1. Introduction
Huntite [Mg
3Ca(CO
3)
4] and hydromagnesite [Mg
5(CO
3)
4(OH)
2.4H
2O] are classified as metastable carbonate
min-erals (
Kinsmann
1967,
sánchez-Román
et al. 2011).The
formation of huntite has been attributed to several
mecha-nisms and environments some of which are listed below:
!
Precipitation from percolating waters moving through
magnesium-rich rocks such as magnesite, dolomite
and/or hydromagnesite deposits (e.g.
Faust
1953,
sKinneR
1958,
tRailKill
1965,
zachmann
1989).
!Bacterial activities during the initial formation of
sab-kha (
PeRthuisot
et al. 1990).
!
Precipitation from Mg-bearing pore waters during
early diagenesis (
Kinsman
1967), at the expense of
do-lomite which becomes unstable when the Mg/Ca ratio
is higher than required for dolomitization (e.g.
iRion
&
mülleR
1968,
mülleR
et al. 1972).
!
Near-surface weathering of serpentinite and highly
serpentinized rocks (e.g.
DamoDaRan
&
somaseKaR
1975,
nemec
1981,
stangeR
&
neal
1994,
BashiR
et al. 2009,
eslamizaDeh
et al. 2014,
losos
et al.
2013).
Although huntite is a rare carbonate mineral, it has
been found in a wide range of geological settings,
includ-ing weathered volcanic tuff sequences, coastal sabkhas,
karstic terrains, continental lacustrine environments,
highly alkaline carbonate playas and weathered
serpenti-nized rocks (
F
aust1953,
cole
&
lanchucKi
1975,
calvo
et al. 1995,
zeDeF
et al. 2000,
BashiR
et al. 2009).
Similarly, hydromagnesite and magnesite occur in a
variety of geological settings and environments. Several
interactive mechanisms such as supergene, hypogene,
and combined supergene-hypogene processes have been
proposed to explain the origin of magnesite in ultramafic
settings (
zachmann
1989,
stamataKis
1995,
Russell
et al.
1999,
FRanK
&
F
ielDing2003). Ultramafic rocks are
con-sidered to be the source of the Mg for the fluid (
o’neil
&
BaRnes
1971,
zeDeF
et al. 2000,
miRnejaD
et al. 2008).
In these settings, several carbonate sources can be
distin-On the occurrence of Mg- and Fe-rich carbonate mineral
assemblages hosted in the Nain ophiolite mélange, Central
Iran and their industrial potential
Alireza Eslami, Michael G. Stamatakis, Maria Perraki, Charalampos Vasilatos and
Luke Hollingbery
With 5 figures and 2 tables
Abstract: In the Nain ophiolite mélange, central Iran, off-white mineral assemblages occur as nodular magnesium rich carbon-ates and thin veinlets disseminated within an earthy serpentinite groundmass. They are related to tectonically disturbed, strongly weathered zones of the ultramafic rocks. Combined XRD, SEM and TG/DTA analysis revealed that the mineralogy of the Mg-rich carbonate is varied. Ten distinct paragenetic assemblages containing hydromagnesite, pyroaurite, manasseite, brugnatellite, hydro-talcite, aragonite, and/or huntite were found. The mineral assemblages formed as the result of precipitation from percolating Mg-rich meteoric waters through brecciated serpentinites. The source of Mg in excess in the groundwater is attributed to the hydrolysis of Mg-rich minerals in the predominant serpentinized ultramafic rocks. Selected hydromagnesite-rich samples were tested as fire retardants. Even though hydromagnesite is the predominant mineral phase, the economic importance of the mineral assemblages in total is limited mainly because of the insufficient whiteness and the presence of Fe-rich minerals that cause undesirable thermal reactions.
Key words: nodular Mg-rich carbonates, serpentinite, hydromagnesite, huntite, pyroaurite, fire retardants, economic importance, Nain ophiolite mélange, Iran
Published online October 2014; published in print January 2015
60
A. Eslami et al.guished : i) atmospheric (e.g.
o’
neil&
B
aRnes1971); ii)
decarboxylation of organic rich sediments (e.g.
F
allicKet
al. 1991); iii) thermal decarbonation of limestones (
F
al-licK
et al. 1991); iv) decomposition of organic material in
soil (e.g.
P
etRov1967); v) regional metamorphic reactions
above 300 °C (
a
Bu-
j
aBeR&
K
imBeRley1992); vi)
vol-canogenic sources (
i
lich1968); vii) deep-seated source
(
K
Reulen1980); viii) combinations of all these processes.
In the Nain ophiolite mélange, Iran,
hydromagnesite-rich mineral assemblages in close relation with brecciated
zones within serpentinites have been reported recently.
Natural mixtures of huntite and hydromagnesite, as well
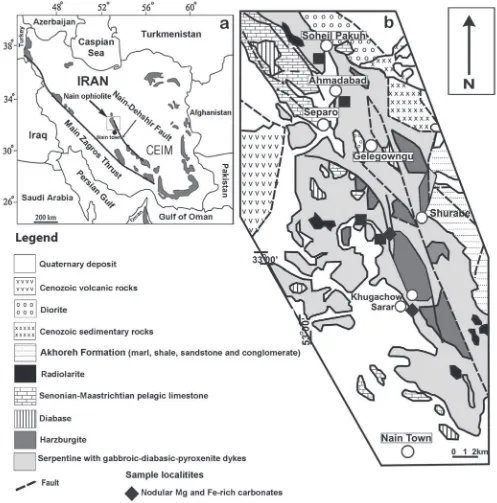
Fig. 1. a – Distribution of different ophiolite complexes in Iran; b – Simplified geological map of north of Nain town (modified after
DavouDzaDeh 1972).
F-0271_njma_192_1_0059_0071_Eslami_0271.indd 60 11.11.2014 15:01:50
as pure hydromagnesite have found applications as
envi-ronmentally friendly fire retardants (
R
othon2003,
l
ioDa -Kis&
t
souKala2010,
h
ollingBeRy&
h
ull2010,
h
ol-lingBeRy
&
h
ull2012a).
The aim of this paper is to characterize the nodular
hy-dromagnesite-rich assemblages and the white carbonate
samples in highly sheared ultramafic rocks from the Nain
ophiolite mélange. It will also discuss their economic
po-tential as fire retardants.
