Changes in phenolic compounds in Litchi (
Litchi chinensis
Sonn.) fruit during postharvest storage
Donglin Zhang, Peter C. Quantick * , John M. Grigor
Food Research Centre,Uni6ersity of Lincolnshire and Humberside,Nuns Corner,61Bargate Grimsby,North East Lincolnshire,
DN34 5BQ,UK
Received 21 July 1999; accepted 8 February 2000
Abstract
Litchi (Litchi chinensisSonn. cv. Huaizhi) fruit were stored at ambient temperature (20 – 25°C) for up to 7 days and at 4°C for up to 35 days for separation, purification and identification of individual phenolic compounds and investigation of their changes during postharvest storage. Results indicate that flavan-3-ol monomers and dimers were major phenolic compounds representing about 87.0% of the phenolic compounds that declined with storage or browning. Cyanidin-3-glucoside was a major anthocyanin and represented 91.9% of anthocyanins. It also declined with storage or browning. Small amounts of malvidin-3-glucoside were also found. Therefore, the major substrates for enzymatic oxidation were apparently flavan-3-ol monomers and dimers and cyanidin-3-glucoside. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Litchi fruit; Phenolic compounds; Browning; Polyphenol oxidase; Storage
www.elsevier.com/locate/postharvbio
1. Introduction
Litchi (Litchi chinensis Sonn.) is a subtropical fruit. Within 2 or 3 days after harvest its pericarp becomes desiccated and turns brown; it decays and its flavour is lost gradually. It is difficult to store the fruit for any longer than 3 or 4 days at ambient temperature without proper treatment (Nip, 1988). Browning in the pericarp reduces its commercial value dramatically and has long been
considered the main postharvest problem
(Akamine, 1960). Litchi fruit pericarp browning after harvest is the result of a complicated process caused by polyphenol oxidase (PPO) activity (Guangdong Postharvest Research Group, 1975; Tan and Li, 1984; Tan and Zhou, 1987; Lin et al., 1988a), peroxidase (Lin et al., 1988b), desiccation (Scott et al., 1982), changes in anthocyanins (Un-derhill and Critchley, 1994), attack by pathogens (Chen, 1984), and other unknown factors.
The bright red colour of litchi fruit is due to the anthocyanin pigments present in the fruit skin. Cyanidin-3-glucoside, cyanidin-3-rutinoside and malvidin-3-glucoside were identified as the major
monomeric anthocyanin pigments (Lee and
* Corresponding author. Tel.:+44-1472-874140; fax:+ 44-1472-315099.
E-mail address:[email protected] (P.C. Quantick)
Wicker, 1990). Besides monomeric pigments, polymeric pigments may also contribute to the visual appearance of litchi fruit. However, litchi pigments, like all other anthocyanins, are rela-tively unstable and lose their bright attractive colour during storage (Campbell, 1959).
Enzymatic browning is mainly associated with PPO, which is able to act on phenolic com-pounds in the presence of oxygen (Nicolas et al., 1994). Polyphenol oxidase and its substrates varies and changes markedly in fruits and veg-etables (Mayer and Harel, 1991). Although the substrate specificity and affinity for PPO have been determined previously in litchi fruit (Tan and Li, 1984; Jiang et al., 1997), there are very little published data on changes in individual phenolic compounds of litchi fruits (Lee and Wicker, 1990), probably due to lack of appro-priate means of extraction, purification and sep-aration. Therefore, the objective of this research was to investigate the changes in individual phe-nolic compounds as the substrates for enzymatic browning during storage so as to further under-stand the browning process in litchi fruit. This would be helpful for developing methods to re-duce browning of the fruit.
2. Materials and methods
2.1. Plant material
Litchi (Litchi chinensis Sonn. cv. Huaizhi)
fruit were obtained from Guangdong, People’s Republic of China by express air transfer. They arrived in England within 24 h of harvest. Bright and full red mature fruits of uniform size (about 20 g), free of physical damage and injury from insects or fungal infection, were used and distributed randomly into groups of ten fruit. Each group represented one replicate, and for
each temperature treatment, three replicates
were used for each data point. Three individual experiments were set up. The fruits were dipped in 0.1% TBZ (Thiabendazole, Decco Chemicals), air-dried for 1 h, packed in 0.03 mm thick
LDPE bags (30×20 cm) and closed with rubber
bands, and stored at ambient temperature (20 –
25°C) for up to 7 days and at 4°C for up to 35 days, and sampled at different storage times. The fruit peel at various browning stages was sampled from the fruit stored at 4°C. Browning was assessed by the extent of the browned area on each fruit’s pericarp (Zhang and Quantick, 1997), using the following scale: browning stage 0, no browning; 1, 51/4 browning; 2, 1/4 – 1/2 browning; 3, 1/2 – 3/4 browning; 4, 3/4-complete browning.
