Uji Toksisitas Formula Jamu Untuk Asma
Galuh Ratnawati and Zuraida Zulkarnain
Medicinal Plant and Traditional Medicine Research and Development Centre Email: [email protected]
ABSTRAK
Asma adalah gangguan pernapasan yang mempersempit saluran udara karena hiperaktivitas terhadap rangsan-gan tertentu, yang menyebabkan peradanrangsan-gan; penyempitan ini bersifat sementara. Meningkatnya jumlah orang yang tertarik dalam penggunaan pengobatan dengan menggunakan tanaman obat dan herbal. Tanaman Obat dan Penelitian Obat Tradisional dan Pusat Pengembangan (B2P2TO2T) telah mengembangkan rumus jamu yang ter-diri dari sembung (Blumea balsamifera L. DC.), kemukus (Piper cubeba L.), patikan kebo (Euphorbia hirta L.), dan rumput teki (Cyperus rotundus L.) untuk pengobatan asma. Sembung secara empiris digunakan sebagai obat asma. Kemukus yang berisi dihidrokubebin memiliki efek melonggarkan pernafasan. Patikan kebo digunakan untuk men-gobati asma, gangguan bronkial kronis, radang selaput lendir hidung hidung akut, dan sebagai ekspektoran. Rum-put teki digunakan sebagai anti-inflamasi. Penelitian ini dilakukan untuk mengetahui toksisitas herbal yang terdiri sembung, kemukus, patikan kebo, dan rumput teki pada tikus Wistar. Uji toksisitas akut menggunakan 3 kelompok; kelompok kontrol, kelompok dosis 5.000 dan 2.500 mg/kg bb. Sedangkan uji toksisitas subkronis menggunakan empat kelompok; kelompok kontrol, dosis 0,144; 0,288; 0,576 g /200 g bb. Data dikumpulkan untuk gejala toksik dan kematian, berat badan, kimia darah dan pemeriksaan histologis dari organ-organ vital. Hasil uji toksisitas akut menunjukkan hingga dosis maksimum 5.000 mg/kg bb menunjukkan tidak toksik. Uji toksisitas subkronis, histologi menunjukkan organ vital tidak menunjukkan perubahan tertentu dalam kedua kelompok kontrol dan dosis kelom-pok.
Kata kunci: toksisitas,Blumea balsamifera L. DC., Piper cubeba L., Euphorbia hirta L.,and Cyperus rotundus L.
ABSTRAC
Asthma is a respiratory disorder that narrows the airway because of hyperactivity to certain stimuli, which cause
by inflammation; this narrowing is temporary. Increasing number of people are interested in the use of treatment using medicinal plants and herbs. Medicinal Plant and Traditional Medicine Research and Development Centre
(B2P2TO2T) has develop jamu formula consisting of blumeae folium (Blumea balsamifera L. DC.), cubebs (Piper
cubeba L.), asthma weed (Euphorbia hirta L.), and nutgrass (Cyperus rotundus L.) for the treatment of asthma. Blumeae folium empirically was used as an asthma medication. Cubebs which contained dihydrocubebin has the
effect of tracheospasmolitic. Asthma weed used to treat asthma, chronic bronchial disorders, acute nasal catarrh,
and as an expectorant. Nutgrass are used as anti-inflammatory. The study was conducted to determine the toxic
-ity of herbs consisting blumeae folium, cubebs, asthma weed, and nutgrass in Wistar rats. Acute toxicity test using
the 3 groups; control group, dose groups 5.000 and 2.500 mg/ kg bw. While the subchronic toxicity test using four groups; control group, a dose of 0.144; 0.288; 0.576 g/200 g bw. Data were collected for toxic symptoms and
mortality, body weight, blood chemistry and histological examination of vital organs. Acute toxicity test results
show up to a maximum dose of 5.000 mg/kg bw showed practically not toxic. Subchronic toxicity test, histology showed the important organs showed no specific changes in both the control group and dose groups.
Asthma including five major causes of death in the world, reaching 17.4 percent.
While in Indonesia, the disease is among the top ten causes of morbidity and mortality. The
World Health Organization (WHO) noted that there are currently 300 million asthma suffer
-ers worldwide. Indonesia alone has 12.5 mil
-lion people with asma (Anonim, 2008; Sarbini, 2009). Asthma is a respiratory disorder that is
narrowed because of hyperactivity to certain
stimuli, which cause inflammation, this condi -tion is temporary. The increasing number of people who are interested in the use of treat-ment using herbs and spices makes a research
and development regarding the efficacy and
safety of herbal medicinal plants becoming very important. Blumeae folium, cubebs , asth-ma weed, and nutgrass are some medicinal plants that have been used for the treatment of asthma individually. Combination using the four medicinal plants are being developed with the hope of synergism interaction increased
the efficacy as antiasthma. Scientific evidence
about the safety of this herbs is absolutely nec-essary.
