Professor Wei-Shou Hu
Spring 2007
Cell Culture Bioreactors
Cell Culture Bioreactors
Basic Types of Bioreactors . . . .1
Segregated Bioreactors (Dead Zone Present)\Compartmentalized Bioreactors . 4
Homogenous Reactor vs . Heterogeneous Reactor . . . . 4
Batch and Continuous Processes . . . . 4
The Operating Mode of Reactors . . . . 5
Batch Cultures . . . . 5
Fedbatch Cultures . . . . 5
Continuous Cultures . . . . 7
Material Balance on Bioreactors . . . .8
Material Balance Equation for Reactor . . . . 8
Tissue culture and disposable cell culture systems . . . .10
Microcarriers . . . . 2
Cell Aggregates . . . . 5
Microsphere Induce Cell Aggregates . . . . 5
Agarose . . . . 6
Microencapsulation . . . . 6
Cell Culture Bioreactors. . . .17
Simple Stirred Tank Bioreactor . . . . 7
Airlift Bioreactor . . . . 8
Spin Filter Stirred Tank . . . . 9
Vibromixer . . . . 20
Fluidized Bed Bioreactor . . . . 2
Basic Types of Bioreactors
Mammalian cell bioreactors are generally categorized similarly to chemical reactors according to their mixing characteristics. It is instructive to review two ideal reactors: well-mixed stirred tank and
plug-flow (tubular) reactor. In an ideal well-mixed bioreactor, the mixing is assumed to be intense enough that the fluid is homogeneous
through the reactor. The mathematical description of ideal continuous
flow stirred tank reactor is described by the following first-order
differential equation.
(
A)
i Ai o Ao A
d VC
F C
F C
r V
V is the culture volume in the bioreactor, CA the concentration of nutrient or product A, t is time, F is the flow rate and rA is the
volumetric consumption rate of nutrient or production rate of product
A.
In an ideal stirred tank reactor, there is no flow bypass and no shunt of substrate from inlet to outlet, no dead zones or clumps of undissolved solid substrate floating around. The addition of a
substrate through feeding is instantaneously distributed throughout
the entire reactor, and when gas sparging is employed the agitator
provides an intimately mixed gas-liquid. It also follows from this assumption that th e stream exiting the reactor will have the same
composition as the well mixed fluid in the reactor.
The basic model for the tubular reactor (such as hollow fiber and ceramic systems to be described later in this chapter) specifies that the liquid phase moves as a plug-flow, meaning that there is no
variation of axial velocity over the cross section. The mass balance
for component A in a volume element
S z
∂
that described an idealplug-flow reactor is the following:
A A Z A
r
z
C
v
t
C
+
∂
∂
−
=
∂
∂
where vz is the linear velocity in the z direction along the flow and
S is the cross-sectional area. Note that we assume there is no liquid
dispersion or back mixing. All elements in the fluid move at the same velocity. At steady state (i.e., cell concentration and cellular activities at a given position are not changing with time), the equation
becomes
F
S
r
z
c
A A⋅
=
∂
∂
which describes changes of concentration of A along the direction of fluid flow. It is clear that the nutrient concentration will decrease from inlet to the distal end of the reactor, while metabolite concentration
increases. The length of the reactor is limited because eventually nutrient depletion or metabolite accumulation inhibits growth and metabolism.
These ideal cases of completely mixed tanks or plug flow tubular
reactors are situations that can be approximated in small-scale laboratory conditions. The conditions in larger scale process reactors
deviate significantly from these ideal conditions.
In a well-mixed bioreactor, there are no concentration gradients in either the gas or the liquid phase. In other words, none of the
chemical species or cells is segregated in the reactor. The other extreme of mixing is total segregation where there is no interaction
between different volume elements in the bioreactor. An ideal plug flow reactor is assumed to be under conditions of total segregation.
Most bioreactor systems have a mixing pattern between the two extremes and are under partially segregated conditions.
In general, laboratory and small pilot plant bioreactors are used for process development and optimization. The fluid mixing
characteristics are rather sensitive to the scale of the reactor.