2.2. Extraction and purification of phenolic compounds
Phenolic compounds were extracted and
purified according to the methods of Amiot et al. (1992) and Mayen et al. (1997) with some modifications. A total of 10 g of peel was cut into small pieces and homogenised with an YS-TRAL GMBH D-7801 Dottingen homogenizer in 100 ml cold ethanol (65%) containing sodium metabisulphite (0.5%) in an ice bath and ex-tracted for 30 min. The homogenate was filtered through four layers of cheesecloth and the residue was added, with 50 ml of the same ex-traction solvent for two successive re-exex-tractions of the residue. The collected filtrate was
cen-trifuged at 7000×g for 15 min and residue was
discarded. Ethanol was removed from the super-natant by evaporation under vacuum at 35 – 40°C, and pigments were eliminated by two successive extractions with petroleum ether (2:1, v:v). After addition of ammonium sulphate (20%) and metaphosphoric acid (2%) to the aqueous phase, phenolic compounds were ex-tracted three times by ethyl acetate (1:1, v:v). The three organic phases were collected, evapo-rated and dried under vacuum at 35°C. The residue was re-dissolved in 10 ml of methanol and the methanolic extract was filtered through
Gelman Nylon Acrodisc 13 (0.45 mm) and
stored at −20°C before analysis by high
perfor-mance liquid chromatography.
2.3. Extraction and purification of anthocyanin
(1992) with some modifications. A total of 10 g peel was cut into small pieces and homogenised as described above in 100 ml of methanol (90%) containing HCl (1%) in an ice bath and extracted for 30 min. The homogenate was filtered through four layers of cheesecloth and the residue was added to 50 ml of the same extraction solvent for two successive re-extractions of the residue. The
collected filtrate was centrifuged at 7000×g for
15 min. The supernatant was evaporated to re-move methanol and dried under vacuum at 35°C. The residue was re-dissolved in 10 ml of methanol and the methanolic extract was filtered through
Gelman Nylon Acrodisc 13 (0.45mm) and stored
at −20°C before analysis by high performance
liquid chromatography.
2.4. HPLC analyses
The chromatographic conditions used were as
follows: an Alltima C18 column (250×4.6 mm, 5
mm size particle, Alltech Associates Applied
Sci-ence Ltd) was equipped with a Merck – Hitachi L-6200A Intelligent Pump at a flow rate of 1
ml/min and peaks were detected and analysed by
a Merck – Hitachi L-4500 Diode Array Detector at 520 nm for anthocyanins and 280 nm for other
phenolic compounds and a computer with
Merck – Hitachi Model D-6500 Chromatography Data Station Software-DAD system manager was used for data recording and processing. The mo-bile phase was (A) acidic water (2% acetic acid) and (B) acetonitrile – methanol (10:15, v/v). The best separation was obtained using the following gradient elution: at 0 min 90% A and 10% B, at 10 min 80% A and 20% B, at 15 min 70% A and 30% B, at 25 min 60% A and 40% B, at 30 min 50% A and 50% B and at 40 min 50% A and 50% B. Identification of the phenolic compounds was achieved by comparison with retention times of standards, UV spectra and calculation of UV absorbance ratios after co-injection of samples and standards. Commercial standards were
pur-chased from Sigma Chemicals and Fluka
Chemika-BioChemika. Each phenolic compound was quantified by comparison with a multipoint calibration curve obtained from the correspond-ing standard.
2.5. Substrate affinity for partially purified polyphenol oxidase from litchi peel
Partial purification of polyphenol oxidase was carried out according to the methods of Jiang et al. (1997) and Fujita et al. (1991) with some modifications using ammonium sulphate fraction-ation and two grades of gel filtrfraction-ation with a Sephadex G-150 column. The dialysed enzyme solution was applied to a Sephadex G-150 column. Enzyme activity was determined accord-ing to the method of Tan and Li (1984), by measuring the oxidation of 4-methylcatechol. The fractions with highest enzymatic activity were col-lected and pooled for measuring substrate affinity.