2. Geological setting
Ophiolites in Iran represent the remnants of the Tethyan
ophiolite belt in the Anatolian segment of the Alpine –
Himalayan orogene. The Nain-Dehshir-Baft ophiolitic
belt marks the boundaries of the Central Iranian
micro-continent (CIM) (Fig. 1a). This belt comprises a set of
dis-membered ultramafic, plutonic, volcanic and sedimentary
mélange complexes that crop out along the Nain-Dehshir
fault (Fig. 1a). The northern most part of this ophiolitic
belt is known as the Nain ophiolite mélange, which
is one of the highly dismembered oceanic lithosphere
fragments formed throughout the Mesozoic. It covers
~600 km
2, extends from NNW to SSE and is surrounded
by Cenozoic sedimentary rocks in the east and Cenozoic
volcanic rocks in the west (Fig. 1b) (
D
avouDzaDeh1972).
The main rock units in the Nain area include a Late
Cre-taceous ophiolitic complex and overlying younger rocks.
From bottom to top, this ophiolitic mélange consists of
a basal metamorphic zone, peridotites (dunite,
harzbur-gite) and serpentinized peridotite, layered, isotropic and
pegmatite gabbros, sheeted dykes, pillowed to massive
basalts, pelagic limestone and radiolarite. The ophiolite
sequence is overlain by Turonian – Maastrichtian pelagic
limestones (
D
avouDzaDeh1972). Two strike-slip fault sets
62
A. Eslami et al.have been identified in the Nain region. These developed
during two faulting stages (
n
aDimi&
s
ohRaBi2008): one
in the early Tertiary (late Cretaceous-Miocene) and other
in the late Tertiary (after late Miocene).
n
aDimi&
s
ohRa -Bi(2008), reported strike-slip movements in this area
changed the motion of older faults from reverse or thrust
into oblique-slip or strike-slip. Due to strong deformation,
ophiolitic rocks especially serpentinites are highly folded
and sheared within thrust sheets of the study area. These
tectonic structures have facilitated alteration of the
ophi-olitic units. Conjugation of the NNW-SSE and
NE-SW-trending fault systems made active smaller blocks with
rotation. Movement of the blocks associated with
rota-tion has caused cutting and rotarota-tion of this ophiolite as
well as younger Quaternary sediments; it has also caused
uplift in this region. The Late Albian age (
∼
100 Ma) has
been reported for the genesis of the Nain ophiolite (
h
as -saniPaK&
g
hazi2000). Recently, uranium-lead zircon
dating revealed that the Nain ophiolite was emplaced
101.2 ± 0.2 Ma (
s
haFaiim
oghaDamet al. 2013).
3. Field observations and sampling
Peridotites in the Nain ophiolite mélange are mostly
ser-pentinized and occur as massive serpentinite, sheared
and fractured serpentinite, and/or serpentinite veins.
The sheared and fractured serpentinite is the commonest
in the Nain ophiolite mélange. It resulted from slight to
strong deformation of massive serpentinite. In general,
serpentinites are mostly weathered into greenish white to
off-white assemblages of Mg-and Fe-rich carbonates. In
some cases tiny spherical aggregates are distributed on
the surface and in the fractures of these weathered rocks
(Fig. 2a). These nodular Mg-rich carbonates (samples
HNIR01, HNIR02, HNIR03, HNIR04, HNIR06, HNIR07,
HNIR10, HNIR11, IHNIR13 and HNIR14) were collected
in four outcrops of the Nain ophiolite mélange (Fig. 1b).
a)
Northeast of the Separo village (53° 01′ 57″ E,
33° 07′ 07″ N).
b)
Southwest of Soheil-Pakuh village (53° 01′ 32″ E,
33° 09′ 42″ N).
c)
North of the Khugachow village (53° 03′ 33″ E,
33° 02′ 59″ N).
d)
East of Sarar (53° 043′ 29″ E, 33° 01′ 36″ N).
Fractured cauliflower-shaped aggregates ranging in
diameter from 0.5 to 1 cm or pseudo-oolitic masses
(sam-ples HNIR05 and HNIR09) which are very hard were
col-lected from the weathered crust that covers serpentinites
(Fig. 2b). Two samples (sample IRNCH08 and IRNCH12)
were collected from shallow exploration pits excavated
in the serpentinite grounds in the Nain ophiolite mélange
(Fig. 2c).
4. Materials and analytical techniques
After testing of a series of initial samples of the white
mineral assemblages, fourteen representative samples
were selected for detailed analysis. Samples were
min-eralogically analysed by X-ray diffraction using a Bruker
5005X-ray diffractometer in combination with the
DIF-FRACplus software at the National & Kapodistrian
Uni-versity of Athens (UoA), Greece. The diffractometer
was operated using Cu Kα-radiation at 40 kV and 40 mA
scanned with 0.020° step size and 1.0 s step time. The raw
Fig. 3. SEM of blade crystals of hydromagnesite, partially covered by fine-grained pyroauriteaggregates (pyraourite aggregates are shown by arrows).
F-0271_njma_192_1_0059_0071_Eslami_0271.indd 62 11.11.2014 15:01:51
files (XRD diagrams) were evaluated for mineralogical
identifications using the EVA 10.0 program of the Bruker
DIFFRACplus-D5005 software. Samples were examined
micro-chemically and visually by scanning electron
mi-croscopy (SEM-EDS). A JEOL JSM-5600LINK ISIS
in-strument at the University of Athens was used. This was
combined with a microanalyzer energy dispersive system
OXFORD LINK ISIS 300, with software ZAF correction
quantitative analysis. The system was operating at 20 kV,
0.5 nA and 50 second time of analysis.
To study the thermal stability, thermogravimetry (TG)
was used to measure the magntitude of the mass losses
against temperature and differential thermal analysis
(DTA) was used to measure the magnitude of the
ther-mal changes (exotherm or endotherm) associated with
the mass losses. Twelve magnesium carbonate-rich
sam-ples (HNIR01, HNIR02, HNIR03, HNIR05, HNIR06,
HNIR08, HNIR09, HNIR10, HNIR11, HNIR12, HNIR13
and HNIR14) were characterised using a Mettler Toledo
TGA/SDTA 851 instrument at the School of Mining and
Metallurgical Engineering of The National Technical
University of Athens (NTUA), Greece. The temperature
was raised at a constant rate (10 °C/min) from ambient to
1200 °C. Sample sizes of approximately 40 – 80 mg were
used and the mass constantly monitored alongside the
thermal heat flow.
