Postharvest Biology and Technology 21 (2000) 21 – 37
Heat treatment and fruit ripening
Robert E. Paull *, Nancy Jung Chen
Department of Tropical Plant and Soil Sciences,College of Tropical Agriculture and Human Resources,
Uni6ersity of Hawaii at Manoa,3190Maile Way,Honolulu,HI96822-2279,USA
Received 25 April 2000; accepted 15 August 2000
Abstract
Postharvest heat treatments lead to an alteration of gene expression and fruit ripening can sometimes be either delayed or disrupted. The extent of the alternation of fruit ripening is a function of the exposure temperature and duration and how quickly the commodity is cooled following the heat treatment. The most commonly measured components of fruit ripening affected by heat treatments include fruit softening, membrane and flavor changes, respiration rate, ethylene production, and volatile production. Cell wall degrading enzymes and ethylene production are frequently the most disrupted and are sometimes not produced or their appearance is delayed following heating. Other processes associated with ripening are not altered to the same extent or soon recover. Fruit sensitivity to heat treatments is modified by preharvest weather conditions, cultivar, rate of heating, and subsequent storage conditions. The amount of sensitivity or tolerance to heat stress of a commodity is related to the level of heat protective proteins at harvest and the postharvest production of heat shock proteins. Two types of heat responses are seen. The first is a normal cellular response (B42°C) that can lead to reduced chilling sensitivity, delayed or slowed ripening and a
modification of quality. The second occurs near the threshold for damage \45°C and is modified by the pre-stress
environmental conditions, the cellular response to stress and cellular recovery. Loss of membrane integrity appears to be an effect and not a cause of injury. The site of the injury lesion is still unknown and could be associated with transcription, translation and cellular recovery capacity after an injury threshold has been exceeded. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Heat resistance; Physiology; Biochemistry; Recovery; Injury threshold
www.elsevier.com/locate/postharvbio
1. Introduction
Heat treatments (thermotherapy) have been used for over a century to free plant materials from pathogens, the temperature and exposure
duration having been determined empirically (Grondeau and Samson, 1994). Postharvest heat treatments of fruit are used for insect disinfesta-tion, disease control, to modify fruit responses to other stresses and maintain fruit quality during storage (Armstrong et al., 1989; Couey, 1989; Klein and Lurie, 1990; Paull, 1994; Paull and McDonald, 1994; Lurie, 1998; McDonald et al., 1999). Postharvest exposure to temperatures B * Corresponding author. Tel.: +1-808-9568389; fax: +
1-808-9563993894.
E-mail address:[email protected] (R.E. Paull).
40 – 42°C often increases storage life and improves the flavor of a number of fruit (Barber and Sharpe, 1971; Liu, 1978; Lurie, 1998; Shellie and Mangan, 1998). The symptoms that occur at higher temperatures (\45°C) are skin scald, and failure of the fruit to soften fully, or softening at a reduced rate (Jones, 1942; Akamine and Seo, 1978; Chan et al., 1981; Kerbel et al., 1985; Couey, 1989; Paull and Chen, 1990).
There are many hypotheses to explain heat injury (Crisan, 1973; Alexandrov, 1977; Levitt, 1980). Most of the concepts and experimental evidence involve protein denaturation, disruption of protein synthesis and loss of membrane in-tegrity. Protein denaturation at lethal tempera-tures is regarded as non-reversible, while lower temperatures can lead to a reversible inactivation (Brandts, 1967; Alexandrov, 1977; Bernstam, 1978). However, the temperatures and exposure times involved in protein denaturation in vitro are considerably above those used in postharvest treatments and may not apply to the same protein in vivo. Less than lethal temperatures and dura-tion can lead to short-term reversible disrupdura-tion of transcription and translation steps in protein synthesis (Bernstam, 1978; Vierling, 1991; Lurie et al., 1996b).
Though much is known about the changes that follow heat treatment of fruit, how this leads to cellular death is unanswered. The answer may be different for chronic versus acute exposure to extreme temperature. Other factors that influence the temperature responses include species and va-riety under test and pretreatment heat exposure history. This complexity has led to some difficulty in interpretation of the published studies. The need is to determine the underlying physiological changes that are possibly reversible, and deter-mine when the cellular disruption exceeds the cell’s ability for repair and recovery.
2. Heat responses and criteria
The difficulty faced in evaluation of heat toler-ance limits for fruit ripening is in selection of criteria. The criteria (cytological disturbance, metabolic depression and necrosis) are dependent
upon the temperature chosen and exposure time. However, overlaying these criteria are cellular protection mechanisms, ability to repair damage and the mechanism by which these are influenced by heat stress. Tied closely to these criteria is the timing of observations.
Cytological changes are well suited for small tissues but may not reflect the situation in larger plant organs such as a fruit. At the other extreme is the most often measured criterion, necrosis. Though necrosis is an integrative measure, its development reflects a cascading effect that may take days to develop, then remains constant. Tis-sue necrosis is often associated with
polyphe-noloxidase (PPO) activity, although, PPO
expression and activity are also affected by heat treatments. In leaf tissue, heat treatments from 45 to 55°C for B1 min significantly decrease PPO activity along with the synthesis of phenolic com-pounds (Loaiza-Velarde et al., 1997). Since nu-merous stresses can lead to the oxidation of polyphenols, this metabolic response provides lit-tle information on methods to minimize damage or an explanation of the initial events during and immediately following heat stress.
3. Temperature stress
pre-R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 23
harvest thermal history of a fruit. Surface temper-atures of dark green fruit may reach 24°C above ambient air temperatures while light-colored fruit may only be 10 – 12°C above ambient (Barber and Sharpe, 1971). The factors involved in field-in-duced thermotolerance have been reviewed by Woolf and Ferguson (2000) in this issue. The role of cultivar in heat responses has been less well studied, though differences are found for grape-fruit (Miller and McDonald, 1991), and observa-tions suggest ‘Kapoho’ papaya is more susceptible than ‘Sunrise’ (Paull, 1995), while no difference was found for two apples varieties (Klein and Lurie, 1990).
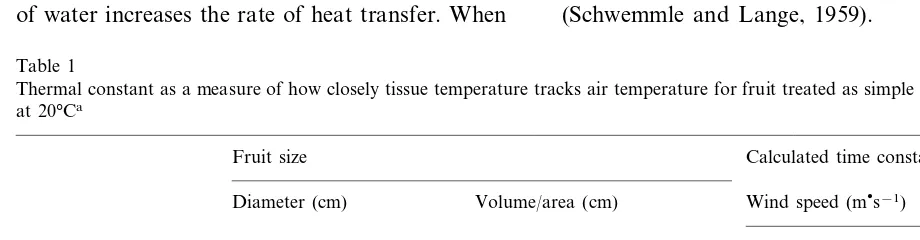
Fruit size, shape, and morphology, play a sig-nificant role by affecting the uniformity of tissue heating. Uniformity of heating is related to the rate of heat conduction, which in plant tissue is low, being similar to water. For apple fruit the thermal conductivity is B0.5 Wm−2K−1and as for many fruit, is less than water 0.6 Wm−2K−1
(Sweat, 1974). The time constant to heat the fruit center will normally be longer than the calculated time (Table 1) and is measurably longer than the surface. For example it is less than a few minutes for small fruit and 300 min for a jack fruit. The time constant is further reduced at higher wind speeds that reduce the boundary layer thickness, thereby affecting heat transfer. If water is used as the heat transfer medium, the boundary layer is reduced even further and the greater heat capacity of water increases the rate of heat transfer. When
both water and air heating is used, the mass of fruit tissue is not uniformly treated, there being a gradient from surface to the center in both tem-perature and duration. These differences are cru-cial in commercru-cial heat treatments and reflect a need to uniformly heat a mass of fruit.
Another factor may be involved that overlies the field induction of thermotolerance. Ferguson et al. (1998) have shown that the development of
thermotolerance associated with heat shock
protein (HSP) in apple fruit follows a daily cycle. The high levels of HSPs in shaded fruit are main-tained over the night period and drop after the onset of daytime. The minimum for HSP-70 mes-sage occurs about midday. Part of the cycle of HSP RNA levels reflects the lag in response shown in fruit due to buffering of thermal changes as the highest fruit temperatures are achieved about 15:00 h. The same loss kinetics is found for papayas where HSP RNAs decline 8 – 24 h after the HS induction treatment (Paull and Chen, 1990). However, this may also reflect diur-nal rhythms. Piechulla and Gruissem (1987) have shown that such rhythms in gene expression do occur in developing tomato fruits. As found for chilling injury (Patterson et al., 1978), a circadian rhythm in thermotolerance may exist, aside from the diurnal ambient temperature changes. Some crop plants are less injured by a 5 min exposure to 50°C during noon than during the rest of the day (Laude, 1939). Other plants are less injured by
46°C, if treated during the dark period
(Schwemmle and Lange, 1959).