Cell Culture Bioreactors
nutrients, oxygen in particular, will inevitably become limiting in the
downstream region of the reactor. In considering the selection of
bioreactors for mammalian cell cultures, the mixing characteristics
and their relationship to scale-up have to be kept in mind.
Stirred Tank (Well Mixed) vs. Tubular Reactor
(Plug Flow)
The distinction between a well mixed continuous stirred tank reactor
(CSTR) and plug flow reactor (PFR) is best illustrated by comparison of their response in the outlet to a step change in feed concentration, consider a continuous reactor that has an inlet stream (feed) and an outlet stream that are equal in volumetric flow rate, the volume of the reactor is thus constant. For the case that the feed stream is colorless,
but at time 0 the stream is changed to a feed with red color at a
concentration of COIf the reactor is well mixed as in a CSTR, as soon
as the feed stream is switched, the color will be seen immediately in the effluent stream, since the color is distributed instantaneously everywhere including the fluid that is taken out in the effluent
stream. The plots shown are the colors seen at the outlet. The red
dye concentrate will increase gradually. If the reactor volume is V, it will take longer than the time needed to flow through one reactor volume to reach the same concentration as in the feed, since the dye is also being taken out from the reactor from the beginning. In fact, by solving the differentiation equation, tIcan be shown that it takes
about three holding times (3t0) to reach almost the same concentration
as in the feed.
Now examine the case of PFR. According to the model of PFR, the red color dye will move downstream like a sharp band, since there is
no backmixing or diffusion to blur the sharp boundary between the color and colorless streams. So the detector at the exit will detect no color right after the switch to dye solution in the feed. It will not see any color until the “front” of the color feed solution reaches the
outlet. The time it will take will be a “hold time”, the exact time of flow a reactor volume into the reactor to displace all the original clear solution in the reactor. As soon as the color comes out in the outlet, the concentration will be equal as in the feed.
If the reactor is not idealized, obviously the pattern of the dye concentration will be different. For a tubular reactor, the “front” may not be as sharp, rather the appearance in the exit will be more gradual. Similarly in a stirred tank, there will be deviation to the
perfect mixing curve.
In more severe cases, a reactor may be compartmentalized or
the feed stream passing right through, or some portions of the reactor
hardly see any feed stream.
Segregated Bioreactors (Dead Zone Present)\
Compartmentalized Bioreactors
Concentrations in different compartments may be different
Most reactors are not ideally all mixed or plug-flow; segregated zone
is not a completely dead zone
Implication When Growth or Reaction Occurs
in the Reactor
Flow and mixing behavior may have a profound effect on the reaction or growth. Consider a nutrient stream entering the reactor. If the reactor is a PFR, the cells in the upstream will have abundant nutrient. As the fluid moves downstream more nutrients get
consumed and their concentration decreases. The cells downstream may not have enough nutrients or face starvation. One way to solve the problem is of course to increase the supply rate by using a higher
nutrient concentration in the feed or by operating at a higher flow rate. But there are limits on both nutrient concentration and flow
rate. Eventually the size of the reactor will be restricted.
In a CSTR model, all cells in the reactor see the same environment.
The nutrients that feed into the reactor will be distributed uniformly
everywhere, either all have abundant or suboptimal levels.
Homogenous Reactor vs. Heterogeneous
Reactor
Heterogeneous reactor—with a solid phase, e.g., microcarriers in stirred tank, tubular reactor packed with foam. A typical tissue has
a cell concentration of about 5X108/ml. Unless a rector have a very high cell concentration in the middle of 107/ml, cell mass is only a
small fraction of the culture volume. So, even though almost all cell culture reactors have all three phases, liquid medium, gas bubbles and cell mass, they are often treated as homogenous bioreactors. On the other hand, in addition to high cell density culture there are
cases where the bioreactor must be treated as heterogeneous. The
solid phase constitutes a large fraction of the culture volume. An
examples is the microcarrier culture. Microcarrier beads often
constitute 10-30% of the culture volume. In such cases even cell concentration needs to be well defined, for example, whether 107 per
milliliter is referring to total culture volume or liquid volume needs
to be specified.