The whiteness of selected samples was measured by a
LANGE instrument, using barium sulfate as a reference
material for 100 % whiteness (UoA).
5. Results
5.1. Carbonate mineralogy
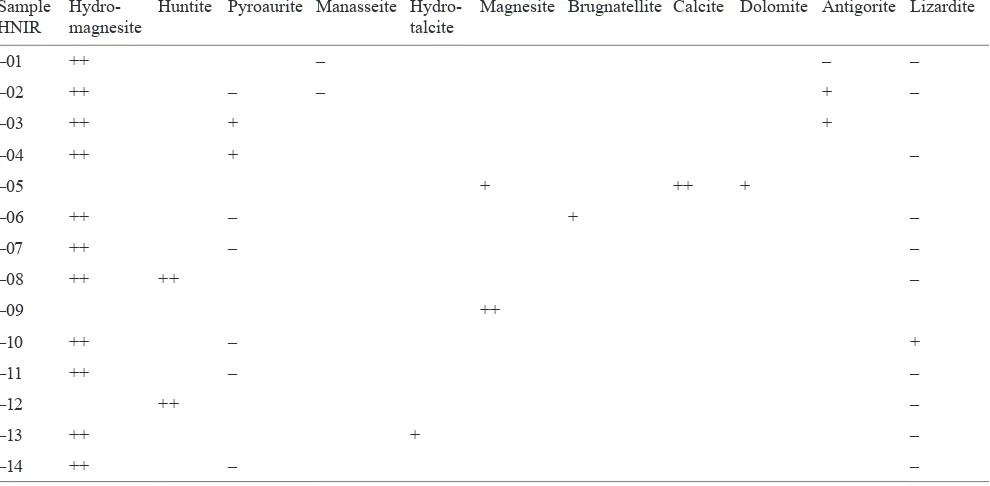
Semi-quantitative mineral analyses of bulk samples
ex-amined by XRD are given in Table 1. Ten distinct
carbon-ate parageneses were determined in the studied samples:
a) Hydromagnesite-manasseite (HNIR01).
most dominant Mg-rich carbonate in these assemblages.
SEM-EDS analysis revealed that all carbonate
min-erals above are microcrystalline. Out of the needle-like
aragonite crystals, the most distinguishable minerals are
hydromagnesite and pyroaurite (Ni(6-x)Mgx)Fe
2(CO
3)
(OH)
16.4H
2O. Hydromagnesite occurs as platy 5 – 20 µmlarge laths and/or blades arranged in parallel (Fig. 3). In
contrast, pyroaurite occur as fine-grained platy and
needle-like crystals formed on the surface of hydromagnesite
crys-tals (Fig. 3). Brugnatellite Mg
6Fe
3+(CO
3)(OH)
13.4(H
2O)
and manasseite Mg
6Al
2(CO
3)(OH)
16.4(H
2O) occur as
dis-seminated on a hydromagnesite groundmass platy and
fi-brous very fine-grained crystals respectively.
5.2. TG/DTA
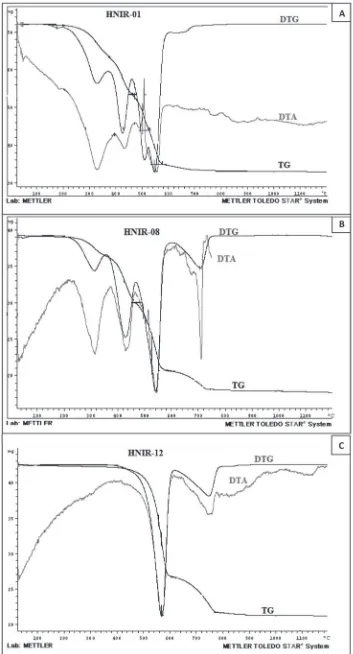
An endothermic peak at ~330 °C was observed for the
DTA analysis of sample HNIR01, which corresponds
to a mass loss of ~12 % or the mass of four water
mol-ecules (Fig. 4a). This suggested that approximately
80 mass% of sample HNIR01 were hydromagnesite
[Mg
5(CO
3)
4(OH)
2· 4H
2O]. At approximately 400 °C, the
release of CO
2initiates. At around 520 °C, a mass loss
occurs which is associated with an exothermic reaction,
followed by an endothermic reaction. It has been shown
that this exothermic reaction is due to the formation of
crystalline magnesium carbonate after the initial loss of
some CO
2(
hollingBeRy
&
hull
2010). The remaining 20
mass% was lizardite, which exhibits overlapping
endo-thermic peaks. Sample HNIR02 shows a similar thermal
behavior.
Endothermic peaks at ~330 °C and at ~730 °C were
observed for the DTA analysis of sample HNIR08, which
correspond to a loss of ~7.5 mass% or the mass of the four
hydromagnesite H
2O molecules and to the release of one
CO
2molecule in huntite, respectively (Fig. 4b). This
sug-gested that approximately 50 mass% of sample HNIR08
were hydromagnesite [Mg
5(CO
3)
4(OH)
2· 4H
2O] and 50
the DTA analysis of sample HNIR12, which corresponds
to a loss of ~37 mass% (Fig. 4c). This suggested that
more than 98 mass% of Sample HNIR12 were huntite
[Mg
3Ca(CO
3)
4]. An endothermic peak at ~580 °C was
ob-served for the DTA analysis of sample HNIR05, which
corresponds to a loss of ~6 mass%. This suggested that
approximately 10 mass% of sample HNIR05 were
mag-nesite (
smyKatz-Kloss
1974). The remaining 90 mass%
64
A. Eslami et al.Fig. 4. TG (black line)/DTG (blue line)/DTA (red line) curves of (a) sample HNIR01 (hydromagnesite), (b) sample HNIR08 (hydromagne-site-huntite) and (c) sample HNIR12 (huntite).
F-0271_njma_192_1_0059_0071_Eslami_0271.indd 64 11.11.2014 15:01:52
~590 °C was observed for the DTA analysis of sample
HNIR09, which corresponds to a loss of 49 mass%. This
suggests that approximately 95 % magnesite (
s
myKatz-K
loss1974).
The DTA curves of the samples HNIR03, -06, -10,
-11, -13, -14 show overlapping endothermic peaks of
hy-dromagnesite and other minor phases such as pyroaurite,
antigorite, lizardite, manasseite, mineral phases that have
also been detected by XRD (Table 1).
5.3. Whiteness and screening tests
The samples HNIR01, HNIR02 and HNIR03 were
ana-lyzed to determine their whiteness, as all commercial
hy-dromagnesite-based fire retardants require a brightness of
> 95 %. All three samples have undesirable low whiteness
values, ranging between 85 and 90 %.
In order to identify the possibility to separate
mechani-cally the nodules from the greenish groundmass,
prelimi-nary tests were performed by using laboratory sieves of
200 mesh (Laboratory of Sedimentology, Geology
De-partment of University of Isfahan, Iran). The separation
of the nodules was poor because they broke easily and
mixed with the earthy serpentine phases as they passed
through the sieve. The recovery of the nodules was
esti-mated at about 50 %.
6. Discussion
6.1. Serpentinite weathering and origin of the
Mg-carbonates
Besides in lacustrine sedimentary basins, hydromagnesite
has been reported in serpentinized ultramafic rocks
affect-ed by strong tectonic activity, in thrust and shear zones
and areas where serpentine has been weathered to an
earthy groundmass (
m
umPton&
t
omPson1966,
B
RiDeauet al. 2007). Commonly, brucite is the early-formed
sec-ondary mineral at the expense of serpentine, turning to
metastable hydromagnesite and/or pyroaurite by the
re-action of brucite with CO
2-bearing groundwater at
shal-low depths and wet surfaces (
m
umPton&
t
omPson1966,
h
ostetleRet al. 1966). Laboratory measurements have
shown that hydromagnesite can precipitate directly from
Mg- and Na-HCO
3-rich solutions (
a
lDeRman1965,
B
eth -Ke1996). Huntite and magnesite have been reported as
direct precipitates, or diagenetic products of an aragonite
and/or hydromagnesite precursor (
a
lDeRman1965,
K
ins -mann1967,
s
tamataKis1995). The precipitation of the
magnesium carbonates depends on four main parameters:
a) alkalinity; b) temperature; c) the partial pressure of CO
2and d) the amount of Ca
2+and Mg
2+ions in solution.
For pyroaurite formation the concentration of Fe
3+66
A.
Eslam
i et al.
Table 2. Suggested reactions for the formation of carbonate minerals in the Nain ophiolite mélange.
Paragenesis Possible reactions
Hydromagnesite-mannaseite and
hydromagnesite-hydrotalcite
a) MgAl2O4+8H2O +9CO2+5Mg3Si2O5(OH)4 → Mg6Al2(CO3)(OH)16 · 4H2O +2Mg5(CO3)4(OH)2 · 4H2O +10SiO2
spinel +8H2O +9CO2+5 serpentine → hydrotalcite or manasseite +2 hydromagnesite +10SiO2
b) 10MgCO3+17H2O+MgAl2O4 → Mg6Al2(CO3)(OH)16 · 4H2O+Mg5(CO3)4(OH)2 · 4H2O +5CO2
10 magnesite +17H2O+spinel→hydrotalcite or manasseite+hydromagnesite +5CO2
c) 4MgCO3 +2Mg3Si2O5(OH)4 +13H2O+MgAl2O4 +CO2→Mg6Al2(CO3)(OH)16 · 4H2O+Mg5(CO3)4(OH)2 · 4H2O +4SiO2
4 magnesite +2 serpentine +13H2O+spinel+CO2 → hydrotalcite or manasseite+hydromagnesite +4SiO2
hydromagnesite-pyroaurite-manasseite
a) 7Mg3Si2O5(OH)4+16H2O +10CO2+Fe2SiO4+1/2O2+MgAl2O4 → Mg6Fe2(CO3)(OH)16 · 4H2O +2Mg5(CO3)4(OH)2 · 4H2O+Mg6Al2(CO3)
(OH)16 · 4H2O +15SiO2
7 serpentine +16H2O +10CO2+fayalite +1/2O2+spinel → pyroaurite +2 hydromagnesite+manasseite +15 SiO2
b) 16MgCO3 +29H2O+MgAl2O4+Fe2SiO4+1/2O2 → Mg6Al2(CO3)(OH)16 · 4H2O+Mg6Fe2(CO3)(OH)16 · 4H2O+Mg5(CO3)4(OH)2 · 4H2O +10CO2 +SiO2
16 magnesite +29H2O+spinel+fayalite +1/2O2 → manasseite+pyroaurite+hydromagnesite +10CO2+SiO2
c) MgCO3+19H2O +5CO2 +5Mg3Si2O5(OH)4+Fe2SiO4+1/2O2+MgAl2O4 → Mg6Al2(CO3)(OH)16 · 4H2O+Mg6Fe2(CO3)
(OH)16.4H2O+Mg5(CO3)4(OH)2 · 4H2O +11SiO2
magnesite +19H2O +5CO2+5serpentine+fayalite +1/2O2+spinel → hydrotalcite or manasseite+pyroaurite+hydromagnesite +11SiO2
hydromagnesite-pyroaurite
a) Fe2SiO4+7Mg3Si2O5(OH)4+5H2O +13CO2+1/2O2 → Mg6Fe2(CO3)(OH)16 · 4H2O +3Mg5(CO3)4(OH)2 · 4H2O +15SiO2
fayalite +7serpentine +5H2O +13CO2+1/2O2 → pyroaurite +3hydromagnesite +15 SiO2
b) 11MgCO3+17H2O +1/2O2+Fe2SiO4 → Mg6Fe2(CO3)(OH)16 · 4H2O+Mg5(CO3)4(OH)2 · 4H2O+SiO2+6CO2
11 magnesite +17H2O +1/2O2+fayalite → pyroaurite+hydromagnesite+SiO2+6CO2
hydromagnesite-huntite 5MgCO5 magnesite+calcite +4H3+CaCO3+4H2O+Mg3Si2O5(OH)4+5/2O2 → Mg3Ca(OH)2(CO3)4+Mg5(CO3)4(OH)2 · 4H2O +2SiO2
2O+serpentine +5/2O2 → huntite+hydromagnesite +2SiO2
huntite 3MgCO3 magnesite+calcite+H3+CaCO3+H2O +1/2O2 → Mg3Ca(OH)2(CO3)4
2O +1/2O2 → huntite
F-0271_njma_192_1_0059_0071_Eslami_0271.indd 66
11.11.2014 15:01:52
nite precipitates, followed by huntite and finally
hydro-magnesite (
s
tamataKis1995). If the concentration of Mg
and the partial pressure of CO
2are both high, magnesite
forms, whereas low partial pressure of CO
2favours the
formation of hydromagnesite for a constant Mg/Ca ratio
(
s
tamataKis1995). We assume several phases of
evolu-tion, weathering and tectonics of the ultramafic rocks.
Hence, at periods with high extensional stresses,
pCO
2was high and hence magnesite was formed. At a later
stage, when the pCO
2was low, hydromagnesite formed.
The area studied is tectonically affected as shown by the
presence of shear zones and detachments. These tectonic
structures facilitated alteration of ultramafic host rocks.
Hence, we assume a similar mechanism for the
forma-tion of the Mg-rich carbonates. Most of the nodular
oc-currences are hosted in a wet earthy serpentinite,
indicat-ing their direct relationship with the groundwater. The
Mg source in excess in the groundwater for the Mg-rich
carbonates is associated with the presence of vast masses
of serpentinized ultrabasic rocks in this area. Hydrolysis
of Mg-rich minerals caused Mg
2+leaching from its
ultra-mafic host rocks. The experimental work of
e
Delsteinet
al. (1982) and relatively high solubility of serpentine
min-erals in pure water at normal pressures and temperatures
(
l
esKo1972) supports this interpretation. The carbonate
may originate from a variety of sources. For determining
all possible sources of the CO
2, stable isotope analyses by
the authors are in progress.
6.2. Mineral paragenesis
Hyrdotalcite-group minerals are layered double
hydrox-ides or anion clays, which are natural lamellar mixed
hydroxides with interlayer spaces containing
exchange-able anions (
m
illset al. 2012). On the basis of an X-ray
investigation,
a
minoF&
B
Roome(1930) recognized two
polytypes of hydrotalcite: pyroaurite and manasseite as
rhombohedral polytypes and hydrotalcite as the
hexago-nal polytype. In the Nain ophiolite mélange these
min-erals are associated with hydromagnesite and occur as
nodular assemblages and thin, discontinuous veinlets in
heavily sheared serpentinized rocks. For the formation of
nodular assemblages, several balanced reactions can be
written (see Table 2). However, the absence of
thermody-namic and stability data for these minerals makes it
dif-ficult to specify the exact reaction that took place. These
equations assume the involvement of the precursor phases
of serpentine and magnesite, as a source of Mg. Olivine
(fayalite) and spinel are assumed as sources of Fe and Al,
respectively. This is necessary for the formation of
hy-dromagnesite and hydrotalcite-like minerals. The absence
of quartz, talc or other silica-rich phases revealed that Si
must have been transported away from body.
Huntite is a soft and fine-grained chemical precipitate,
which has been re-dispersed in water. It can form at low
temperature at surface and near-surface conditions by
di-rect precipitation from Mg-rich solutions or by interaction
of Mg-rich waters with preexisting carbonate minerals
(
D
ollase&
R
eeDeR1986). It has been mined as pigment
in low depth pits, since the ancient times. Commercially,
a natural mixture of huntite and hydromagnesite is sold
under the trade name “UltraCarb” which has an industrial
economic value as an industrial fire retardant. In
compari-son to the hydrotalcite mineral associated with
hydromag-nesite, huntite does not occur as nodular assemblages but
as lumps and coatings in fissures of the weathered
serpen-tinite immediately below the soil profile.
6.3. Thermogravimetry
Both hydromagnesite and huntite decompose
endother-mically. This endothermic decomposition and release of
inert gases H
2O and CO
2gives them their fire retardant
properties (
h
ollingBeRy&
h
ull2012b). During thermal
decomposition hydromagnesite may undergo an
exo-thermic structural rearrangement (
h
ollingBeRy&
h
ull2012a,
s
awaDaet al. 1978 a and b,
s
awaDaet al. 1979a, b,
c). The rearrangement of the crystal structure results in a
thermally more stable form, which releases CO
2at a
high-er temphigh-erature than the initial arrangement.
Hydromagne-site thermally decomposes by first losing H
2O, followed
by release of OH and finally release of CO
2. The thermal
decomposition of hydromagnesite has been proposed to
occur via the following reactions (e.g.
h
aliKiaet al. 1998,
i
nglethoRPe&
s
tamataKis2003):
Mg
5(CO
3)
4(OH)
2· 4H
2O → Mg
5(CO
3)
4(OH)
2+4H
2O
(< 250 °C)
Mg
5(CO
3)
4(OH)
2→ 2MgCO
3+3MgO +2CO
2+H
2O
(250 – 350 °C)
68
A. Eslami et al.The mass loss associated with the decomposition of
the hydroxide ion probably occurs somewhere between
330 °C and 430 °C but is overshadowed by the larger
mass loss associated with the decomposition of the
car-bonate ions. These three decompositions would result in
losses of 15.45 mass%, 3.86 mass%, and 37.77 mass%
re-spectively and result in a total loss of 57.08 mass%. Using
hydromagnesite’s molecular mass of 467.5 g mol
–1it can
be calculated that loss of the four H
2O molecules account
for a loss of 15.40 mass%. The loss of a further H
2O
mole-cule from the decomposition of OH
–and loss of four CO
2molecules would account for a further 41.50 mass% (3.85
mass% and 37.65 mass% respectively) loss (
h
ollingBeRy&
h
ull2012a).
It has also been shown that in the third stage of
decom-position, the release of four CO
2molecules is strongly
af-fected by the partial pressure of CO
2in the atmosphere and
the rate of heating (
h
ollingBeRy&
h
ull2012a,
s
awaDaet al. 1978 a and b,
s
awaDaet al. 1979a, b, c). An
exo-thermic rearrangement of the crystal structure to a more
thermally stable form may occur under certain conditions
such as high heating rates or high partial pressures of CO
2.
