849
TiBS
Aux/IAA Aux/IAA
Aux/IAA Aux/IAA
SCFTIR1
Downstream genes
SCF + auxin
TIR1
Figure 5
IN 1931, Stephenson and Stickland showed that the facultative anaerobic colon bacterium Escherichia colicould ac-tivate hydrogen thanks to enzymes they termed hydrogenases1. More recently, these enzymes have been shown to play a central role in the hydrogen metabolism of many microorganisms of great biotech-nological interest, such as methanogenic, acetogenic, nitrogen-fixing, photosyn-thetic and sulfate-reducing bacteria.
Knowledge of the functional and structural properties of hydrogenases might help in the design of less expen-sive catalysts for fuel cells. Existing fuel cells use platinum catalysts and these severely restrict the use of hydrogen-driven vehicles in spite of the urgent need to switching to clean fuels2.
Hydrogenases catalyse the reversible re-action H2↔2H112e2(Refs 3–6). The first
step in the reaction is the heterolytic cleav-age of H2into H1 and H2. The hydrogen-uptake reaction results in protons and electrons, which are subsequently used to generate ATP and reducing power; the hydrogen-producing reaction in-volves the reduction of protons by low-redox-potential electrons generated by fermentation. Most of the hydrogenases described to date are metalloproteins containing Ni or Fe, or both. Of these, the NiFe hydrogenases are the most exten-sively studied and the three-dimensional structures of several enzymes belonging to this group have been reported7. A sur-prising feature of these enzymes is that their active sites contain, in addition to a Ni ion, a redox-inactive, low-spin Fe21 cen-ter with CO and CN2coordination8,9.
The unrelated Fe-only hydrogenases have been less well studied and, until very recently, no three-dimensional structure was available for this class of enzyme. However, in the past year or so, the structures of the Fe-only hydro-genases from Desulfovibrio desulfuricans and Clostridium pasteurianumhave been solved to 1.6 and 1.8 Å resolution, re-spectively10,11. Here, we compare these structures and discuss their analogies to the NiFe-containing enzymes.
Structural comparisons
Both amino acid sequence analyses and electronic paramagnetic resonance (EPR) studies have indicated that Fe-only hydrogenases generally contain two [4Fe–4S] clusters (the F-clusters) in a ferredoxin-like domain. In addition, an unusual EPR signal has been attributed to a novel 6Fe cluster that was proposed to be the active site and was called the H-cluster5. The positions of the F- and clusters in the respective F- and H-domains of C. pasteurianumhydrogenase I (CpI) and D. desulfuricans hydrogenase (DdH) are shown in Fig. 1. DdH is a dimeric periplasmic protein of 53 kDa (43 kDa and 10 kDa for the large and small subunits, respectively) and CpI is a monomeric cytoplasmic enzyme of 61 kDa. In addition to the clusters described above, CpI contains two FeS centers co-ordinated by domains found at the N terminus of the molecule. One of these domains bears a striking structural simi-larity to [2Fe–2S] plant-type ferrodoxins (green in Fig. 1b,c).A short domain (pink in Fig. 1b,c) connects the N-terminal [2Fe–2S] ferrodoxin-like module with the F-domain and consists of just two a
helices separated by a loop region that coordinates an additional [4Fe–4S] clus-ter through three cysteinyl ligands and the Nering atom of a histidine residue.
As was expected from the high degree of amino acid sequence identity (Fig. 1a)12,13, CpI and DdH display exten-sive structural similarities at the H- and F-domains (Fig. 1b,c)10,11. The root-mean-square deviation (rmsd) for the superposition between the H-domains is 1.0 Å (338 Ca’s) and that between the F-domains is 1.7 Å (57 Ca’s). However, the relative orientations of the H- and F-domains are not the same in the two enzymes: the rmsd for the combined H-and F-domains (381 Ca’s) is 2.2 Å. This is probably a consequence of differences elsewhere in the molecules, such as the two extra N-terminal domains in CpI, the numerous insertions in the F-domain in CpI and the longer C-terminal region of
27 Ulmasov, T. et al. (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9, 1963–1971
28 Ulmasov, T. et al. (1997) ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865–1868
29 Ulmasov, T. et al. (1999) Dimerization and DNA binding of auxin response factors. Plant J. 19, 309–319
30 Leyser, H.M.O. et al. (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 10, 403–413
31 Rouse, D. et al. (1998) Changes in auxin response
from mutations in an Aux/IAA gene. Science 279, 1371–1373
32 Tian, Q. and Reed, J.W. (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126,
711–721
33 Oeller, P.W. and Theologis, A. (1995) Induction kinetics of the nuclear proteins encoded by the early indoleacetic acid-inducible genes, IAA4/5 and PS-IAA6, in pea (Pisum sativum L.). Plant J. 7, 37–48
34 Cernac, A.C. et al. (1997) The SAR1 gene of Arabidopsis acts downstream of the AXR1 gene in auxin response. Development 124, 1583–1591
35 Li, S.J. and Hochstrasser, M. (1999) A new protease required
for cell-cycle progression in yeast. Nature 398, 246–251
36 Jones, A.M. et al. (1998) Auxin-dependent cell expansion mediated by overexpressed Auxin-Binding Protein 1. Science 282, 1114–1117
37 Winston, J.T. et al. (1999) The SCFb-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkBaand b -catenin and stimulates IkBaubiquitination in vitro. Genes Dev. 13, 270–283
38 Mizoguchi, T. et al. (1994) Characterization of two cDNAs that encode MAP kinase homologues in Arabidopsis thaliana and analysis of the possible role of auxin in activating such kinase activities in cultured cells. Plant J. 5, 111–122
A novel FeS cluster in Fe-only
hydrogenases
Yvain Nicolet, Brian J. Lemon, Juan C.
Fontecilla-Camps and John W. Peters
Many microorganisms can use molecular hydrogen as a source of electrons or generate it by reducing protons. These reactions are catalysed by metalloenzymes of two types: NiFe and Fe-only hydrogenases. Here, we review recent structural results concerning the latter, putting special emphasis on the characteristics of the active site.
Y. Nicoletand J.C. Fontecilla-Campsare at the Laboratoire de Cristallographie et de Cristallogenèse des Protéines, Institut de Biologie Structurale Jean-Pierre Ebel, CEA-CNRS, 41 Avenue des Martyrs, 38027, Grenoble Cedex 1, France; and B.J. Lemon
and J.W. Petersare at the Dept of Chemistry and Biochemistry, Utah State University, Logan, UT 84322-0300, USA.