Blumeae folium (Blumea balsamifera
L. DC.) contains flavonoids, borneol, cineol,
limonene, camphor, a-pinene, Camphene,
b-pinene, 3-Carene, sesquiterpenes, monoter -penes, triterpene, and cryptomeridiol, blumea-lactone A, B, C. Blumeae folium empirically used in the treatment of respiratory diseases such as sinusitis, colds, asthma and bronchitis
as an expectorant (Globinmed, 2014).
Cubebs (Piper cubeba L.) is character-ized by lignan cubebin, essential oil com-pounds such as cadinol, a tricyclic sequiter-pene, alcohol, a mixture of isomer cardinenes,
1,4-cineol and terpineol-4 (Wagner, 1996). Di
-hydrocubebin have tracheospasmolytic effect, reducing the effect of the contraction of meta
-kolin (Wahyono dkk., 2003).
Asthma Weed (Euphorbia hirta L.) con-tains quersetin, quercitrin, xanthorhamnin,
leucocyanidin, 3,5-diglucoside, tanning sub -stances with gallic acid as a constituent com-ponent, inocyte, sterols, terpenoids such as taraxerol, friedelin, n-alkanes, and triacontane. Euphorbia hirta var. procumbens (Boiss)
Small-containing herfirdelin, β-amarin, β-cytosterin, hentriakontan (BPOM, 2006). This herb used
for the treatment of lung abscess, chronic
bronchitis, asthma, dysentery, inflammation of
the mammary gland or breast and typhoid
ab-dominalis (Hariana, 2006).
Nutgrass (Cyperus rotundus L.) contains
alkaloids, flavonoids, tannins, starch, glyco -sides, furochromones, monoterpenes, sesqui-terpenes, cytosterol, fatty oil containing waxy substance that is neutral, glycerol, linoleic acid,
myristic and stearic acid (Singh et al., 2012). The aim of this study was to make sure the safety of anti asthma herbs formula.
MATERIALS AND METHOD Materials
This research was exploratory
experi-mental design (CRD) with one factor infusion
dose variation. The study was conducted in the Animal Laboratory, Medicinal Plant &
Tradi-tional Medicine R&D Center, Tawangmangu in April-December 2012. Fourty two male and fe
-male Wistar rats, age 2-3 months with a body weight of 180-230 g. Mice were obtained from the Animal House Center for R & D TO & OT Tawangmangu. Determination of the number of samples used the formula: T (n-1)> 15. “T”
was the number of groups, and “n” was the number of samples in each group.
Methods
Acute Toxicity Test
Herbs consists of blumeae folium, cubebs, asthma weed, and nutgrass, with a
ra-tio of 5: 3: 3: 5.
Eighteen Wistar rats males and females were
randomly divided into 3 groups, namely:
Control group was given distilled water
Group 1: given a dose of herb 5.000 mg / kg
bw.
Group 2: given a dose of herb 2.500 mg/ kg
bw.
Each rats were weighed to determine the volume of a given infusion. Infusion was given
on the first day and then was observed 1-4
hours after infusion of the presence of symp-toms of vomiting, diarrhea, tremors, seizures,
losing appetite, weakness, difficulty breathing,
etc. Further observations were made every day
for 14 days. Every two days weighing is done to determine the effect of body weight on the
test material body weight gain. If found death necropsy performed, then made observation to vital organs, such as liver, kidney, heart, spleen, lungs and stomach both macroscopic and microscopic. Histopathological prepara-tions stained with hematoxylin eosin.
Subchronic Toxicity Test
Twenty four male and females Wistar
rats were randomly divided into 4 groups,
namely:
Control group was given distilled water
Group 1:herbs infusion dose 0.144 g/200g bw Group 2:herbs infusion dose 0.288 g/200g bw Group 3:herbs infusion dose 0.576 g/200g bw
Each rats were weighed to determine the volume of a given infusion. Infusion was
given for 90 consecutive days. Observations
were made on the presence of symptoms of vomiting, diarrhea, tremors, seizures, losing
appetite, weakness, difficulty breathing, etc..