As mentioned above (see Fig. 4a), TG/DTA analyses
of the hydromagnesite-rich samples of this area clearly
show three decomposition steps (most evident in the
al-most pure hydromagnesite sample HNIR01), compared
to only two steps of the commercial hydromagnesite of
Turkish origin. However, under high rates of heating the
Nain hydromagnesite shows a decomposition profile very
similar to that of Turkish hydromagnesite of sedimentary
origin (Fig. 5,
h
ollingeRy& h
ull2012a).
Huntite decomposes through two stages as clearly
shown in Figure 4 c. The first stage occurs between about
400 °C and 630 °C with an associated loss of ~37 mass%
(release of three CO
2molecules) and the second stage
oc-curs between about 630 °C and 750 °C with a further loss
of ~12.5 mass% (further release of one CO
2molecule,
h
ollingBeRy& h
ull2012a).
The three decomposition stages detected in the
reevesite-pyroaurite series (
F
Rost& e
RicKson2004) are:
Fig. 5. Thermal decomposition of selected Nain hydromagnesite-rich samples in comparison with commercial Turkish hydromagnesite (hollingBeRy & hull2012a)(Ni
(6-x)Mg
x) Fe
2(CO
3)(OH)
16· 4H
2O → (Ni
(6-x)Mg
x) Fe
2(CO
3)(OH)
16+4H
2O
(150 –165 °C)
(Ni
(6-x)Mg
x) Fe
2(CO
3)(OH)
16→(Ni
(6-x)Mg
x)O
5Fe
2O
3+CO
2+8H
2O
(245 – 340 °C)
(Ni
(6-x)Mg
x)O
5Fe
2O
3→(Ni
(6-x)Mg
x)Fe
2O
3+O
2(341– 455 °C)
F-0271_njma_192_1_0059_0071_Eslami_0271.indd 68 11.11.2014 15:01:53
The first step represents the dehydration step with the
consequent loss of H
2O. This step occurs generally in the
150 °C to 165 °C temperature range. Since most
thermo-plastic polymers are processed within this temperature
range or higher, the presence of this mineral will be a
lim-iting factor in their use as fire retardants in thermoplastic
polymers. The second step involves the simultaneous loss
of CO
2and H
2O. It is assumed that oxides are formed. The
third mass loss step involves oxygen loss and the
reduc-tion in the moles of oxygen in the mixed metal oxide. The
release of oxygen is obviously a negative effect in terms
of fire retardancy. In the samples studied, pyroaurite
oc-curs in small amounts, as shown by the overlapping weak
endothermic peaks of pyroaurite with those of
hydromag-nesite and of other minor phases such as antigorite,
lizar-dite and manasseite.
7. Conclusions
The white mineral assemblages occurring in the Nain
ophiolite mélange are varied and consist mainly of
hy-dromagnesite and/or huntite as well as minor quantities
of pyroaurite, manasseite, hydrotalcite and brugnatellite.
The Mg-rich carbonates formed by weathering of highly
tectonised ultramafic rocks. From TG/DTA data, we
ob-served that the Nain Mg-rich carbonates show an initial
mass loss between about 200 °C and 400 °C which is
associated with loss of H
2O. Between about 400 °C and
600 °C, the mass loss associated with the loss of CO
2var-ies. When heated at high heating rates, the mixed
Mg-rich carbonates from Nain are decomposed in three steps,
similar to that shown by Turkish sedimentary pure
hydro-magnesite:
1. Endothermic loss of water of crystallization.
2. Dehydroxylation and formation of amorphous MgCO
3.
3. Endothermic MgCO
3decarbonation.
The presence of minor amounts of hydrotalcite-group
minerals associated with the hydromagnesite may cause
undesirable thermal reactions.
Possible industrial uses
Hydromagnesite and huntite have already been
character-ized as fire retardant additives for polymers (
h
ollingBeRy& h
ull2012b). However, in the deposits studied here,
hydromagnesite is accompanied by hydrotalcite-group
minerals as unusual minor Mg- and Fe-rich
carbon-ate phases. The low decomposition temperature of the
reevesite-pyroaurite will limit the use of mixtures
con-taining hydromagnesite and huntite to polymers that can
be processed below this temperature. One of the largest
areas of application for mineral fire retardants is in
poly-olefin and PVC wire and cable sheathing, but these types
of compounds are typically processed at temperatures
higher than the 150 °C decomposition temperature of the
reevesite-pyroaurite minerals. Thus, the presence of these
minerals will be a limiting factor in their use as fire
retard-ants in thermoplastic polymers. In addition, the economic
importance of these mineral assemblages is limited due to
the undesirable color (brightness ranging from 85-90 %)
and the difficulty to mechanically separate the cotton
balls from the earthy serpentinite groundmass.
Acknowledgments
Analytical support of the National & Kapodistrian
Uni-versity of Athens, Greece, National Technical UniUni-versity
of Athens, Greece and LKAB Minerals Ltd. is
grateful-ly acknowledged. The authors are especialgrateful-ly obliged to
Dr. M.A.
m
acKizaDehfrom Geology Department of the
University of Isfahan for his fruitful and logistical
sup-port during fieldwork. Thanks are also expressed to Prof.
j
ose-P
eDRoc
alvo(Complutense University, Madrid) and
an anonymous reviewer for their fruitful commends and
suggestions.
References
aBu-jaBeR, N. S. & KimBeRley, M. M. (1992): Origin of
ultramafic-hosted magnesite on Margarita Island, Venezuela. – Mineral. De
-posita 27: 234 – 241.
alDeRman, A. R. (1965): Dolomite sediments and their environ-ment in the south-east of South Australia. – Geochim.
Cosmo-chim. Acta 29: 1355 –1365.
aminoFF, G. & BRoome, B. (1930): Contributions to the knowledge
of the mineral pyroaurite. – Kungl. Sv. Vet. Akademiens Han
-dlingar. 9: 23 – 48.
BashiR, E., naseem, S., sheiKh, S. A. & Kaleem, M. (2009):
Miner-alogy of Kraubath-type magnesite deposits of the Khuzdar area, Balochistan, Pakistan. – Journal of the Earth Sciences Applica-tion and Research Centre of Hacettepe University, Yerbilimleri
30: 169 –180.