Operating Mode of Bioreactors
Batch and Continuous Processes
A reactor is called continuous when the feed and product streams are continuously being fed and withdrawn from the system. In principle, a reactor can have a continuous recirculating flow, but no continuous feeding of nutrient or product harvest; it is still a batch reactor. A
fed-batch bioreactor usually has intermittent feed. It may or may not have medium withdrawal during the run.
Cell Culture Bioreactors 5
Example:
For instance, Yeast cells (saccharomyces cereviciae)can metabolize glucose either to ethanol, or to oxidize it to carbon dioxide, mammalian cells can convert glucose mostly to lactate, or oxidize it to carbon dioxide. Cells in two such types of metabolism
are in two different metabolic states. The two metabolic states are
characterized by different specific glucose consumption rates, lactate or ethanol production and the yield coefficient for biomass, i.e.
different stoichiometric ratio.
Example:
For instance, a 1 l culture has 0.3 of solid microcarriersand 0.7 l of medium, with 109 cells in it. The cell concentration
is 109 cells/L-culture or 1.43 x 109 cells/L-medium. If the glucose
concentration in the culture medium decreases from 2.10 g/L
(medium) over one day, then the specific glucose consumption rate is (2.10-1.90) g/L-medium ÷ (1.43 x 109 cells/L-medium) = 1.40 x
10-10 g/cell-hr. The specific rate calculated would have been very
different if one concentration is based on liquid volume and the other is based on total culture volume.
The Operating Mode of Reactors
Batch Cultures
Batch processes are simple and are widely used, especially in the vaccine industry and in pre-production scales of rDNA protein production. Fedbatch processes are widely used in multi-purpose, multi-product facilities because of their simplicity, scalability, and flexibility. A variety of fedbatch operations, ranging from very simple to highly complex and automated, are seen in current production
facilities.
Fedbatch Cultures
Intermittent Harvest
In general, fedbatch processes do not deviate significantly from batch cultures. For both intermittent-harvest and traditional fedbatch cultures, cells are inoculated at a lower viable cell density in a
medium that is usually very similar in composition to a typical batch
medium. Cells are allowed to grow exponentially with essentially
no external manipulation until nutrients are somewhat depleted and
an intermittent-harvest fedbatch process, a portion of the cells and product are harvested, and the removed culture fluid is replenished
with fresh medium. This process is repeated several times. This simple strategy is commonplace for the production of viral vaccines
produced by persistent infection, as it allows for an extended
production period. It is also used in roller bottle processes with adherent cells.
Fedbatch
For production of recombinant proteins and antibodies, a more
traditional fedbatch process is typically used. While cells are
still growing exponentially, but nutrients are becoming depleted, concentrated feed medium (usually a 10-15 times concentrated basal medium) is added either continuously (as shown) or intermittently to supply additional nutrients, allowing for a further increase
in cell concentration and the length of the production phase. In
contrast to an intermittent-harvest strategy, fresh medium is added
proportionally to cell concentration without any removal of culture
broth. To accommodate the addition of medium, a fedbatch culture
is started in a volume much lower than the full capacity of the
bioreactor (approximately 40% to 50% of the maximum volume).
The initial volume should be large enough to allow the impeller to
be submerged, but is kept as low as possible to allow for a maximum
extension of the cultivation period.
Fed-batch Culture with Metabolic Shift
In batch cultures and most fedbatch processes, lactate, ammonium,
and other metabolites eventually accumulate in the culture broth over
time, inhibiting cell growth. Other factors, such as high osmolarity and accumulation of reactive oxygen species, are also likely to be growth inhibitory, and certainly contribute to the eventual loss of viability
and productivity. The effects of lactate and ammonia on cultured
cells are complex. Detectable changes in growth, productivity, and metabolism have all been documented. Additionally, metabolite
accumulation has been found to affect product quality. In recombinant
Cell Culture Bioreactors 7
has been reported to affect glycoform of the product.By minimizing metabolite accumulation, the duration of a fedbatch
culture can be even further extended and higher cell and product
concentrations can be achieved. Reduced metabolite accumulation
in fedbatch culture is traditionally accomplished by limiting the availability of glucose and glutamine using controlled feeding
strategies that maintain glucose at very low levels. After extended exposure to low glucose concentrations, cell metabolism is directed to a more efficient state, characterized by a dramatic reduction in
the amount of lactate produced. Such a change in cell metabolism from the normally observed high lactate producing state to a much reduced lactate production state is often referred to as metabolic shift. The observation of such changes in metabolism was made more
than two decades ago, yet its application in fedbatch culture was not
realized until much later. Extending the methodology to controlling
both glucose and glutamine at low levels, both lactate ammonium
accumulations can be reduced. By applying such a control scheme
in fedbatch culture, lactate concentration was reduced by more than three fold, and very high cell concentrations and product titers were
achieved in hybridoma cells.