3. Results
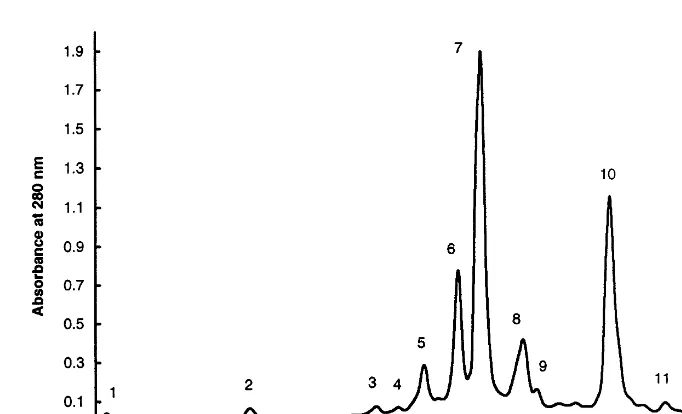
Litchi peel contained mainly flavan-3-ol
monomers and dimers (Fig. 1), such as (+
)-cate-chin, (+)-gallocatechin, (−)-epigallocatechin,
(−)-epicatechin, (−)-epicatechin 3-gallate, pro-cyanidin B1, propro-cyanidin B2 and propro-cyanidin B4, representing 87.0% of the total phenolic
com-pounds detected (Table 1). (−)-Epicatechin,
(−)-epicatechin 3-gallate, (−)-epigallocatechin and procyanidin B2 were found in very high concentrations. Gallic acid was the only hydroxy-benzoic derivative found and no hydroxycinnamic derivatives were detected.
Among the anthocyanins, the litchi peel con-tained mainly cyanidin-3-glucoside, representing 91.9% of the total with a small amount of malvidin-3-glucoside present (Table 1).
Unknown peak 9 had a spectrum similar to flavan-3-ol monomers and dimers and therefore possibly is in the flavan-3-ol family, while un-known peaks 11 and 12 could be derivatives of flavan-3-ols (Fig. 2). Unknown peak 13 may be a polymer (phenol oxidation product) that in-creased with browning and storage (Tables 2 – 4). The content of gallic acid and unknown peak 13 increased with storage at ambient and 4°C (Tables 2 and 3). At ambient temperature, gallic acid at day 7 was 2.7 times greater than at day 0. The contents of (+)-catechin, (+)-gallocatechin,
pro-cyanidin B1, propro-cyanidin B2, propro-cyanidin B4 and unknown peaks 9 and 11 declined with storage at ambient temperature and 4°C (Tables 2 and 3).
(−)-Epicatechin declined by 77.7% over 7 days at
ambient temperature. However, the contents of
peak 12 and (−)-epicatechin 3-gallate declined at ambient temperature and increased and then de-clined with storage at 4°C.
The peel at various browning stages was sam-pled from the fruit stored at 4°C. The content of
Fig. 1. Typical HPLC chromatogram of phenolic compounds of litchi peel. Peak identification: (1) gallic acid; (2) procyanidin B1; (3) (+)-catechin; (4) (+)-gallocatechin; (5) procyanidin B4; (6) procyanidin B2; (7) (−)-epicatechin; (8) (−)-epigallocatechin; (9) unknown peak; (10) (−)-epicatechin 3-gallate; (11) unknown peak; (12) unknown peak; (13) unknown peak.