Table 1
Thermal constant as a measure of how closely tissue temperature tracks air temperature for fruit treated as simple geometric shapes at 20°Ca
Fruit size Calculated time constant (min)
Wind speed (ms−1)
Volume/area (cm) Diameter (cm)
1 4
0.6 0.1
Rowan berry 0.71 0.3
3
6 1
Crab apple 16 7.7
60
Jack fruit 10 300 170
A short exposure to near-lethal temperature is a ‘crisis’ situation that relates to survival and the capacity to recover, while longer exposure to lower temperatures is more difficult to quantify. Growth and metabolism can also be adversely affected below such ‘crisis’ temperatures. For ex-ample, avocado fruits stored at 30 and 34°C ripened abnormally, showed considerable surface pitting, and poor flavor. Ethylene treatment in-creases the threshold temperature for injury in avocado (Lee and Young, 1984). Exposure of pears (Maxie et al., 1974), and tomatoes (Ogura et al., 1976; Yoshida et al., 1984; Biggs et al., 1988; Picton and Grierson, 1988; Lurie and Klein, 1992) to 30°C for 48 h or more results in a delay of various aspects of ripening such as pigmentation, softening, and by a marked short-term decline in ethylene production (Maxie et al., 1974; Yoshida et al., 1984; Biggs et al., 1988; Picton and Grier-son, 1988; An and Paull, 1990), and reduced volatiles (McDonald et al., 1999).
The maximum tolerance of plants, normally in the range of 42 – 60°C, correlates roughly with the original evolutionary habitat (Kappen, 1981). The lethal temperature has been reported to be 45°C for tomatoes, 63°C for grapes, and 49 – 52°C for apples (Huber, 1935). This variation in lethal temperature can be traced in part to exposure duration. Couey (1989) grouped fruits into heat-tolerant and heat-sensitive to hot water treatment. The heat-tolerant fruits were bananas, papaya, peppers and cantaloupes. The difficulty with the division is that thermotolerance is dependent upon cultivars, season, fruit size, maturity and postharvest handling (Jones, 1942; Sinclair and Lindgren, 1955; Sharp et al., 1989; McGuire and Reeder, 1992). Heat tolerance also differs signifi-cantly between different organs on one plant, stems being more tolerant than leaves, and the leaf epidermis and stomata being more tolerant than mesophyll. However, the differences re-ported in heat tolerance may reflect, in part, actual thermal adaptation. This difference in ther-mal tolerance is displayed by papaya fruit har-vested at different times of the year (Paull, 1995) and apples harvested from different locations (Ferguson et al., 1998).
4. Response to heat treatment
4.1. Cellular manifestations
The gross cytological changes that follow high
temperature exposure (\45°C) have been
de-scribed (Belehradek, 1957) and include coagula-tion of the cytoplasm, cytolysis, nuclear changes and altered mitosis. Protoplasmic streaming is inhibited, and there is increased protoplasmic vis-cosity and a loss of membrane permeability
(Alex-androv, 1977). Lower temperatures (B40°C)
have no observed effects on cytology (Cheng et al., 1988). Heating fruit also changes the cuticle structure by reducing the number of cracks in apples (Lurie et al., 1996a), cuticle permeability in peaches (Phillips and Austin, 1982), and enhanced water loss in grapefruit (Porat et al., 2000), though no similar change is found for oranges (Williams et al., 1994).
Cellular membranes have been stressed with respect to heat injury (Alexandrov, 1977), and the role of membranes in cell death induced by heat has been reviewed (Blum, 1987). A sigmoidal curve describes electrolyte leakage by tomato discs induced by increasing temperature (Inaba and Crandall, 1988). Critical exposures times for leakage were 34 min at 55°C, 105 min at 50°C and 166 min at 45°C. The tomatoes did not, however, exhibit visible heat injury. A 50% higher elec-trolyte leakage was found from apple fruit discs isolated after being held at 38°C for 2 days (Lurie and Klein, 1990); after the disks were transferred to 20°C, leakage declined within 2 days, to con-trol levels. However, little change has been re-ported in the first few hours of exposure, suggesting membrane changes may be an effect, not a cause of damage.
R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 25
desaturase was silenced have lower levels of trienoic fatty acids and are better able to accli-mate to higher temperature (Murakami et al., 2000). The resistance in these transgenic plants to heat is not transient and is unlike the protection conferred by induction of HSPs. The tentative conclusion is that the loss of cellular membrane integrity with increasing temperatures is gradual, with repair and reversibility possible until a lethal cell temperature is reached. The acclimation to high temperature stress does involve the mem-branes and develops following a number of days of exposure to elevated temperatures.
4.2. Physiological manifestations
4.2.1. Respiration
The respiration rate of ripening fruit is initially increased by exposure to higher temperatures (Jones, 1939; Akamine, 1966; Maxie et al., 1974; Ogura et al., 1976; Inaba and Chachin, 1988, 1989; Klein and Lurie, 1990; Lurie and Klein, 1991; Mitcham and McDonald, 1993). The re-sponse to temperature varies with the physiologi-cal age of the tissue. The temperature coefficient (Q10) of apple fruit declines from 2.8 in young
fruit to 1.6 in fruit 6 weeks later over the range 10 – 30°C (Jones, 1981). Though misleading if Q10
is applied outside the range tested, the difference in physiological age is of significance. After the heat treatment, the respiration rate declines to near or below the level of the non-heated control. Twelve days or more at ca. 33°C results in the suppression of respiration in tomato fruit and this action is not completely reversed after returning the tomato fruit to ambient temperatures (Ogura et al., 1976; Yoshida et al., 1984; Cheng et al., 1988; Inaba and Chachin, 1988). Heat treatments do have an impact on the subsequent climacteric respiratory rise. The increase between the initial preclimacteric level and the climacteric peak in avocado fruit was 250% at 25°C, and only 30% at 30°C (Biale and Young, 1971). During heat treat-ments at 43 – 48°C, ripening fruit initially show an elevated respiration rate in papayas (Jones, 1939; Paull and Chen, 1990) and mangoes (Mitcham and McDonald, 1993) that declines to the same level or below of fruit that received no heat
treatment. The extent and timing of the initial peak in papayas depend upon the relative humid-ity of the heated air (Jones, 1939). The heat-treated fruit climacteric peak is delayed about 2 days (Mitcham and McDonald, 1993). Similarly in apples, the respiration is lower upon return of heated fruit to ambient temperatures compared to non-heated fruit (Klein and Lurie, 1990).
The respiratory control ratio is higher in tomato mitochondria isolated from fruit stressed for 3 days at 37°C (Cheng et al., 1988) and mitochondrial Ca2+-ATPase activity is also very significantly reduced by the same stress. There is a shift with chronic heat exposure to the cyanide insensitive pathway (Inaba and Chachin, 1989). The shift to alternative oxidative phosphorylation may reflect disruption of the thermoregulatory role of this pathway in order to maintain cellular stasis (Breidenbach et al., 1997).
4.2.2. Ethylene
Ethylene synthesis is reversibly inhibited at higher temperatures (Biale, 1960; Field, 1984). Fruit exposed to long periods at high temperature quickly recover their ability for ethylene synthesis (Ogura et al., 1976; Biggs et al., 1988; Dunlap et al., 1990). The conversion of ACC to C2H4 is
ethyl-ene-related mRNA during heat treatment (Picton and Grierson, 1988), or reactivation of pre-exist-ing mRNA and/or proteins.
Temperatures \35°C cause an accumulation in endogenous ACC content in apple and tomato tissue (Yu et al., 1980; Atta Aly, 1992) and re-duced ethylene production in both fruits (Biggs et al., 1988; Klein, 1989). The accumulation of ACC does not occur in fruit subjected to higher temper-atures or for longer duration. ACC synthase is therefore regarded as less sensitive to loss due to heat stress than ACC oxidase in tomato and apple (Klein, 1989; Atta Aly, 1992). ACC synthase ac-tivity is lost in mangoes and only partially recov-ers on return to ambient temperatures (Ketsa et al., 1999). The differences in response between the oxidase and the synthase may be related to differ-ences in turnover rates.
Wound-induced ethylene synthesis is less sensi-tive to heat stress inactivation than climacteric-re-lated ethylene synthesis (Biggs et al., 1988). Pears, tomatoes and bananas do not respond to exoge-nous ethylene during heat treatment (Maxie et al., 1974; Seymour et al., 1987; Yang et al., 1990), and it has been suggested (Yang et al., 1990; Lurie, 1998) that this loss of sensitivity is associated with inactivation of ethylene receptors. Result with the ethylene receptor inhibitor, 1-methycyclopropene, suggest that recovery of ethylene sensitivity in plum and banana occurs in 4 – 5 days (Abdi et al., 1998; Golding et al., 1998), which does not agree with the recovery time found for tomatoes heated for 12 h (Biggs et al., 1988). Disruption of ethyl-ene synthesis may mediate heat-induced ripening inhibition (Lurie, 1998), but its effect is more likely to be part of an injury response, related possibly to recovery of ethylene synthesis and membrane disruption. Heat treatments that cause injury do engender a burst of ‘wound’ ethylene 1 – 2 days after treatment that is followed by a subsequent advanced ethylene climacteric peak four days later, and sooner than the peak in papaya fruit not subject to an injurious heat treatment (Paull and Chen, 1990).
4.2.3. Softening and cell wall metabolism
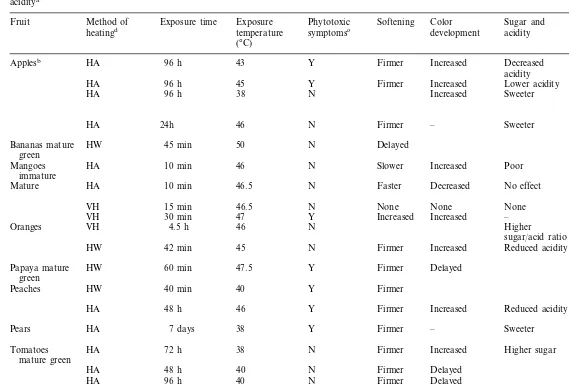
Flesh softening is often slowed following expo-sure to 38 – 40°C (Table 2), even if the treatment is
applied for an extended period (4 days) before storage (Klein and Lurie, 1990, 1992; Lurie and Nussinovitch, 1996). Following disinfestation temperatures of 45 – 50°C softening is faster (Mitcham and McDonald, 1992; Shellie and Man-gan, 1994) or disrupted (Paull and Chen, 1990).
Exposure of apples to 38°C for 4 days resulted in fruit with less soluble and more insoluble pectin (Klein et al., 1990; Ben-Shalom et al., 1993, 1996). Tomatoes had less soluble polyuronides, and had less galactose and arabinose loss after 96 h at 40°C (Mitcham and McDonald, 1992). Heat-in-jured papaya fruit that fail to soften, also show little change in soluble pectin amounts or molecu-lar weights during ripening (Qiu et al., 1995). The softening disruption has been ascribed to reduc-tion of cell wall hydrolytic enzymes. Though heat disruption of cell wall breakdown has been pro-posed as the cause of delayed or poor softening, the actual enzyme having the central role in soft-ening has not been determined (Lashbrook et al., 1998; Rose et al., 1998). The findings with differ-ent cell wall degrading enzymes do show that the disruption may be associated with mRNA
synthe-sis and stability, or protein synthesis and
degradation.
In the range 35 – 60°C, considerable (87%) loss of activity of purified glucanase from tomato fruit can occur (Hinton and Pressey, 1980), and 50% glucanase activity is lost by exposure to 50°C for 5 min (Pressey, 1983). Tomato endo-mannase and galactosidase are rapidly lost and the activity returns 4 and 14 days, respectively, after heating at 40°C for 2 days (Sozzi et al., 1996). The role of these enzymes in fruit softening is unclear. Simi-larly, pectinesterase is suppressed in tomatoes held at 33°C (Ogura et al., 1975), though there is little difference in the degree of apple pectin ester-ification after treatment at 38°C for 4 days (Klein et al., 1995).
R
Response of fruits to various exposure times and temperatures with respect to the development of phytotoxic symptoms, softening, skin color development, sugar and aciditya
Method of Exposure time
Fruit Exposure Phytotoxic Softening Color Sugar and Source temperature symptomsc development acidity
heatingd
(°C)
Y Firmer Increased Decreased
96 h Klein and Lurie
Applesb HA 43
(1990) acidity
Increased Lower acidity Liu (1978)
45 Y Firmer
96 h HA
HA 96 h 38 N Increased Sweeter Lurie and Nussinovitch (1996)
– Sweeter Klein and Lurie Firmer
N
HA 24h 46
(1992)
HW 45 min N Delayed Armstrong (1982)
Bananas mature 50 green
Slower Increased Poor
Mangoes HA 10 min 46 N Jacobi et al. (1995) immature
Decreased No effect Jacobi and Giles Faster
N Mature HA 10 min 46.5
(1997)
VH 15 min 46.5 N None None None Jacobi et al. (1995) Increased –
VH 30 min 47 Y Increased
Higher
VH 4.5 h 46 N Shellie and
Oranges
sugar/acid ratio Mangan (1998) HW 42 min 45 N Firmer Increased Reduced acidity Williams et al.
(1994) Firmer Delayed
47.5 Y
Papaya mature HW 60 min Paull and Chen
green (1990)
HA 48 h 46 Y Increased Reduced acidity Kerbel et al. (1985) – Sweeter
Pears HA 7 days 38 Y Firmer Spotts and Chen (1987)
Firmer Increased Higher sugar
38 Lurie and Klein
Tomatoes HA 72 h N
(1992) mature green
N Firmer Delayed Sozzi et al. (1996) 48 h 40
72 h 37 Inaba and Chachin
HA N Firmer
(1989)
aSelected recent references — for earlier references refer to Paull (1990).
bDamage to fruit is very dependent upon cultivar, size, shape, maturity and handling. cPhytotoxicity: Y–Yes; N–No.
mesocarp shows no carotenoids (Paull and Chen, 1983). This suggests that the inner mesocarp is at a more advanced stage of ripening and at a stage which may be more susceptible to heat stress than the less mature outer region (Paull and Chen, 1990). Papaya cell wall polygalacturonase and xylanase production occurs over a short period during ripening that contrasts with the continual production of polygalacturonase in tomatoes (Grierson, 1986) and cellulase in avocado (Christ-offersen et al., 1982).
Chan et al. (1981) observed that papaya fruit with 25 or 50% of the skin yellow had less poly-galacturonase than fruit at the color-break stage after 65 min exposure to 46°C. A similar reduc-tion was found for tomatoes held for several days at 33°C (Ogura et al., 1975). These results could be due to either denaturation or disruption of polygalacturonase synthesis. Since polygalactur-onase shows first order heat denaturation kinetics and is 60% denatured by 20 min at 70°C (Chan and Tam, 1982), exposure of fruit to 49°C would be expected to induce minimal denaturation in papaya. The failure to soften, therefore, is possi-bly attributable to suppression of the mRNA for wall softening enzymes, as found in tomatoes (Picton and Grierson, 1988). The loss of poly-galacturonase mRNA in tomatoes is also not overcome by exogenous ethylene (Picton and Gri-erson, 1988). Polygalacturonase activity, however, does return after a 6 day lag when returned to 25°C (Ogura et al., 1975; Yoshida et al., 1984). A similar reversibility in fruit softening has been found in apples held at 38°C for 4 days (Lurie and Klein, 1990).
4.2.4. Chlorophyll and carotenoid metabolism Heating leaves to 44°C leads to loss of chloro-plast activity, especially electron transport by photosystem II (Berry et al., 1975). An associated characteristic, delayed light emission, declines in papaya skin with first order kinetics when fruit are heated between 42 and 48°C (Chan and For-bus, 1988). At higher temperatures (49 – 51°C), heat inactivation is biphasic.
Above 25°C, bananas (Burg, 1962; Blackbourn et al., 1989) fail to degreen, while above 30°C papaya fruit fail to ripen normally, with the pulp
becoming soft and watery, although the skin chlorophyll changes are not affected (An and Paull, 1990). Degreening is accelerated in apples (Liu, 1978; Klein et al., 1990; Lurie and Klein, 1990), plantain and tomato (Seymour et al., 1987; Lurie and Klein, 1991) at 35 – 40°C for 4 days and delayed with a shorter treatment of 2 days at 40°C in tomato (Sozzi et al., 1996). The difference in tomato response suggests that a threshold dura-tion has been exceeded. This is supported from studies with maple leaves (McCain et al., 1989) where the damage occurred over a narrow range from 53 to 57°C. In soybean leaves, 1 min at 53°C causes no damage, while at 54°C chlorosis occurs, and necrosis at greater than at 55°C (Coleman et al., 1988). Alternatively, at all temperatures, dehy-dration at the higher temperature alters the de-greening rate.
Carotenoid synthesis is inhibited by
tempera-tures \30°C (Goodwin and Jamikorn, 1952;
Cheng et al., 1988; Lurie et al., 1996b; Sozzi et al., 1996). The inhibition of lycopene synthesis in tomatoes (Cheng et al., 1988; Lurie et al., 1996b; Sozzi et al., 1996) is due to inhibition of mRNA transcription of lycopene synthesis that recovers after removal of the heat.
4.2.5. Fla6or and 6olatile production
The impact on flavor varies with species, tem-perature and duration of the heat treatment. Av-ocado flavor is adversely affect by treatment at 43°C for longer than 5 h (Kerbel et al., 1987). Juice from heat-treated grapefruit (46°C — 5 h or 48°C — 3 h) from early and mid-season fruit was not different from unheated fruit (McGuire and Reeder, 1992), while juices from heated late-sea-son fruit or grapefruit placed in a constant tem-perature water bath instead of gradual heating
were judged significantly inferior (McGuire,
sub-R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 29
jected to a high humidity heat treatment (Jacobi et al., 1995; Jacobi and Giles, 1997).
The sugar to acid ratio is often a measure used to determine the effect of heat treatments on quality (Table 2). At lower treatment tempera-tures (38°C, 4 days), apples have a higher ratio immediately following treatment due mainly to reduced acid levels (Liu, 1978; Porritt and Lidster, 1978; Klein and Lurie, 1990). Titratable acidity is also reduced in nectarines subjected to treatments of 41 – 46°C for 24 – 48 h (Lay-Yee and Rose, 1994), a similar reduction occurring in strawberry acidity (Garacia et al., 1995). There is generally no significant effect on soluble solids. Mango soluble solids are not affected by an insect vapor heat treatment (Jacobi et al., 1995; Jacobi and Giles, 1997). Grapefruit (46 – 50°C for up to 7 days) and oranges (46 – 47.5°C plus storage) showed a similar effect: reduced acidity, no effect on soluble solids and a higher sugar/acid ratio (McGuire, 1991; Miller and McDonald, 1991; McGuire and Reeder, 1992; Shellie and Mangan, 1998). Heat treatments, either water or hot air (38 – 48°C for 1 h to 3 days) have no effect on tomato soluble solids or acidity (Lurie and Klein, 1991; Lurie and Sabehat, 1997; McDonald et al., 1999). The variable effects of heat treatments on sugars and acidity, most often titratability acidity, depends upon the temperature used and duration. Apple volatile production, though enhanced during a 38°C treatment is immediately inhibited after heat treatments and recovers slowly (Fallik et al., 1997). Tomato volatile flavor level and profile is altered by temperature treatments of 1 h increasing from 39 – 48°C; two components in-creased and five dein-creased, out of the 15 analyzed (McDonald et al., 1996, 1999)
4.2.6. Protein metabolism
Exposure of ripening pear fruit to 40°C leads to a disruption of protein synthesis via a loss of polysomes (Romani and French, 1977). This re-sponse is readily reversible and establishes a close correlation between the protein synthesizing ma-chinery and the progression of ripening. Apples held at 38°C for 4 days had reduced S35
-methion-ine incorporation into proteins (Lurie and Klein, 1990). HSP synthesis in pear cells is also greater at
39°C than above 40°C, when the treatment is extended for up to 8 h (Ferguson et al., 1994). Papaya fruit protein synthesis changes following exposure to 38°C for 2 h and there is no accumu-lation in the control fruit held at 22°C for 10 h. Translated polysomal RNA confirmed that new polypeptides are synthesized following heat shock induction (Paull and Chen, 1990). Thermotoler-ance decreases with continued exposure of fruit to 42°C (Paull and Chen, 1990) suggesting that 42°C is the limit for induction of HS tolerance and protein degradation is enhanced, as found for pear cells (Ferguson et al., 1994). Ubiquitin may not be involved in specific protein degradation above 40°C. Continued exposure to 42°C, though still allowing HS polypeptide synthesis, may also cause damage. These results agree with those for tomato fruit treated for long periods at 35°C (Picton and Grierson, 1988). Apple held for 4 days at 38°C had a change in protein synthesis profiles (Lurie and Klein, 1990). The new protein bands on electrophoresis gels at the low molecular weight (14 – 22 kD) and high molecular weight (68 and 92 kD) ranges (Lurie and Klein, 1990) corre-spond with HSP positions and agree with the results obtained with papaya (Paull and Chen, 1990).
Thermal injury (46°C, 65 – 90 min) to papaya fruit softening during ripening is correlated with a 90% decrease in normal polygalacturonase levels (Chan et al., 1981), the injury being more severe in riper fruit. These researchers also noted the same conditions as those observed in our labora-tory (Paull and Chen, 1990) of heated fruit show-ing delayed ripenshow-ing and leavshow-ing a 1 – 1.5 cm thick area of hard tissue. Partial recovery to 25% of normal polygalacturonase levels occurred after 6 days at 24°C (Chan et al., 1981). The conclusion from this work was that transcription is disrupted by heat. This disruption could possibly occur by mRNA being released from the ribosomes at 40°C (Romani and French, 1977; Stuger et al., 1999). Protein degradation, particularly of rate limiting enzymes, may continue at a higher rate due to the higher temperature (Vierstra, 1994; Callis, 1995). This degradation could lead, if a threshold was
exceeded, to disruption of metabolic fluxes
4.2.7. Nucleic acid metabolism
Synthesis of ripening-specific mRNAs and new enzymes is induced during ripening (Christof-fersen et al., 1982; Tucker and Grierson, 1982; Spiers et al., 1984; Grierson, 1986). Elevated stress temperatures may lead to inhibition and/or re-duced expression of softening and other ripening-related mRNA (Picton and Grierson, 1988). Transcriptional regulation is probably modified by mRNA stability. Post-translational modifica-tion may also be involved in regulating the overall accumulation of cell wall degrading enzymes (Biggs and Handa, 1988). The inhibition of ripen-ing above 30°C has been ascribed to disruption of the enzymes involved in ethylene synthesis (Maxie et al., 1974; Ogura et al., 1975; Lurie, 1998), loss of cell wall degrading enzymes (Chan et al., 1981), and to suppression of ripening-related mRNA synthesis (Picton and Grierson, 1988). Heat-stressed cells re-program their translational
ma-chinery in favor of HSPs (Nover, 1991).
Translation of other transcripts is almost com-pletely suppressed. However, most untranslated messages are not degraded (Nover et al., 1989). These messages may be secreted into storage par-ticles within small molecular weight HSPs that appear after prolonged stress (Stuger et al., 1999) The failure of heated fruit to soften suggests (a) a failure to transcribe, (b) a loss of mRNAs coding for wall softening enzymes, or (c) a failure to translate mRNAs. In addition, heating could activate enzymes or enhance enzyme turnover leading to cellular damage (Swartz et al., 1956; Callis, 1995) directly, or indirectly by destroying cellular compartmentation.
5. Mechanism of responses to high temperature
HSPs produced in response to high temperature are believed to prevent irreversible protein denat-uration that would be detrimental to the cell (Parsell and Linquist, 1993) and this activity may be enhanced in plants by small HSPs (Lee and Vierling, 2000). HSPs and associated thermotoler-ance have been reported for field-grown cotton leaves (Burke et al., 1985), soybean leaves (Kim-pel and Key, 1985), sorghum (Ougham and
Stod-dart, 1986), papaya fruit (Paull and Chen, 1990) and apples (Klein and Lurie, 1990; Ferguson et al., 1998). These proteins are produced within 30 min after exposure to temperatures in the range 34 – 42°C (Kanabus et al., 1984). The lag period for induction for HS response is slower than other stress responses (Trewavas, 1999), and there is a synergistic interaction with ABA (Xiong et al., 1999). The decay of HSPs occurs, with a corre-sponding loss in thermotolerance. This phe-nomenon appears to confer a temporary, acquired
heat resistance to sub-lethal temperatures
(Altschuler and Mascarenhas, 1982). There is a fundamental role for HSPs in cellular function during high temperature stress (Vierling, 1991; Queitsch et al., 2000). Arabidopsis plants express-ing less than usual amounts of HSP101, as a result of mutation, antisense or co-suppression, grow at normal rates but have severely diminished capacity to acquire heat tolerance after mild con-ditioning pretreatments (Queitsch et al., 2000; Hong and Vierling, 2000). The smaller MW HSPs protect cellular proteins from thermal aggregation (Vierling, 1997) and protein folding activity and HS-induced protein aggregation preventive activ-ity are integrated (Lee and Vierling, 2000). Other HSPs have unique functions, such as the reactiva-tion of proteins that have already aggregated or promote the degradation of misfolded proteins. Different mechanisms of tolerance seem to be induced by sudden HS and that acquired by sus-tained growth at a moderately high temperature (Wu and Wallner, 1984). Membrane fatty acid desaturation plays a role in acclimation to higher temperatures (Murakami et al., 2000).
HS may induce reactive oxygen intermediates that can cause membrane and protein damage. The creation of oxygen radical scavengers, such as superoxide dismutases, peroxidases, and catalases, is induced by HS (Holmberg and Bulow, 1998). HS also induces ascorbate peroxidase in tomato associated with a HS cis-element in the promoter (Storozhenko et al., 1998). The reported effect of reactive oxygen is supported by HSPs being pro-duced at higher temperature when the tempera-ture is raised slowly (3°C h−1) than when the
in-R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 31
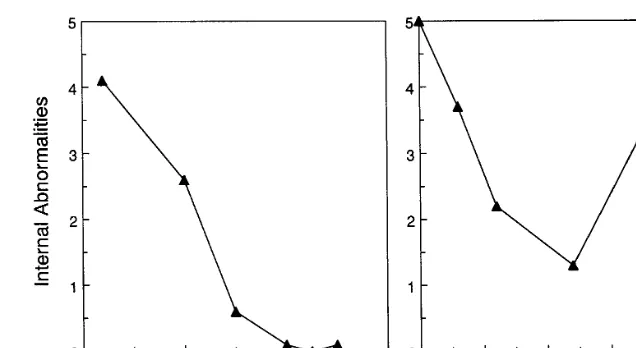
Fig. 1. Effect of pretreatment temperature for one hour (A) and pretreatment time at 38°C (B) in hot water on the development of internal abnormalities in papaya flesh. The internal abnormalities are due to a failure of the flesh to soften, the procedures used are described in Paull and Chen (1990). After pretreatment, fruit were held for 3 h at 22°C before exposure to 70 min at 49°C.
crease allows adaptation, and emphasizes the im-portance of the heat-stress profile (final tempera-ture and duration). It has also been suggested that the amount of free iron present in a cell reduces oxidative damage, as transgenic tobacco plants expressing alfalfa ferritin show reduced damage to abiotic and pathogenic stress (Deak et al., 1999). Selections of mutant plants have also shown that a number of different types of thermostability may exist (Mullarkey and Jones, 2000).
Many procedures have been empirically devel-oped to reduce the injury caused by heat treat-ment. The disruption of papaya fruit softening by the injurious heat treatment is reduced or pre-vented by a pretreatment at 42°C for 1h (Fig. 1A) or varying the pretreatment time at 38°C (Fig. 1B), these treatments being followed by 3 h at 22°C (Paull and Chen, 1990). The greater damage to papaya heated at 38°C for longer than 2 h may be due to fruit undergoing ripening and riper fruit are more sensitive to damage than mature green fruit. The 8 h approach time to 44°C for the papaya vapor heat treatment (Seo et al., 1974) is designed to reduce subsequent injury when the fruit is exposed to 44°C for 8.75 h. This vapor heat treatment approach time would be expected to provide the conditions necessary to develop HSP and associated thermotolerance. The rapid
rise of temperature to 49°C using vapor heat always damages ‘Valencia’ oranges and grapefruit (Sinclair and Lindgren, 1955), but these fruit are tolerant when heated for 8 h at 43°C, before the 49°C treatment. Cucumber can be preconditioned by a 24 h treatment at 32.5°C to tolerate subse-quent exposure for 50 min to hot water at 46°C (Chan and Linse, 1989). Though 32.5°C is lower than the temperature normally used to induce HSP, the longer (24 h) duration would probably induce HSPs and thermotolerance. An under-standing of the conditions necessary to induce HSPs and thermotolerance are essential to opti-mize heat treatments used for insect disinfestation and disease control.
6. Conclusions
Table 3
Proposed impact of two levels of heat stress,B40–42°C, and\45°C, on the immediate effects on plant metabolism including heat
shock (HS) protein synthesis and upon return to ambient temperaturesa
Return to ambient temperatures Heat stress (°C) Immediate effects
–
B42 Respiration Increased Returns to control levels
Ethylene – Decreased Recovers slowly
Transcription – HS-mRNA produced No longer transcribed
– Other mRNA suppressed Returns to normal transcription Translation – HS proteins produced Decline over 18 h
mRNA – Sequestered Returns for translation
– Degradation continues at a higher rate
Protein Returns to normal degradation rate
Reactive – Increased Returns to normal levels oxygen
–
\45 Respiration Increased Returns to control levels
Ethylene – Decreased Very slowly returns over days – HS mRNAs poorly inducted
Transcription No longer transcribed
– mRNA synthesis reduced Returns depending upon stress Translation – Polysomes lost Returns depending upon stress Protein – Degradation continues at a higher rate, Returns depending upon stress. Beyond
threshold, unbalanced metabolism leads to especially rate limiting enzymes that may
have higher turnover rates necrosis
Reactive – Increases damage to proteins and If threshold for damage is exceeded, leads to necrosis
membranes oxygen
aNecrosis is defined as cell death due to environmental stress beyond a threshold level for which the cells’ cellular machinery is
unable to maintain integrity or recover during or following removal of the stress.
R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 33
possibly diurnal variations are modifying factors. The second type of response occurs when the stress exceeds a threshold and the cells’ ability to recover is lost (Fig. 2). This response is associated with the disruption of various aspects of ripening. The site of the lesion may be associated with either or both transcription and translation and a cellular threshold of metabolic disruption.
There are similarly two areas in need of an-swers, and an understanding of these should en-able us to develop better approaches to avoiding injury and maintaining fruit quality. The first area is the role of adaptation including the effect of HSP, fruit physiological status and cultivar differ-ences. The second area focuses on the heat lesion. The question evolves around the impact of heat treatments on transcription, translation, enzyme turnover and cellular recovery capacity.
Acknowledgements
We thank Mrs Gail Uruu and Dr Ching Cheng Chen for technical help. This is the College of Tropical Agriculture and Human Resource, Jour-nal Series 4490.
References
Abdi, N., McGlasson, W.B., Holford, P., Williams, M., Mizrahi, Y., 1998. Responses of climacteric and sup-pressed-climacteric plums to treatment with propylene and 1-methycyclopropene. Postharvest Biol. Technol. 14, 29 – 39.
Akamine, E.K., 1966. Respiration of fruits of papaya (Carica papayaL., var. Solo) with reference to the effect of quaran-tine disinfestation treatments. Proc. Am. Soc. Hort. Sci. 89, 231 – 236.
Akamine, E.K., Seo, S.S., 1978. Progress report on search for alternative disinfestation treatments for export papaya. Proceedings of the Fourteenth Annual Hawaii Papaya Industry Association Conference. University of Hawaii. Cooperative Extension Service. Misc. Publ. 167.
Alexandrov, Ya V., 1977. Cells, Molecules and Temperature. Translated from the Russian by V.A. Bernstam. Springer-Verlag, Berlin, Heidelberg, NY.
Altschuler, M., Mascarenhas, J.P., 1982. Heat shock proteins and effects of heat shock in plants. Plant Mol. Biol. 1, 103 – 115.
An, R.F., Paull, R.E., 1990. Storage temperature and ethylene on the ripening of papaya fruit. J. Am. Soc. Hort. Sci. 115, 949 – 953.
Armstrong, J.W., 1982. Development of a hot-water immer-sion quarantine treatment for Hawaiian grown ‘Brazilian’ bananas. J. Econ. Entomol. 75, 787 – 790.
Armstrong, J.W., Hansen, J.D., Hu, B.K.S., Brown, S.A., 1989. High-temperature, forced air quarantine treatment for papaya infested with Tephritid fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 82, 1667 – 1674.
Atta Aly, M.A., 1992. Effects of high temperature on ethylene biosynthesis by tomato fruits. Postharvest Biol. Technol. 2, 19 – 24.
Barber, H.N., Sharpe, P.J.H., 1971. Genetics and physiology of sunscald of fruits. Agric. Meteorol. 8, 175 – 191. Belehradek, J., 1957. Physiological aspects of heat and cold.
Annu. Rev. Physiol. 19, 59 – 82.
Ben-Shalom, N., Hanzon, J., Klein, J.D., Lurie, S., 1993. A postharvest heat treatment inhibits cell wall degradation in apples during storage. Phytochemistry 34, 955 – 958. Ben-Shalom, N., Hanzon, J., Pinto, R., Lurie, S., 1996. Cell
wall changes and partial prevention of fruit softening in pre-storage heat treated ‘Anna’ apples. J. Sci. Food Agric. 72, 231 – 234.
Bernstam, V.A., 1978. Heat effects on protein biosynthesis. Annu. Rev. Plant Physiol. 29, 25 – 46.
Berry, J.A., Fork, D.C., Garrison, S., 1975. Mechanistic stud-ies of thermal damage to leaves. Carnegie Inst. Washington Year Book 74, 751 – 759.
Biale, J.B., 1960. Respiration of fruits. In: Ruhland, W. (Ed.), Handbuch Der Pflanzenphysiologie. Springer-Verlag, Berlin, pp. 536 – 592.
Biale, J.B., Young, R.E., 1971. The avocado pear. In: Hulme, A.C. (Ed.), The Biochemistry of Fruits and Their Products. Academic Press, New York, pp. 1 – 63.
Biggs, M.S., Handa, A.K., 1988. Temporal regulation of poly-galacturonase gene expression of fruits of normal, mutant, and heterozygous tomato genotypes. Plant Physiol. 89, 117 – 125.
Biggs, M.S., Woodson, W.R., Handa, A.K., 1988. Biochemical basis of high temperature inhibition of ethylene biosynthe-sis in ripening tomato fruit. Physiol. Plant 72, 572 – 578. Blackbourn, H., John, P., Jeger, M., 1989. The effect of high
temperature on degreening in ripening bananas. Acta Hor-tic. 258, 271 – 278.
Blum, A., 1987. Plant Breeding for Stress Environments. CRC Press, Boca Raton, Florida.
Brandts, J.F., 1967. Heat effects on proteins and enzymes. In: Rose, A.H. (Ed.), Thermobiology. Academic Press, NY, pp. 25 – 72.
Breidenbach, R.W., Saxton, R.J., Hansen, L.D., Criddle, R.S., 1997. Heat generation and dissipation in plants: Can the alternative oxidative phosphoylation pathway serve a ther-moregulatory role in plant tissue other than specialized organs? Plant Physiol. 114, 1137 – 1140.
Burke, J.J., Hatfield, J.L., Klein, R.R., Mullet, J.E., 1985. Accumulation of heat shock proteins in field grown cot-ton. Plant Physiol. 78, 394 – 398.
Burmeister, D.M., Ball, S., Green, S., Woolf, A.B., 1997. Interaction of hot water treatments and controlled atmo-sphere storage on quality of ‘Fuyu’ persimons. Posthar-vest Biol. Technol. 12, 71 – 81.
Callis, J., 1995. Regulation of protein degradation. Plant Cell 7, 845 – 857.
Chan, H.T., 1986a. Effects of heat treatments on the ethyl-ene-forming enzyme system in papaya. J. Food Sci. 51, 581 – 583.
Chan, H.T., 1986b. Heat inactivation of the ethylene form-ing enzyme system in cucumber. J. Food Sci. 51, 1491 – 1493.
Chan, H.T., Jr., Tam, S.Y.T., 1982. Partial separation and characterization of papaya endo- and exo-polygalactur-onase. J. Food Sci. 47, 1478 – 1483.
Chan, H.T., Forbus, W.B., 1988. Delayed light emission as a biochemical indicator of papaya heat treatment. J. Food Sci. 53, 1490 – 1492.
Chan, H.T., Linse, E., 1989. Conditioning cucumbers for quarantine heat treatments. HortScience 24, 985 – 989. Chan, H.T., Jr., Tam, S.Y.T., Seo, S.T., 1981. Papaya
poly-galacturonase and its role in thermally injured ripening fruit. J. Food Sci. 46, 190 – 197.
Cheng, T.S., Floros, J.D., Shewfelt, R.L., Chang, C.J., 1988. The effect of high-temperature stress on ripening of tomatoes (Lycopersicon esculentum). J. Plant Physiol. 132, 459 – 464.
Christoffersen, R.E., Warm, E., Laties, G.E., 1982. Gene expression during fruit ripening in avocado. Planta 155, 52 – 57.
Coleman, W.J., Govindjee, Gutowsky, H.S., 1988. The effect of chloride on the thermal inactivation of oxygen evolu-tion. Photosynth. Res. 16, 261 – 275.
Couey, H.M., 1989. Heat treatment for control of posthar-vest disease and insect pests of fruit. HortScience 24, 198 – 202.
Crisan, E.V., 1973. Current concepts of thermophilism and the thermophilic fungi. Mycologia 65, 1171 – 1198. Deak, M., Horvath, G.V., Danietova, S., Torok, K., Sass,
L., Vass, I., Barna, B., Kiraly, Z., Dudits, D., 1999. Plants ectopically expressing the iron-binding protein, fer-ritin, are tolerant to oxidative damage and pathogens. Nat. Biotechnol. 17, 192 – 196.
Dunlap, J.R., Lingle, S.E., Lester, G.E., 1990. Ethylene pro-duction in netted muskmelon subjected to postharvest heating and refrigerated storage. HortScience 25, 207 – 209.
Fallik, E., Archbold, A.A., Hamilton-Kemp, T.R., Loughrin, J.H., Collas, R.W., 1997. Heat treatment temporarily in-hibits aroma volatile compound emission from ‘Golden Delicious’ apples. J. Food Chem. 45, 4038 – 4041. Ferguson, I.B., Lurie, S., Bowen, J.H., 1994. Protein
synthe-sis and breakdown during heat shock of cultured pear
(Pyrus communis. L.) cells. Plant Physiol. 104, 1429 – 1437.
Ferguson, I.B., Snelgar, W., Lay-Yee, M., Watkins, C.B., Bowen, J.H., 1998. Expression of heat shock proteins in apple fruit in the field. Aust. J. Plant Physiol. 25, 155 – 163.
Field, R.J., 1984. The effect of temperature on ethylene pro-duction by plant tissue. In: Roberts, J.A., Tucker, G.A. (Eds.), Ethylene and Plant Development. Butterworths, London, pp. 47 – 69.
Garacia, J.M., Aguilera, C., Albi, M.A., 1995. Postharvest heat treatment in Spanish strawberry (Fragaria x Ananassa cv. Tudla). J. Agric. Food Chem. 43, 1489 – 1492.
Golding, J.B., Shearer, D., Wyllie, S.G., McGlasson, W.B., 1998. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87 – 98.
Gombos, Z., Wada, H., Hideg, E., Murata, N., 1994. The unsaturation of membrane lipids stabilizes photosynthesis against heat stress. Plant Physiol. 104, 563 – 567.
Goodwin, T.W., Jamikorn, M., 1952. Biosynthesis of carote-nes in ripening tomatoes. Nature 170, 104 – 105.
Grierson, D., 1986. Molecular biology of fruit ripening. In: Miflin, B.J. (Ed.), Plant Molecular and Cell Biology. Ox-ford University Press, London, pp. 363 – 383.
Grondeau, C., Samson, R., 1994. A review of thermopathy to free plant materials from pathogens, especially seeds from bacteria. Crit. Rev. Plant Sci. 13, 57 – 75.
Hinton, D.M., Pressey, R., 1980. Glucanases in fruits and vegetables. J. Am. Soc. Hort. Sci. 105, 499 – 502. Holmberg, N., Bulow, I., 1998. Improving stress tolerances
in plants by gene transfer. Trends Plant Sci. 2, 61 – 66. Hong, S-W., Vierling, E., 2000. Mutants of Arabidopsis
thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Nat. Acad. Sci. 97, 4392 – 4397. Huber, H., 1935. Der Warmehaushalt der pflanzen.
Natur-wiss. Landwirtsch. 17, 1 – 148.
Inaba, M., Chachin, K., 1988. Influence of and recovery from high-temperature stress on harvested mature green tomatoes. HortScience 23, 190 – 192.
Inaba, M., Chachin, K., 1989. Electrolyte leakage as an indi-cator of high temperature injury to harvested mature green tomatoes. J. Am. Sci. Hort. Sci. 113, 96 – 99. Inaba, M., Crandall, P.G., 1988. Electrolyte leakage as an
indicator of high temperature injury to harvested mature green tomatoes. J. Am. Soc. Hort. Sci. 113, 96 – 99. Jacobi, K.K., Giles, J.E., 1997. Quality of ‘Kensington’
mango (Mangifera indica L.) fruit following continued vapor heat disinfestation and hot water disease control treatments. Postharvest Biol. Technol. 12, 285 – 292. Jacobi, K., Giles, E., MacRae, E., Wegrzyn, T., 1995.
Con-ditioning ‘Kensington’ mango with hot air alleviates hot water disinfestation injuries. HortScience 30, 562 – 565. Jones, H.G., 1981. Carbon dioxide exchange of developing
apple fruits. J. Exp. Bot. 321, 1203 – 1210.