Continuous Cultures
Simple Continuous Stirred Tank Reactor (CSTR)
Steady state Grow up the culture in batch mode. Then turn on both in and
out flow of medium. Cell and product concentration reach
steady state. Transient
Same as that for steady state except that cell and product
nutrient concentration fluctuate.
Continuous Culture with Cell Retention (Recycle)
– Perfusion Culture
Transient
Same as CSTR, some cells are retained in bioreactor to reach high cell concentration. Product throughput is higher per reactor volume, but not the concentration. Typically cell, nutrient and product concentrations fluctuate.
Steady state
Same as that for transient except that steady state is achieved. This rarely happens.
Continuous Culture with a Metabolic Shift
This is the same as simple continuous culture except in the start-up.
Instead of starting from a batch culture, a fed-batch culture with a metabolic shift is used. After cells reach a high concentration and the metabolic shift is affected, the culture is shifted to a continuous culture. Because no (or low) lactate and ammonia is produced,
the concentrations of cells and products are substantially higher
concentration approaches that of perfusion cultures. However, the medium usage is substantially reduced, and the product concentration
is higher.
Material Balance on Bioreactors
Material Balance Equation for Reactor
Batch Culture
v v
s v
dx
V V x
dt ds
V Vq x
dt
m =
= −
Fed-batch Culture
( d(sv)
( )
dt
v
v s v
d x V V
F t x V q x V
dt dt
m
Fedbatch Culture and Dynamic Nutrient Feeding 45
of antibiotic production capacity have process engineers played such a key role in bringing a large array of products to therapeutic use in such a short time. The increased output required to meet the expanding market was not accomplished by merely increasing the total culture volume. A large part was achieved through improving yields by process renovation, as opposed to pro-cess innovation. Only a decade ago, an antibody titer in the hundreds of milligrams per liter was the norm. Now, concentrations of a few grams per liter are common. With the increasing development of new products and the growing need for large quantities of each new therapeutic, it is prudent to reassess the technological advances made in the past decade and to pursue innovative ideas that will ease the task of meeting future demands.
The final product concentration is primarily affected by the specific pro-ductivity of cells, the maximum cell concentration, and the duration that high viability can be sustained. For batch processes, the low level of nutrients that can be tolerated by cells limits the final cell and product concentration. Cells are simply unable to attain and sustain high cell concentrations with the resources available in a typical growth medium. To overcome nutrient limitation, fedbatch processes have been widely practiced and are currently the norm for most cell culture processes. In fedbatch cultures, concentrated medium is added during cultivation to prevent nutrient depletion, prolonging the growth phase and increasing cell and product concentrations. Continued addition of medium past the peak of cell concentration also increases the final titer significantly by allowing cells to be kept viable at high concentrations and continue to produce product for a longer time.
Efforts to enhance the performance of fedbatch culture have traditionally focused on medium development, process control, and manipulation of cell metabolism by control of the culture environment. With recent advances in genomic research tools and a more global understanding of cell physiology, metabolic engineering may emerge as a more prominent strategy to increase productivity. Even with the promise of creating superior host cells through cell engineering, pushing the limits of productivity will always require an in-tensive process engineering effort to accommodate the increased demands of higher cell and product concentrations. This review will summarize current practices and articulate the developmental needs of fedbatch culture to meet these future challenges.
2
Different Forms of Fedbatch Culture
46 K.F. Wlaschin · W.-S. Hu
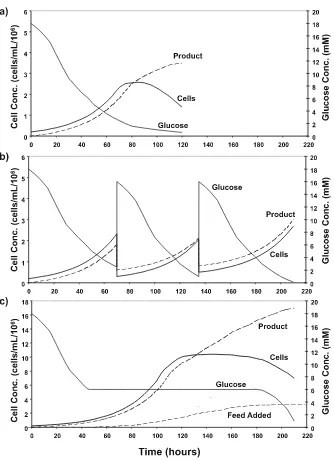
of fedbatch cultures as compared to a typical batch operation, time profiles of cell, nutrient, and product concentrations for batch (Fig. 1a), intermittent-harvest fedbatch (Fig. 1b), and traditional fedbatch cultures (Fig. 1c) are shown.
In general, fedbatch processes do not deviate significantly from batch cul-tures. For both intermittent-harvest and traditional fedbatch cultures, cells are inoculated at a lower viable cell density in a medium that is usually very similar in composition to a typical batch medium. Cells are allowed to grow exponentially with essentially no external manipulation until nutrients are somewhat depleted and cells are approaching the stationary growth phase. At this point, for an intermittent-harvest fedbatch process (Fig. 1b), a por-tion of the cells and product are harvested, and the removed culture fluid is replenished with fresh medium. This process is repeated several times. This simple strategy is commonplace for the production of viral vaccines produced by persistent infection, as it allows for an extended production period. It is also used in roller bottle processes with adherent cells.
For production of recombinant proteins and antibodies, a more traditional fedbatch process (shown in Fig. 1c) is typically used. While cells are still growing exponentially, but nutrients are becoming depleted, concentrated feed medium (usually a 10–15 times concentrated basal medium) is added either continuously (as shown) or intermittently to supply additional nutri-ents, allowing for a further increase in cell concentration and in the length of the production phase. In contrast to an intermittent-harvest strategy, fresh medium is added proportionally to cell concentration without any removal of culture broth. To accommodate the addition of medium, a fedbatch culture is started in a volume much lower than the full capacity of the bioreactor
(ap-proximately40% to50% of the maximum volume). The initial volume should
be large enough for the impeller to be submerged, but is kept as low as pos-sible to allow for a maximum extension of the cultivation period.
In batch cultures and most fedbatch processes, lactate, ammonium, and other metabolites eventually accumulate in the culture broth over time, in-hibiting cell growth. Other factors, such as high osmolarity and accumulation of reactive oxygen species, are also likely to be growth inhibitory, and cer-tainly contribute to the eventual loss of viability and productivity. The effects of lactate and ammonia on cultured cells are complex. Detectable changes in growth, productivity, and metabolism have all been documented [1]. Addit-ionally, metabolite accumulation has been found to affect product quality. In recombinant erythropoietin producing CHO cells, high ammonia concentra-tion has been reported to affect the glycoform of the product [2].
Fedbatch Culture and Dynamic Nutrient Feeding 47
Fig. 1 Representative cell, nutrient, and product concentrations for a typical a batch
culture, b intermittent-harvest fedbatch culture, and c fedbatch culture with dynamic
48 K.F. Wlaschin · W.-S. Hu
After extended exposure to low glucose concentration, cell metabolism is directed to a more efficient state, characterized by a dramatic reduction in the amount of lactate produced. Such a change in cell metabolism from the normally observed high lactate producing state to a much reduced lactate production state is often referred to as metabolic shift. The observation of such changes in metabolism was made more than two decades ago [3–7], yet its application in fedbatch culture was not realized until much later [8]. Ex-tending the methodology to controlling both glucose and glutamine at low levels, both lactate and ammonium accumulation can be reduced [7, 9–11]. By applying such a control scheme in fedbatch culture, lactate concentration was reduced by more than three fold, and very high cell concentrations and product titers were achieved in hybridoma cells [8].
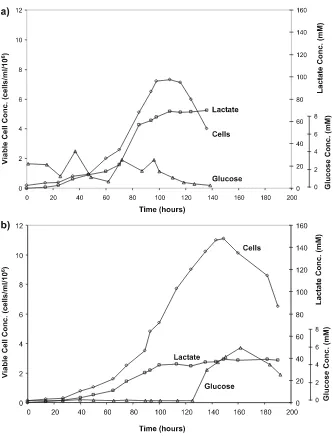
Figure 2 compares the time profile of cell growth, glucose concentration and lactate concentration for two hybridoma fedbatch cultures growing under different metabolic states. Shown in Fig. 2a is a culture in which the glucose
level was controlled in the range of 1.0–4.0mM, a relatively low
concentra-tion. In many cultures, glucose concentration is controlled at even higher
levels, in the range of10mM. In these ranges of glucose concentration, cells
behave very similarly, having a high lactate production rate. As a result, the level of lactate accumulated eventually requires the addition of base to main-tain pH. To supply nutrients to the culture, feed medium was added approxi-mately proportionally with the base addition rate, since lactate production is indicative of the metabolic demands of the culture. This feeding strategy will be discussed in more detail in Sect. 4.2.2. A final cell concentration of
7.5×106cells mL–1was obtained with lactate accumulating to nearly70mM
in the final culture volume. In the culture shown in Fig. 2b, the set point of
glucose concentration was at 0.03mM. Feed medium was added based on
the oxygen uptake rate (OUR), which is estimated on-line. This strategy will also be discussed further in a later section (4.2.4). The continuous exposure to very low glucose concentrations allowed cells to shift their metabolism to a state where little lactate was produced. The final lactate concentration
only accumulated to40mM. With the control of glucose concentration at low
levels, the reduced lactate concentration, and the elimination of base
add-ition, a final viable cell concentration of more than11.5×106cells mL–1was
achieved.
be-Fedbatch Culture and Dynamic Nutrient Feeding 49
Fig. 2 Time profiles of cell, lactate, and glucose concentration for a hybridoma fedbatch
culture with cells growing with ahigh-lactate producing metabolism, andbmetabolic
shift. Metabolic shift was achieved by control of glucose concentrations at0.03mM
50 K.F. Wlaschin · W.-S. Hu
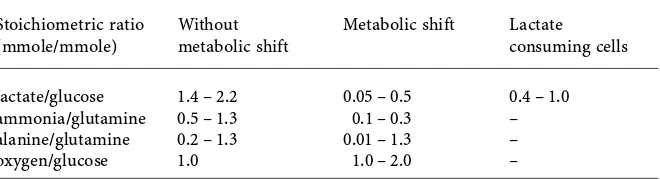
Table 1 Characteristic Stoichiometric Ratios of Key Nutrients for Cells Growing in Differ-ent Metabolic States
Stoichiometric ratio Without Metabolic shift Lactate
(mmole/mmole) metabolic shift consuming cells
lactate/glucose 1.4 – 2.2 0.05 – 0.5 0.4 – 1.0
ammonia/glutamine 0.5 – 1.3 0.1 – 0.3 –
alanine/glutamine 0.2 – 1.3 0.01 – 1.3 –
oxygen/glucose 1.0 1.0 – 2.0 –
in Table 1, showing a dramatic reduction from 0.5–1.3 moles ammonium per mole of glutamine to 0.1–0.3 mole per mole under metabolically shifted conditions. In later stages of fedbatch cultures, lactate consumption, as op-posed to production, is occasionally observed, although this phenomenon is not well documented in published literature. In such cases, an
approx-imate ratio of lactate to glucose consumption is between ∼0.4–1.0 moles
of lactate consumed per mole of glucose consumed. While this observation seemingly contradicts the role of lactate as an inhibitory molecule, it illus-trates the flexibility of mammalian cells to adapt their behavior for survival under a wide range of conditions. With this repertoire of available cell behav-ior, fedbatch culture strategies that provide conditions that reduce metabolite accumulation is a field of fedbatch culture technology still warranting further development.
3
Designing Feed Medium for Fedbatch Cultures
The design of feed medium is critical for the implementation of a success-ful fedbatch process. A well-designed feed medium should ensure cell growth and product formation are not limited by depletion of any medium compon-ent or inhibited by excessive nutricompon-ent conccompon-entration or metabolite accumula-tion. To achieve this, a good estimate of the rates of consumption of medium components is required. For most processes, a feed medium that is 10 to 15 times the nutrient concentration of basal medium is used. With this simple design, the consumed nutrients are replenished, and the growth and produc-tion phases are prolonged; however, many components will likely be supplied in excess, while others will be in limited supply [13].