Further observations were made every day for
90 days.
Each body weight weighing is done once a
week to determine the effect of test materi -als on body weight gain. Blood sampling was
performed on day 0, 45th, and 90th for blood
chemistry values include urea, creatinine, SGPT, SGOT. If found death necropsy performed, then made observation to vital organs, such as liver, kidney, heart, spleen, lungs and stomach both macroscopic and microscopic. Histopatho-logical preparations stained with hematoxy-lin eosin. At the end of the study all animals
sacrificed and then necropsy performed, then
made observation to vital organs, such as liver, kidney, heart, spleen, lungs and stomach both macroscopic and microscopic. Histopathologi-cal preparations stained with hematoxylin eo-sin.
Data were analyzed using one-way ANO -VA test.
RESULTS AND DISCUSSION
Acute toxicity test results showed no
Subchronic toxicity test results showed
no significant difference in body weight be -tween the dose groups and the control group
(Table 2). No symptoms of vomiting, diarrhea,
trembling, seizures, not eating, lethargy, short-ness of breath found on all test animals. There was no death during the period of testing and observation.
Examination of renal function param-eters were the levels of urea and creatinine
in the blood. The results showed significantly difference in urea levels in female animals be
-tween dose group 0.576 g/200 g bw with the control group on day 45 and on day 90, and
still in the normal range. Creatinine levels of
males dose groups 0.144 and 0.288 g/200 g bw with the control group at day 45, were still
in the normal range. Similarly, the creatinine
levels on day 90 male dose groups 0.288 and 0.576 g/200 g bw.
Table 2. Rats Body Weight at Subchronic Toxicity Test
Body weight (g)
Day 0 Day 30 Day 60 Day 90 Bw gained
Male
Control 247.00±36.17 332.67±55.15 380.33±54.98 400.00±52.05 153.00±16.52
Dose 0.144 g/200 g bw 228.33±16.17 317.33±22.03 367.00±33.78 385.67±28.29 157.33±13.58
Dose 0.288 g/200 g bw 222.67±8.50 325.00±9.54 344.00±6.93 366.33±4.93 143.67±10.12
Dose 0.576 g/200 g bw 236.33±18.77 335.67±25.38 364.33±38.89 381.33±33.50 145.00±19.08
Female
Control 198.67±3.79 230.00±6.00 248.67±3.21 252.67±1.53 54.00±5.29
Dose 0.144 g/200 g bw 184.00±3.61 218.33±7.57 236.33±10.02 241.67±1.53 57.67±3.06
Dose 0.288 g/200 g bw 173.33±8.96 220.33±23.07 232.33±28.11* 241.33±33.53 68.00±25.63
Dose 0.576 g/200 g bw 172.00±14.11 209.00±18.73 217.33±24.85* 224.33±23.97* 52.33±12.50
Data was Mean ± SD
*Significantly different compared to control, p<0.05
Body weight (g)
Day 0 Day 6 Day 14 Bw Gained
Male
Control 264.33±3.21 261.67±2.89 313.33±4.16 49.00±5.57
Dose 2500 mg/kg bw 251.67±41.43 242.67±9.61 286.00±8.54 34.33±4.62
Dose 5000 mg/kg bw 249.33±19.50 246.67±23.29 292.67±21.39 43.33±2.52
Female
Control 187.33±17.21 190.00±14.18 207.67±13.58 20.33±4.73
Dose 2500 mg/kg bw 172.00±12.77 179.00±16.09 195.00±12.49 23.00±1.00
Dose 5000 mg/kg bw 182.67±12.42 184.00±10.15 205.67±10.41 23.00±9.54 Data was Mean ± SD
Examination of liver function param-eters were SGPT and SGOT levels in the blood.
The results showed significantly difference in the levels of SGPT at doses of 0.144 g/200, 0.288, 0.576 g/200 g bw group of females with the control group on day 90. However, SGPT
levels were higher than the range since before
giving jamu formulas for asthma (Day 0).