R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 35
Jones, W.W., 1942. Respiration and chemical changes of the papaya fruit in relation to temperature. Plant Physiol. 17, 481 – 486.
Kanabus, J., Pikaad, C.S., Cherry, J.H., 1984. Heat shock proteins in tobacco cell suspension during growth cycle. Plant Physiol. 75, 639 – 644.
Kappen, L., 1981. Ecological significance of resistance to high temperature. In: Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H. (Eds.), Physiological Plant Ecology. I. Re-sponses to the physical environment. Springer Verlag, Berlin, pp. 439 – 474.
Kerbel, E.L., Mitchell, F.G., Mayer, G., 1985. Effect of postharvest heat treatments for insect control on the qual-ity and market life of peaches. HortScience 20, 725 – 727. Kerbel, E.L., Mitchell, F.G., Mayer, G., 1987. Effect of
postharvest heat treatments for insect control on the qual-ity and market life of avocados. HortScience 22, 92 – 94. Ketsa, S., Chidtragool, S., Klein, J.D., Lurie, S., 1999.
Ethyl-ene synthesis in mango fruit following heat treatment. Postharvest Biol. Technol. 15, 65 – 72.
Kimpel, J.A., Key, J.L., 1985. Presence of heat shock mRNA’s in field grown soybeans. Plant Physiol. 79, 672 – 678. Klein, J.D., 1989. Ethylene biosynthesis in heat treated apples.
In: Clijsters, H., de Proft, M., Marcelle, R., Van Pouche, M. (Eds.), Biochemical and Physiological aspects of ethyl-ene production in lower and higher plants. Kluwer, Dor-drecht, The Netherlands, pp. 184 – 190.
Klein, J.D., Hanzon, J.D., Irwin, P.L., Ben-Shalom, N., Lurie, S., 1995. Pectin esterase activity and pectin methyl esterfi-cation in heated ‘Golden Delicious’ apples. Phytochemistry 39, 491 – 494.
Klein, J.D., Lurie, S., 1990. Prestorage heat treatment as a means of improving poststorage quality of apples. J. Am. Soc. Hort. Sci. 115, 265 – 269.
Klein, J.D., Lurie, S., 1992. Prestorage heating of apple fruit for enhanced postharvest quality: Interaction of time and temperature. HortScience 27, 326 – 328.
Klein, J.D., Lurie, S., Ben-Arie, R., 1990. Quality and cell wall components of ‘Anna’ and ‘Granny Smith’ apples treated with heat, calcium and ethylene. J. Am. Soc. Hort. Sci. 115, 954 – 958.
Lashbrook, C.C., Brummell, D.A., Rose, J.K.C., Bennett, A.B., 1998. Non-pectolytic cell wall metabolism during fruit ripening. In: Giovannoni, J.J. (Ed.), Fruit Molecular Biology, Harvard Academic Press, Reading, U.K. (In press-Preprint provided by senior author).
Laude, H.H., 1939. Diurnal cycle of heat resistance in plants. Science 89, 556 – 557.
Lay-Yee, M., Rose, K.J., 1994. Quality of ‘Fantasia’ nec-tarines following forced-air heat treatments for insect disin-festation. HortScience 29, 663 – 666.
Lee, G.J., Vierling, E., 2000. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 122, 189 – 197. Lee, S.K., Young, R.E., 1984. Temperature sensitivity of
avocado fruit in relation to C2H4 treatment. J. Am. Soc.
Hort. Sci. 109, 689 – 692.
Levitt, J., 1980. Responses of Plants to Environmental Stresses, second ed, vol. I. Academic Press, New York. Liu, F.W., 1978. Modification of apple quality by high
tem-perature. J. Am. Soc. Hort. Sci. 103, 730 – 732.
Loaiza-Velarde, J.G., Tomas-Barbera, F.A., Saltveit, M.E., 1997. Effect of intensity and duration of heat shock treat-ments on wound-induced phenolic metabolism in Iceberg lettuce. J. Am. Soc. Hort. Sci. 122, 873 – 877.
Lurie, S., 1998. Postharvest heat treatments. Postharvest Biol. Technol. 14, 257 – 269.
Lurie, S., Klein, J.D., 1990. Heat treatment of ripening apples: Differential effects on physiology and biochemistry. Phys-iol. Plant 78, 181 – 186.
Lurie, S., Klein, J.D., 1991. Acquisition of low temperature tolerance in tomatoes by exposure to high temperature stress. J. Am. Soc. Hort. Sci. 116, 1007 – 1012.
Lurie, S., Klein, J.D., 1992. Ripening characteristics of toma-toes stored at 12 and 2°C following a prestorage heat treatment. Scientia Hortic. 51, 55 – 64.
Lurie, S., Nussinovitch, A., 1996. Compression characteristic, firmness and texture perception of heated and unheated apples. Intern. J. Food Sci. Technol. 31, 1 – 5.
Lurie, S., Sabehat, A., 1997. Prestorage temperature manipu-late to reduce chilling injury in tomatoes. Postharvest Biol. Technol. 11, 57 – 61.
Lurie, S., Othman, S., Borochov, A., 1995. Effects of heat treatment on plasma membrane of apple fruit. Postharvest Biol. Technol. 5, 29 – 38.
Lurie, S., Fallik, E., Klein, J.D., 1996a. The effect of heat treatment on apple epicuticular wax and calcium uptake. Postharvest Biol. Technol. 8, 271 – 277.
Lurie, S., Handros, A., Fallek, E., Shapira, R., 1996b. Re-versible inhibition of tomato fruit gene expression at high temperature. Plant Physiol. 110, 1207 – 1214.
Maxie, E.C., Mitchell, F.G., Sommer, N.F., Snyder, R.G., Rae, H.L., 1974. Effects of elevated temperatures on ripen-ing of ‘Bartlett’ pearsPyrus communisL. J. Am. Soc. Hort. Sci. 99, 344 – 349.
McCain, D.C., Groxdale, J., Markey, J.L., 1989. Thermal damage to chloroplast envelope membranes. Plant Physiol. 90, 606 – 608.
McDonald, R.E., McCollum, T.G., Baldwin, E.A., 1996. Prestorage heat treatments influence free sterols and flavor volatiles of tomatoes stored at chilling temperature. J. Am. Soc. Hort. Sci. 121, 531 – 536.
McDonald, R.E., McCollum, T.G., Baldwin, E.A., 1999. Tem-perature of water treatments influences tomato fruit quality following low temperature storage. Postharvest Biol. Tech-nol. 16, 147 – 155.
McGuire, R.G., 1991. Market quality of grapefruit after heat quarantine treatments. HortScience 26, 1393 – 1395. McGuire, R.G., Reeder, W.F., 1992. Predicting market quality
of grapefruit after hot air quarantine treatment. J. Am. Soc. Hort. Science 117, 90 – 95.
Mitcham, E.J., McDonald, R.E., 1992. Effect of high tempera-ture on cell wall modifications associated with tomato fruit ripening. Postharvest Biol. Technol. 1, 257 – 264.
Mitcham, E.J., McDonald, R.E., 1993. Respiration rate, inter-nal atmosphere, ethanol and acetaldehyde accumulation in heat-treatment mango fruit. Postharvest Biol. Technol. 3, 77 – 86.
Monteith, J.L., 1981. Coupling of plants to the atmosphere. In: Grace, J., Ford, E.D., Jarvis, P.G. (Eds.), Plants and their Atmospheric Environment. Blackwell, Oxford. UK, pp. 1 – 29.
Mullarkey, M., Jones, P., 2000. Isolation and analysis of thermotolerance mutants of wheat. J. Exp. Bot. 51, 137 – 146.
Murakami, Y., Tsuyama, M., Kobayashi, Y., Kodama, H., Iba, K., 2000. Trienoic fatty acids and plant tolerance of high temperature. Science 287, 476 – 479.
Nover, L., 1991. Translational control. In: Nover, L. (Ed.), Heat Shock Response. CRC Press, Boca Raton, Florida, pp. 299 – 324.
Nover, L., Scharf, D., Neumann, D., 1989. Cytoplasmic heat shock granules are formed from precursors particles and are associated with specific set of mRNAs. Mol. Cell Biol. 9, 1298 – 1308.
Ogura, N., Nakagawa, H., Takehana, H., 1975. Effect of storage temperature of tomato fruits on changes of their polygalacturonase and pectinesterase activities accompa-nied with ripening. J. Agric. Chem. Soc. Japan 49, 271 – 274.
Ogura, N., Hayashi, Ogishima, T., Abe, Y., Nakegawa, H., Takehana, H., 1976. Ethylene production by tomato fruits at various temperatures and effect of ethylene treatment on fruits. J. Agric. Chem. Soc. Japan 50, 519 – 523.
Ougham, H.J., Stoddart, J.L., 1986. Synthesis of heat shock proteins and acquisition of thermotolerance in high tem-perature tolerant and high temtem-perature susceptible lines of sorghum. Plant Sci. 44, 163 – 167.
Parsell, D.A., Linquist, S., 1993. The function of heat shock proteins in stress tolerance degradation and reactivation of proteins. Annu. Rev. Genet. 27, 437 – 496.
Patterson, B.D., Paull, R.E., Smillie, R.M., 1978. Chilling resistance inLycopersicon hirsutumHumb & Bonpl., a wild tomato with altitudinal distribution. Aust. J. Plant Physiol. 5, 609 – 617.
Paull, R.E., 1990. Postharvest heat treatments and fruit ripen-ing. Postharvest News Inf. 1, 355 – 361.
Paull, R.E., 1994. Response of tropical commodities to insect disinfestation treatments. HortScience 29, 16 – 24. Paull, R.E., 1995. Preharvest factors and the heat sensitivity of
field grown ripening papaya fruit. Postharvest Biol. Tech-nol. 6, 167 – 175.
Paull, R.E., Chen, N.J., 1983. Postharvest variation in cell wall-degrading enzymes of papaya (Carica papayaL.) dur-ing fruit ripendur-ing. Plant Physiol. 72, 382 – 385.
Paull, R.E., Chen, N.J., 1990. Heat shock response in field grown ripening papaya fruit. J. Am. Soc. Hort. Sci. 115, 623 – 631.
Paull, R.E., McDonald, R.E., 1994. Heat and cold treatments. In: Paull, R.E., Amstrong, J.W. (Eds.), Insect Pests and Fresh Horticultural Products: Treatments and Responses. CAB International, Wallingford, UK, pp. 191 – 222. Phillips, D.J., Austin, R.K., 1982. Changes in peaches after
hot water treatment. Plant Dis. Rep. 66, 487 – 488. Piechulla, B., Gruissem, W., 1987. Diurnal mRNA
fluctua-tions of nuclear and plastid genes in developing tomato fruits. EMBO J. 6, 3593 – 3599.
Picton, S., Grierson, D., 1988. Inhibition of expression of tomato-ripening genes at high temperature. Plant Cell En-viron. 11, 265 – 272.
Porat, R., Pavencello, D., Peretz, J., Ben-Yohoshua, S., Lurie, S., 2000. Effects of various heat treatments on the induc-tion of cold tolerance and on the post harvest qualities of ‘Star Ruby’ grapefruit. Postharvest Biol. Technol. 18, 159 – 165.
Porritt, S., Lidster, P., 1978. The effect of prestorage heating on ripening and senescence of apples during cold storage. J. Am. Soc. Hort. Sci. 103, 584 – 587.
Pressey, R., 1983. beta-Galactosidases in ripening tomatoes. Plant Physiol. 7l, 132 – 135.
Qiu, Y., Nishina, M.S., Paull, R.E., 1995. Papaya fruit growth, calcium uptake and fruit ripening. J. Am. Soc. Hort. Sci. 1204, 246 – 253.
Queitsch, C., Hong, S-W., Vierling, E., Linquist, S., 2000. Heat Shock Protein 101 plays a crucial role in thermotoler-ance in Arabidopsis. Plant Cell 12, 479 – 492.
Romani, R., French, K., 1977. Temperature-dependent changes in the polysomal population of senescent (ripen-ing) pear fruit. Plant Physiol. 60, 930 – 932.
Rose, J.K.C., Hadfield, K.A., Labavitch, J.M., Bennett, A.B., 1998. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiol. 117, 345 – 361. Schwemmle, B., Lange, O.L., 1959. Endogen-tagesperiodische schwankungen der hitzeresistenz bei Kalanchoe blossfeldi-ana. Planta 53, 134 – 144.
Seo, S.T., Hu, B.K.S., Komura, M., Lee, C.Y.L., Harris, E.J., 1974. Dacus dorsalisVapor heat treatment in papaya. J. Econ. Entomol. 67, 240 – 242.
Seymour, G.B., John, P., Thompson, A.K., 1987. Inhibition of degreening in the peel of bananas ripened at tropical temperatures II. Role of ethylene, oxygen and carbon dioxide. Annu. Appl. Biol. 110, 153 – 161.
Sharp, J.L., Ouye, M.T., Ingle, S.J., Hart, W.G., Enkerlin, W.R., Celedonio, H.H., Toledo, J., Stevens, L., Quintero, E., Reyes, J., Schwarz, A., 1989. Hot-water quarantine treatment for mangoes from the state of Chiapas, Mexico, infested with Mediterranean fruit fly andAnastrepha ser
-pentina. J. Econ. Entomol. 82, 1663 – 1666.
Shellie, K.C., Mangan, R.L., 1994. Postharvest quality of ‘Valencia’ orange after exposure to hot, moist, forced air for fruit fly disinfestation. HortScience 29, 1524 – 1527. Shellie, K.C., Mangan, R.L., 1998. Navel orange tolerance to
R.E.Paull,N.Jung Chen/Posthar6est Biology and Technology21 (2000) 21 – 37 37
Sinclair, W.B., Lindgren, D.L., 1955. Vapor heat sterilization of California citrus and avocado fruits against fruit-fly insects. J. Econ. Entomol. 48, 133 – 138.
Sozzi, G., Cascone, O., Franschina, A.A., 1996. Effect of a high temperature stress on endo-b-mannase and b -galac-tosidase activities during tomato fruit ripening. Postharvest Biol. Technol. 9, 49 – 63.
Spiers, J., Brady, C.J., Grierson, D., Lee, E., 1984. Changes in ribosome organization and messenger RNA abundance in ripening tomato fruits. Aust. J. Plant Physiol. 11, 225 – 233. Spotts, R.A., Chen, P.M., 1987. Prestorage heat treatment for control of decay of pear fruit. Phytopath 77, 1578 – 1582. Storozhenko, S., DePauw, P., Van Montagu, M., Inze, D.,
Kushnir, S., 1998. The heat shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 118, 1005 – 1014.
Stuger, R., Ranostaj, S., Materna, T., Forreiter, C., 1999. Message RNA-binding properties of non-polysomal ri-bonucleoproteins from heat stressed tomato cells. Plant Physiol. 120, 23 – 31.
Swartz, M.N., Kaplan, N.O., Frech, M.E., 1956. Significance of ‘heat-activated’ enzymes. Science 123, 50 – 53.
Sweat, V.E., 1974. Experimental values of thermal conductiv-ity of selected fruits and vegetables. J. Food Sci. 39, 1080 – 1083.
Trewavas, A., 1999. Le calcium, C’est la vie: Calcium makes waves. Plant Physiol. 120, 1 – 6.
Tucker, G.A., Grierson, D., 1982. Synthesis of polygalactur-onase during tomato fruit ripening. Planta 155, 64 – 67. Vierling, E., 1991. The role of heat shock proteins in plants.
Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 579 – 620.
Vierling, E., 1997. The small heat shock proteins in plants are members of an ancient family of heat induced proteins. Acta Physiol. Plant 19, 539 – 547.
Vierstra, R.D., 1994. Protein degradation in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 385 – 410. Williams, M.H., Bown, M.A., Vesk, M., Brady, C., 1994.
Effect of postharvest heat treatments on fruit quality, surface structure, and fungal disease of Valencia orange. Aust. J. Exp. Agric. 346, 1183 – 1190.
Woolf, A.B., Ferguson, I.B., 2000. Postharvest responses to high fruit temperatures in the field. Postharvest Biol. Tech-nol., In press.
Wu, M.T., Wallner, S.J., 1984. Heat stress responses in cul-tured plant cells. Heat tolerance induced by heat shock versus elevated growing temperatures. Plant Physiol. 75, 778 – 780.
Xiong, L., Ishitani, M., Zhu, J.K., 1999. Interaction of os-motic stress, temperature and abscisic acid in the regula-tion of gene expression in arabidopsis. Plant Physiol. 119, 205 – 211.
Yang, R.F., Cheng, M.T.S., Shewfelt, R.L., 1990. The effect of high temperature and ethylene treatment on the ripening of tomatoes. J. Plant Physiol. 136, 941 – 942.
Yoshida, O., Nakagawa, H., Ogura, N., Sato, T., 1984. Effect of heat treatment on the development of polygalacturonase in tomato fruit during ripening. Plant Cell. Physiol. 25, 505 – 509.
Yu, Y.B., Adams, D.O., Yang, S.F., 1980. Inhibition of ethylene production by 2,4-dinitrophenol and high temper-ature. Plant Physiol. 66, 286 – 290.