BethKe, C. (1996): Geochemical reaction modelling: Concepts and
applications. – Oxford University Press, New York, USA, 397 p. BRiDeau, M. A., steaD, D., Roots, C. & oRwin, J. (2007): Geo-morphology and engineering geology of a landslide in ultramafic
rocks, Dawson City, Yukon. – Eng. Geol. 89: 171–194.
calvo, J. P., stamataKis, M. G. & magganas, A. C. (1995): Clastic
huntite in upper Neogene formations of the Kozani Basin,
Mac-edonia, northern Greece. – J. Sediment. Res. 65: 627– 632.
cole, W. F. & lancucKi, C. J. (1975): Huntite from Deer Park, Vic
-toria, Australia. – Amer. Mineral. 60: 1130 –1131.
DamoDaRan, K. T. & somaseKaR, B. (1975): Huntite-magnesite from the altered serpentinites of Nuggihalli schist belt, Karnataka
state. – In: nagamuRa, C. (Ed.), Studies in Precambrians. –
70
A. Eslami et al.DavouDzaDeh, M. (1972): Geology and petrology of the area North of Nain, Central Iran. – Geological Survey of Iran, Report No. 1.
eDelstein, I. I., melniK, A. D. & PiliPenKo, A. A. (1982): On geo-chemistry of process of weathering of the rock-forming miner-als of ultramafic rocks (according to experimental data). – Geo-chemia 2: 263 – 570.
eslamizaDeh, A. & samaniRaD, S. (2014): Petrology of ultramafic rocks and Mg-rich carbonate minerals in southeast of Dehshir, Central Iran. – Arab. J. Geosci. 7: 3675 – 3682.
FallicK, A. E., ilich, M. U. & Russell, M. J. (1991): A stable iso-tope study of the magnesite deposits associated with the Alpine-type ultramafic rocks of Yugoslavia. – Econ. Geol. 86: 847– 861.
Faust, G. T. (1953): Huntite, Mg3Ca(CO3)4, a new mineral. – Amer.
Mineral. 38: 4 – 24.
FRanK, T. D. & FielDing, C. R. (2003): Marine origin for Precam-brian, carbonate-hosted magnesite? – Geology 31: 1101–1104.
FRost, R. L. & eRicKson, K. L. (2004): Thermal decomposition of synthetic hydrotalcites, reevesite and pyroaurite. – J. Therm. Anal. Calorim. 76: 217– 225. kinetic analysis of the thermal decomposition of magnesium hy-droxide using thermogravimetric data. – Thermochim. Acta. 320:
75 – 88.
hassaniPaK, A. A. & ghazi, A. M. (2000): Petrochemistry, 40Ar– 39Ar ages and tectonics of the Nain Ophiolite, Central Iran. – GSA An-nual Meeting, 237– 238.
hollingBeRy, L. A. & hull, T. R. (2010): A Review of the struc-ture and thermal decomposition of hydromagnesite and huntite. – Thermochim. Acta 509: 1–11.
hollingBeRy, L. A. & hull, T. R. (2012a): The thermal decomposi-tion of natural mixtures of huntite and hydromagnesite. – Ther-mochim. Acta 528: 45 – 52.
hollingBeRy, L. A. & hull, T. R. (2012b): The fire retardant effects of huntite in natural mixtures with hydromagnesite. – Polym. De-grad. Stab 97: 504 – 512.
hostetleR, P. B., coleman, R. C. & evans, B. W. (1966): Brucite in Alpine serpentinites. – Amer. Mineral. 51: 75 – 98.
ilich, M. (1968): Problems of the genesis and genetic classification of magnesite deposits. – Geologicky Zbornik-Geologica Car-pathica XIX, Bratislava, 1: 149 –160.
inglethoRPe, S. D. & stamataKis, M. G. (2003): Thermal decom-position of natural mixtures of hydromagnesite and huntite from Kozani, Northern Greece. – Mineral Wealth., 7–18.
iRion, G. & mülleR, G. (1968): Huntite, dolomite, magnesite and polyhalite of recent age from Tuz Gölü, Turkey. – Nature 220: 1309 –1310.
Kinsman, D. J. (1967): Huntite from a carbonate-evaporite environ-ment. – Amer. Mineral. 52: 1332 –1340.
KReulen, R. (1980): CO2-rich fluids during regional metamorphism
on Naxos(Greece): Carbon isotopes and fluid inclusions. – Amer. J. Sci. 280: 745 –771.
lesKo, I. (1972): Über die Bildung von Magnesitlagerstätten –Mit-teilung aus dem Forschungsinstitut der Veitscher Magnesitwerke A.-G., Leoben. – Miner. Deposita 7: 61–72.
lioDaKis, s. & tsouKala, m. (2010): Environmental benefits of us-ing magnesium carbonate minerals as new wildfire retardants instead of commercially available, phosphate-based compounds. – Environ. Geochem. Health. 32: 391– 399.
losos, Z., KováR, O., houzaR, S., & zeman, J. (2013): Rare hy-drated Mg-carbonate-hydroxide assemblage of serpentinite fis-sures in Hrubsice, western Moravia (Czech Republic): a genetic model of is formation. – N. Jb. Miner. Abh. (J. Min. Geochem.)
190: 253 – 263.
mills, S. J., chRisty, A. G., génin, J. M. R., KameDa, T. & colom -Bo, F. (2012): Nomenclature of the hydrotalcite supergroup: natu-ral layered double hydroxides. – Minenatu-ral. Mag. 76: 1289 –1336.
miRnejaD, H., eBRahimi-nasRaBaDi, E., lalonDe, A. E. & tayloR, B. E. (2008): Mineralogy, stable isotope geochemistry, and par-agenesis of magnesite deposits from the ophiolite belt of eastern Iran. – Econ. Geol. 103: 1703 –1713.
mülleR, G., iRion, G. & FöRstneR, U. (1972): Formation and di-agenesis of inorganic Ca-Mg carbonates in the lacustrine envi-ronment. – Naturwiss. 59: 158 –164.
mumPton, F. A. & tomPson C. S. (1966): The stability of brucite in the weathering zone of the New Idria serpentinite. – Clays Clay Miner. 14: 249 – 257.
naDimi, A. & sohRaBi, A. (2008): Nain tectonic mélange, Central Iran: Strike-slip faulting and tectonics evolution. – Third Interna-tional Geomodelling Conference, Firenze, Italy, p. 84 – 88.
nemec, D. (1981): Huntite from the serpentinite area near Hrubsice, Western Moravia. – Cas. Mineral. Geol. 26: 75 –78.
o’neil, J. R. & BaRnes, I. (1971): C13 and O18 compositions in some fresh-water carbonates associated with ultramafic rocks and ser-pentinites: western United States. – Geochim. Cosmochim. Acta
35: 687– 697.