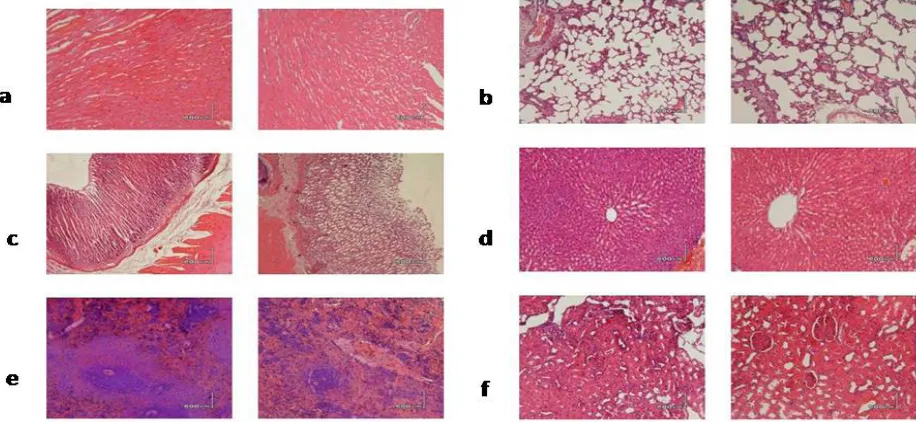
The histologic features in vital organs
showed no specific changes were observed ei -ther in the control group and group dose. The composition of cells and tissues appear normal as shown in Figure 1.
Table 4. Enzyme Activities in Serum
SGPT SGOT
Day 0 Day 45 Day 90 Day 0 Day 45 Day 90
Male
Control 44.67±4.04 35.67±5.03 31.67±7.02 171.00±48.28 143.33±13.01 110.00±16.37 Dose 0.144 g/ 200 g bw 45.33±2.08 39.67±1.53 34.33±7.57 219.00±35.54 147.33±26.27 130.00±49.57 Dose 0.288 g/ 200 g bw 42.67±10.21 51.00±2.00 40.00±7.55 206.67±13.43 112.00±11.53 143.67±31.26 Dose 0.576 g/ 200 g bw 42.00±4.58 43.33±7.77 39.67±0.58 176.33±26.69 177.00±64.86 122.00±14.73
Female
Control 40.67±8.50 45.00±7.00 59.00±13.53 219.00±20.07 190.67±28.29 178.00±50.27 Dose 0.144 g/ 200 g bw 37.00±1.00* 38.67±0.58 35.33±3.79* 209.67±22.01 226.33±54.42 168.33±62.79 Dose 0.288 g/ 200 g bw 40.00±13.08 42.67±10.97 46.67±12.22* 196.00±7.81 183.33±11.93 183.67±62.39 Dose 0.576 g/ 200 g bw 44.33±0.58* 38.67±1.53 46.33±9.02* 211.00±8.72 207.67±67.34 215.00±72.81
Data was Mean ± SD
*Significantly different compared to control, p<0.05
Table 3. Chemical Component in Serum
Urea Creatinin
Day 0 Day 45 Day 90 Day 0 Day 45 Day 90 Male
Satellite 66.33±6.11 35.33±2.08 39.33±1.53 1.17±0.12 0.70±0.00 0.47±0.06 Dose 0.144 g/ 200 g bw 64.67±19.76 48.33±6.11 50.00±1.73 1.40±0.00 0.80±0.17* 0.43±0.06 Dose 0.288 g/ 200 g bw 48.67±4.73 39.00±7.55 51.67±2.08 1.27±0.15 0.90±0.36* 0.40±0.00* Dose 0.576 g/ 200 g bw 58.67±14.22 37.00±7.21 46.33±7.02 1.07±0.06 0.73±0.25 0.50±0.00*
Female
Satellite 44.33±13.28 38.33±5.86 39.67±0.58 0.87±0.12 0 80±0.17 0.53±0.06
Dose 0.144 g/ 200 g bw 39.67±4.51 29.33±2.08 36.67±1.53 0.97±0.15 0.90±0.17 0.47±0.12 Dose 0.288 g/ 200 g bw 43.00±13.45 36.33±2.31 50.00±2.65 1.13±0.06 0.80±0.17 0.43±0.06 Dose 0.576 g/ 200 g bw 40.33±11.59 29.00±0.00* 31.67±2.31* 0.93±0.15 0.63±0.12 0.43±0.12 Data was Mean ± SD
Data was Mean ± SD
Results of acute toxicity tests showed no symptoms of toxicity and mortality in all test animals up to the largest dose given at
5.000 mg/kg bw. Thus classified jamu formu -las for asthma practically not toxic. Liver func-tion tests performed by examinafunc-tion of liver
enzymes in the blood, namely SGOT (Serum
Glutamic oxaloacetic transaminase) and SGPT
(Serum Glutamic Pyruvic Transaminase). In
normal conditions the SGOT produced by the liver, cardiac muscle, kidney, brain, and red blood cells. This enzyme is released into the bloodstream when these tissues are damaged. For example, the blood SGOT levels increased in conditions of muscle injury and heart attack. Therefore, the increase of this enzyme in the blood does not mean that the liver tissue dam-age as it can be elevated in conditions other than liver damage. Unlike the SGOT, SGPT is a
specific indicator of liver cell damage. This en -zyme is also produced in small amounts by the
kidney and striated muscle (Anonim, 2014).