Table 1
Phenolic compounds and anthocyanins in litchi peel at the arrival day
ac Areas (%) Phenolic compounds
Peak Rta Kb
– 0.4
1 Gallic acid 5.94 1.10
1.0 2.70
2.97
2 Procyanidin B1 11.23
(+)-Catechin 15.89 4.61 1.6
3 1.55
(+)-gallocatechin 16.71
4 4.90 1.06 1.1
Procyanidin B4 1.07 5.3
5 17.72 5.26
1.08
5.68 11.4
18.90 Procyanidin B2
6
32.5
(−)-Epicatechin 19.82 6.00 1.06
7
1.09 10.9
(−)-Epigallocatechin
8 21.37 6.55
Unknown peak 21.83
9 6.71 1.02 0.3
7.68 1.14 23.3
24.57 (−)-epicatechin 3-gallate
10
8.39
26.58 1.09
Unknown peak
11 1.7
4.1
Unknown peak 27.67 8.78 1.05
12
1.13 0.8
Unknown peak
13 30.96 9.94
91.9
Cyanidin-3-glucoside 20.61 7.70 –
A
14.35
B Malvidin-3-glucoside 36.38 1.86 3.8
aRt, retention time (min). bK, capacity factor (Rt
r−Rt0)/Rt0, Rt0=2.83. For anthocyanins, Rt0=2.37. ca, separation factor:K
Fig. 2. UV-Visible spectra of unknown peaks 9, 11, 12 and 13 from litchi peel extract. A, peak 9; b, peak 11; c, peak 12 and d, peak 13.
of browning and the contents of peak 13 in-creased. However, the contents of peak 12 and
(−)-epicatechin 3-gallate increased and then
de-clined with development of browning.
The contents of cyanidin-3-glucoside and
malvidin-3-glucoside declined during storage at both ambient temperature and 4°C (Tables 2 and 3). The decline also paralleled browning (Table 4). The affinity of (−)-epigallocatechin, (−
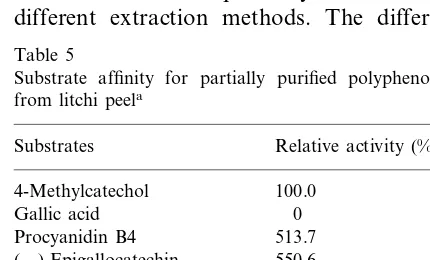
)-epi-catechin, (−)-epicatechin 3-gallate, procyanidin
B1, procyanidin B2 and procyanidin B4 for polyphenol oxidase was five times higher than for
4-methylcatechol. The affinity of (+)-catechin
and (+)-gallocatechin was 1.5 times higher than
4-methylcatechol (Table 5).
4. Discussion
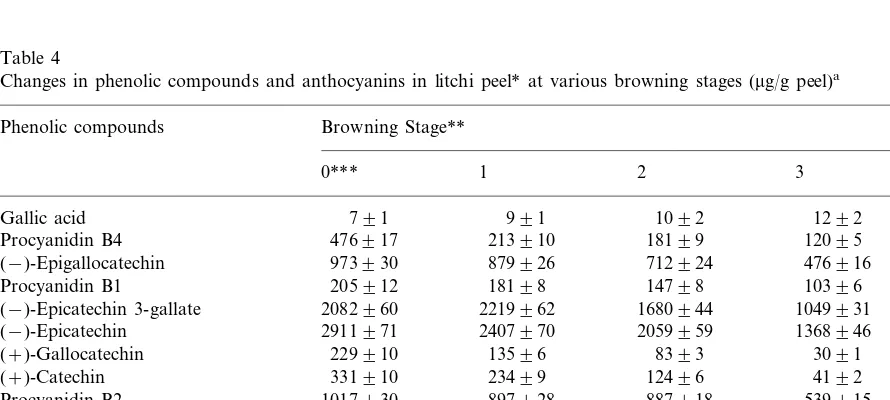
Oxidation of phenolic compounds is the main cause of browning in fruits (Macheix, et al., 1990). Tan and Li (1984) suggested that browning of litchi fruit would result mainly from enzymatic oxidation catalysed by PPO, associated with a loss of cellular compartmentation. When harvested at gallic acid increased with development of
brown-ing (Table 4). At brownbrown-ing stage 4, it was 2.7 times as high as in fresh peel without browning. The contents of (+)-catechin, (+)-gallocatechin,
(−)-epigallocatechin, (−)-epicatechin,
pro-cyanidin B1, propro-cyanidin B2, propro-cyanidin B4, peak 9 and peak 11 decreased with development
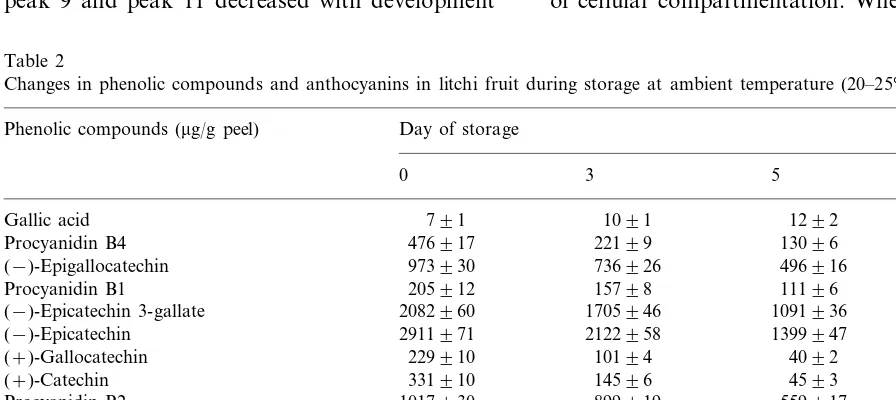
Table 2
Changes in phenolic compounds and anthocyanins in litchi fruit during storage at ambient temperature (20–25°C)a
Phenolic compounds (mg/g peel) Day of storage
3 5 7
0
1091
Gallic acid 791 1292 2091
Procyanidin B4 476917 22199 13096 5794
(−)-Epigallocatechin 973930 736926 496916 425915
9194 11196
15798
Procyanidin B1 205912
(−)-Epicatechin 3-gallate 2082960 1705946 1091936 826930
2911971 2122958
(−)-Epicatechin 1399947 648926
2191 4092
10194
(+)-Gallocatechin 229910
331910 14596
(+)-Catechin 4593 4092
1017930
Procyanidin B2 899919 559917 424913
14896 5393
Peak 9 4092 1991
Peak 11 15696 1091 891 NDb
19196 20597
28699 366910
Peak 12
7394 8694
Peak 13 11295 13796
Cyanidin-3-glucoside 596921 423917 312915 243914
2491 1891
Malvidin-3-glucoside 1491 991
aEach data point represents the mean of three individual experiments9S.E. (n=9). The contents of peaks 9, 11, 12 and 13 were
calculated against (−)-epicatechin.
Table 3
Changes in phenolic compounds and anthocyanins in litchi fruit during storage at 4°Ca
Phenolic compounds (mg/g peel) Day of Storage
7 14 21
0 28 35
891 891 1092 1292 2091
Gallic acid 791
429914 233911 19099
476917 13196
Procyanidin B4 5693
973930
(−)-Epigallocatechin 951929 890930 732929 496919 423915
205912
Procyanidin B1 201910 19199 15198 11397 9695
2113961 2224963 1710947
2082960 1099931
(−)-Epicatechin 3-gallate 830931
2860970
(−)-Epicatechin 2911971 2437971 2129960 1399951 641927
210910 14697 8994
229910 3292
(+)-Gallocatechin 2091
331910
(+)-Catechin 307910 24499 13796 4893 4091
1017930
Procyanidin B2 979931 911929 891919 541917 425915
12395 9695 4593
14896 3592
Peak 9 1991
15696
Peak 11 8893 3392 1291 891 ND*
369910 375911 26097
366910 20296
Peak 12 19196
7793 7894 8594
Peak 13 7394 10995 13397
552920 516920 403915
596921 297914
Cyanidin-3-glucoside 250913
2291 2091 1891
Malvidin-3-glucoside 2491 1491 1091
aEach data point represents the mean of three individual experiments9S.E. (n=9). The contents of peaks 9, 11, 12 and 13 were
calculated against (−)-epicatechin. * ND means not detectable.
Table 4
Changes in phenolic compounds and anthocyanins in litchi peel* at various browning stages (mg/g peel)a
Phenolic compounds Browning Stage**
1 2 3 4
0***
991 1092
Gallic acid 791 1292 2091
Procyanidin B4 476917 213910 18199 12095 4793
973930
(−)-Epigallocatechin 879926 712924 476916 403912
18198 14798
205912 10396
Procyanidin B1 9094
2082960
(−)-Epicatechin 3-gallate 2219962 1680944 1049931 823928
2911971
(−)-Epicatechin 2407970 2059959 1368946 628926
13596 8393
229910 3091
(+)-Gallocatechin 2091
(+)-Catechin 331910 23499 12496 4192 3892
897928 887918
1017930 539915
Procyanidin B2 414915
14896
Peak 9 9494 4392 3392 1791
Peak 11 15696 3092 991 791 ND****
366910 373910 25698
Peak 12 19996 19096
Peak 13 7394 8094 8694 11195 13596
495920 388916
596921 288915
Cyanidin-3-glucoside 231913
1991 1591 1291 990
Malvidin-3-glucoside 2491
a* Litchi peel at various browning stage was collected and sampled from the fruit stored at 4°C. ** Browning stages: 0, no
bright and full red maturation, active metabolism in pericarp tissues continues and cells are able to maintain membrane integrity (Liu et al., 1991). With the extension of storage, cells gradually lose the integrity of the membrane system, which re-sults in the loss of compartmentation allowing enzymes to act on their substrates. Tan and Zhou (1987) suggested that simple phenols similar to catechol are possible substrates for enzymatic oxi-dation. However, flavan-3-ol monomers and dimers were major phenolic compounds in litchi peel (Table 1), representing about 87% of the total phenolic compounds, that declined with storage or browning. The affinity of some flavan-3-ol monomers and dimers to polyphenol oxidase was five times greater than 4-methylcatechol, which is frequently used for measuring PPO activity (Tan and Li, 1984; Lin et al., 1988a; Macheix et al., 1990). Therefore we suggest that flavan-3-ol monomers and dimers are major substrates for enzymatic oxidation rather than simple phenols.
Lee and Wicker (1990) reported that cyanidin-3-glucoside, cyanidin-3-rutinoside and malvidin-3-glucoside were identified as the major monomeric anthocyanin pigments in litchi fruit. In our re-search, we found that cyanidin-3-glucoside was a major anthocyanin in this cultivar and there is also a small amount of malvidin-3-glucoside. The different results are probably due to the use of different extraction methods. The difference in
anthocyanins between our results and those previ-ously reported by Lee and Wicker (1990) could also reflect cultivar differences.
Underhill and Critchley (1994) suggested that changes in anthocyanin is one of the causes of browning. However, degradation of anthocyanins is the result of coupled oxidation in the presence of other phenolics (Kader et al., 1998). Cyanidin-3-glucoside declined with storage or browning (Tables 2 – 4), which implies that anthocyanin con-tributes to the browning possibly through the coupled oxidation.
Partially purified PPO from litchi peel had stronger specificity for flavan-3-ol monomers and dimers (Table 5). Therefore, we suggest that flavan-3-ol monomers and dimers are major phe-nolic compounds as substrates for enzymatic oxi-dation, causing browning during postharvest storage. In addition, flavan-3-ols participate in the structure of proanthocyanidins (condensed tan-nins) as their monomers (Macheix, et al., 1990). Tannins, which are generally considered as proan-thocyanidins and glucose polyesters of gallic acid of hexahydroxydiphenic acids, range in colour from yellowish-white to light brown (Fennema, 1996). Therefore, we suggest that tannins poly-merized and formed from flavan-3-ols may con-tribute to the discoloration of litchi fruit.
Acknowledgements
The authors are grateful to Professor Chen Fang, Professor Dr Jiang Yueming, Associate Professor Liu Shuxian and Li Yuebiao of the first author’s former colleagues in South China Insti-tute of Botany, Guangzhou, People’s Republic of China, who organised the express transfer of litchi fruit from China to England.
References
Akamine, E.K., 1960. Preventing the darkening of fresh litchi prepared for export. Technol. Progr. Rep. Hawaii Agri. Exp. Stat. 127, 1 – 17.
Amiot, M.J., Tacchini, M., Aubert, S., Nicolas, J., 1992. Phenolic composition and browning susceptibility of vari-ous apple cultivars at maturity. J. Food Sci. 57, 958 – 962. Table 5
Substrate affinity for partially purified polyphenol oxidase from litchi peela
Substrates Relative activity (%)
4-Methylcatechol 100.0 0 Gallic acid
513.7 Procyanidin B4
(−)-Epigallocatechin 550.6 Procyanidin B1 501.1 (−)-Epicatechin 3-gallate 546.5 566.6
aAll substrates were presented at a concentration of 1 mM.
Campbell, C.W., 1959. Storage behaviour of fresh Brewster and Bengal lychees. Pro. Florida State Hort. Soc. 72, 356 – 360.
Chen, W.S., 1984. A brief profile on studies of postharvest storage of litchi fruit. Litchi Sci. Bull. 3-4, 47 – 51. Fennema, O.R., 1996. Food Chemistry, Third ed. Marcel
Dekker, New York, pp. 651 – 722.
Fujita, S., Tono, T., Kawahara, H., 1991. Purification and properties of polyphenol oxidase in head lettuce (Lactuca sati6a). J. Sci. Food Agric. 55, 643 – 651.
Guangdong Postharvest Research Group, 1975. Studies on prevention of peel browning of frozen litchi fruit. Acta Bot. Sinica. 17, 303 – 308.
Jiang, Y.M., Zauberman, G., Fuchs, Y., 1997. Partial purifica-tion and some properties of polyphenol oxidase extracted from litchi fruit pericarp. Postharvest Biol. Technol. 10, 221 – 228.
Kader, F., Haluk, J.P., Nicolas, J.P., Metche, M., 1998. Degradation of cyanidin 3-glucoside by blueberry polyphe-nol oxidase-kinetic studies and mechanisms. J. Agri. Food Chem. 46, 3060 – 3065.
Lee, H.S., Wicker, L., 1990. Anthocyanin pigments in the skin of lychee fruit. J. Food Sci. 56, 466 – 468, 483.
Lin, Z.F., Li, S.S., Zhang, D.L., Lin, G.Z., Li, Y.B., Liu, S.X., Chen, M.D., 1988a. The changes of pigments, phenolic contents and activities of polyphenol oxidase and pheny-lalanine ammonia-lyase in pericarp of postharvest litchi fruit. Acta Bot. Sin. 30, 40 – 45.
Lin, Z.F., Li, S.S., Zhang, D.L., Lin, G.Z., Li, Y.B., Liu, S.X., Chen, M.D., 1988b. The changes of oxidation and peroxi-dation in postharvest litchi fruit. Acta Bot. Sin. 30, 382 – 387.
Liu, S.X., Jiang, Y.M., Chen, F., Zhang, D.L., Li, Y.B., 1991. The relationship between the browning in pericarp of litchi (Litchi chinensis Sonn.) fruits and polyphenol oxidase, peroxidase, phenolics and their compartmentation. Acta Bot. Austro Sin. 7, 95 – 98.
Macheix, J.J., Fleuriet, A., Billot, J., 1990. Fruit Phenolics. CRC Press, Boca Raton, FL, pp. 1 – 24.
Mayen, M., Baron, R., Merida, J., Medina, M., 1997. Changes in phenolic compounds during accelerated browning in white wines from cv.Pedro Ximenezand cv.Baladi grapes. Food Chem. 58, 89 – 95.
Mayer, A.M., Harel, E., 1991. In: Fox, P.F. (Ed.), Phenoloxi-dases and their significance in fruit and vegetables, in Food Enzymology, vol. 1. Elsevier, London, pp. 373 – 398. Mazza, G., Velioglu, Y.S., 1992. Anthocyanins and other
phenolic compounds in fruits of red-flesh apples. Food Chem. 43, 113 – 117.
Nip, W.K., 1988. Handling and preservation of lychee (Litchi chinensisSonn.) with emphasis on colour retention. Trop. Sci. 28, 5 – 11.
Nicolas, J.J., Richard-Forget, F.C., Goupy, P.M., Amiot, M.J., Aubert, S., 1994. Enzymatic browning reactions in apple and apple products. C. Rev. Food Sci. Nutri. 34, 109 – 157.
Scott, K.J., Brown, B.I., Chaplin, G.R., Wilcox, M.E., Bain, J.M., 1982. The control of rotting and browning of lychee fruit by hot benomyl and plastic film. Sci. Hort. 16, 253 – 262.
Tan, X.J., Li, Y.B., 1984. Partial purification and properties of polyphenol oxidase in litchi fruit peel. Acta Phytophys. Sin. 10, 339 – 346.
Tan, X.J., Zhou, Y.C., 1987. Studies on the enzymatic brown-ing of Litchi chinensis pericarp by polyphenol oxidase. Acta Phytophys. Sin. 13, 197 – 203.
Underhill, S.J.R., Critchley, C., 1994. Anthocyanin decolouri-sation and its role in lychee pericarp browning. Aust J. Exp. Agri. 34, 115 – 122.
Zhang, D., Quantick, P.C., 1997. Effects of chitosan coating on enzymatic browning and decay during postharvest stor-age of litchi (Litchi chinensisSonn.) fruit. Postharvest Biol. Technol. 12, 195 – 202.