PeRthuisot, J. P., castanieR, S. & mauRin, A. (1990): La huntite (CaMg3(CO3)4) de la Sebkha el Melah (Zarzis, Tunisie). Un
exemple de microbiodiagenese carbonatogene. – Société Géologique de France, Bulletin, 8eme Série 6: 657– 666.
PetRov, V. P. (1967): Osnovy ucheniia o drevnikh korakh vyvetriva -niia (Principles of studying ancient weathering crusts). – Nedra Publisher, Moskva, 343 pp. (in Russian).
Rothon, R. N. (2003): Effects of particulate fillers on flame retard-ant properties of composites. – In: Rothon, R. N. (Ed.): Particu-late Filled Polymer Composites, 2nd ed., Rapra Technology Ltd, Shrewsbury, pp. 263 – 302.
Russell, M. J., ingham, J. K., zeDeF, V., maKtav, D., sunaR, F.,
hall, A. J. & FallicK, A. E. (1999): Search for signs of ancient life on Mars: expectations from hydromagnesite microbialites, Salda Lake, Turkey. – J. Geol. Soc. 156: 869 – 888.
sawaDa, Y., uematsu, K., mizutani, N. & Kato, M. (1978a): Thermal decomposition of hydromagnesite 4MgCO3·Mg(OH)2 · 4H2O. –
J. Inorg. Nucl. Chem. 40, 979 – 982.
sawaDa, Y., uematsu, K., mizutani, N. & Kato, M. (1978b): Thermal decomposition of hydromagnesite 4MgCO3·Mg(OH)2 · 4H2O
under different partial pressures of carbon dioxide. – Thermo-chim. Acta 27: 45 – 59.
sawaDa, Y., yamaguchi, J., saKuRai, O., uematsu, K., mizutani, N. & Kato, M. (1979a): Thermal decomposition of basic magnesium carbonates under high-pressure gas atmospheres. – Thermochim. Acta 32: 277– 291.
sawaDa, Y., yamaguchi, J., saKuRai, O., uematsu, K., mizutani, N. & Kato, M. (1979b): Thermogravimetric study on the decompo-sition of hydromagnesite 4MgCO3·Mg(OH)2 · 4H2O. –
Thermo-chim. Acta 33, 127–140.
sawaDa, Y., yamaguchi, J., saKuRai, O., uematsu, K., mizutani, N. & Kato, M. (1979c): Isothermal differential scanning calorimetry on an exothermic phenomenon during thermal decomposition of hydromagnesite 4MgCO3·Mg(OH)2 · 4H2O. – Thermochim.
Acta 34: 233 – 237.
sánchez-Román, M., RomaneK, C. S., FeRnánDez-RemolaR, D. C.,
sáanchez-navas, A., mcKenzie, J. A., PiBeRnat, R. A. & v ascon-celos, C. (2011): Aerobic biomineralization of Mg-rich
carbon-ates: implications for natural environments. – Chem. Geol. 281: 143 –150.
shaFaiimoghaDam, H., Rahgoshay, M., whitechuRch, H. & mon-tigny, R. (2007): A geochemical scenario for evolution of the
Nain-Baft back-arc basin. – Goldschmidt Conference Abstracts, A920.
shaFaiimoghaDam, H., coRFu, F. & steRn, R. J. (2013): U-Pb zir-con ages of Late Cretaceous Nain-Dehshir ophiolites, central Iran. – J. Geol. Soc. London 170: 175 –184.
sKinneR, B. J. (1958): Huntite from Tea Tree Gully, South Australia. – Amer. Mineral. 43: 159 –162.
smyKatz-Kloss, W. (1974): Differential Thermal Analysis:
Applica-tions and Results in Mineralogy. – Springer-Verlag, Berlin, 185
pp.
stamataKis, M. G. (1995): Occurrence and genesis of huntite-hydromagnesite assemblages, Kozani basin, Greece: important new white fillers and extenders. – Institution Mining Metallurgy, Transactions B 104: 179 –186.
stangeR, G. & neal, C. (1994): The occurrence and chemistry of huntite from Oman. – Chem. Geol. 112: 247– 254.
thRailKill, J. V. (1965): Studies in the excavation of limestone caves
and the deposition of speleothems. – Unpublished Ph.D. thesis, Princeton University, 193 p.
zachmann, D. W. (1989): Mg-carbonate deposits in freshwater en-vironments. – In: mölleR, P. (ed.): Magnesite: Geology, Mineral-ogy, Geochemistry, Formation of Mg-carbonates. – Monograph Series on Mineral Deposits 28: 61– 94.
zeDeF, V., Russell, M. J., FallicK, A. E. & hall, A. J. (2000):Gene-sis of vein stockwork and sedimentary magnesite and hydromag-nesite deposits in the ultramafic terrains of southwestern Turkey: A stable isotope study. – Econ. Geol. 95: 429 – 446.
Manuscript received: April 1, 2014; accepted: August 20, 2014. Responsible editor: G. Franz
Authors’ addresses:
aliReza eslami (corresponding author), Department of Economic Geology, Faculty of Basic Sciences, Tarbiat Modares University, Tehran 14115-175, Iran.
michael g. stamataKis, chaRalamPos vasilatos, Department of Geology and Geoenvironment, Section of Economic Geology &
Geochemistry, National & Kapodistrian University of Athens, Panepistimiopolis, Ano Ilissia, 157 84 Athens, Greece.
maRia PeRRaKi, School of Mining and Metallurgical Engineering, National Technical University of Athens, 9 Heroon Politechniou St., Zografou, 15780, Greece.