SGOT and SGPT describe liver cell damage if
the value is greater than or equal to 3 times the
normal value.
Urea and creatinine are the end product of nitrogen metabolism.
Urea is the primary metabolite derived from protein and tissue protein turnover. Creati-nine is a product of the catabolism of muscle creatine. Both are relatively small molecules
(respectively 60 and 113 daltons) which dis
-tributes the total water in the body (Wikipe
-dia, 2014) . Creatinine levels are within nor
-mal limits is 0.2-0.8 mg/dl (Mitruka, 1981).
Renal conditions can be described by the ra-tio of urea and creatinine. Urea and creatinine
ratio> 100:1 indicates pre-renal damage, 40-100: 1 indicates normal or post-renal damage, <40:1 indicates intrarenal damage (Wikipedia, 2014). The ratio of serum urea and creatinine in subchronic toxicity tests is 92.66: 1 for male and 73.65: 1 for female at the highest dose,
which means that the kidneys in normal con-ditions.
CONCLUSION
Jamu formulas for asthma is practically not toxic and didn’t damage liver also kidney.
ACKNOWLEDGEMENT
Thank to Traditional Medicine and
Me-dicinal Plant Research and Development Cen -ter, Health Ministry of Indonesia for
support-Figure 1. Histology of organs in the control group (left) and dose group (right) with a magnification of 200x, 100x except stomach. No specific changes in
REFERENCES
Anonim,2008. Penyakit Asma 5 Besar
Penyebab Kematian di Dunia. (Cited on Januari 31, 2012), Available from URL: http://www.balita-anda.com/ensik
-lopedia-balita/447-penyakit-asma-5
-besar-penyebab-kematian-di-dunia. html
Anonim,2014.SGOT and SGPT,Med-Helath.net. (Cited on Mei 29, 2014) Available from
URL:www.med-health.net/Sgot-Sgpt. html,
Badan Pengawas Obat dan Makanan (BPOM), 2006. Acuan Sediaan Herbal Vol Keem-pat Edisi Pertama. Jakarta: Badan Pen-gawas Obat dan Makanan Republik In-donesia.
Globinmed, 2014. Blumea balsamifera Linn.
DC. (Cited on Mei 29, 2014) Available
from URL:http://www.globinmed.com/
index.php?option=com_content&view=
article&id=79078:blumea-balsamifera-linn-dc&catid=704:b
Hariana A.,2006. Tumbuhan Obat dan Khasiat-nya. Seri 2. Penebar Swadaya, Jakarta.
hal 19-20 http://www.lintas.me/ar
-ticle/tribunbatam.co.id/Penderita_ Asma_di_Indonesia_125_Juta_Orang/1 Mitruka BM. and Rawnsley HW., 1981. Clinical
Biochemical and Haematological Ref-erence Values in Normal Experimental
Animals and Normal Human 2nd. Masson
Publishing USA Inc.
Sarbini,2009.Penderita Asma di Indonesia 12,5 Juta Orang. (Cited on Januari 31, 2012), Available from URL:
Singh N., Pandey BR., Verma P., Bhalla M., and
Gilca M., 2012. . Phytopharmacother -apeutics of Cyperus rotundus Linn.
(Motha): An overview. Indian Journal of Natural Products and Resources, 3(4):
467-476.
Wagner H. and Bladt S., 1996. Plant Drugs
Analysis, A Thin Layer Chromatography Atlas 2nd. Springer Verlag, Berling. P
272-273.
Wahyono HL., Wahyuono S., Mursyidi A.,
Ve-poorte R., Timmerman H., 2003,
Isola-tion of Tracheospasmolitic Compound from Piper cubeba Fruits. Majalah
Far-masi Indonesia, 14(3): 119-123
Wikipedia, 2012. Asma. (Cited on Januari 31, 2012), Available from URL: http:// id.wikipedia.org/wiki/Asma
Wikipedia, 2014. BUN to creatinine ratio, (Cited on Mei 29, 2014) Available from
URL: