Summary Norway spruce (Picea abies (L.) Karst.) seedlings growing in a growth pouch system were used to investigate the effects of the ectomycorrhizal fungus Hebeloma crustulini-forme (Bull. ex St. Amans) Quél. and various Cs/K ratios on the uptake of 134Cs, expressed as a percentage of the total amount of 134Cs supplied. The amount of 134Cs taken up by seedlings increased with increasing Cs/K ratio. At a Cs/K ratio of 0.1, uptake of 134Cs ranged between 7.2 and 7.3% and was independent of ectomycorrhizal status, whereas at Cs/K ratios
≥ 1 uptake of 134Cs varied from 8.1 to 11.1% for ectomycorrhi-zal and from 10.4 to 14.4% for non-inoculated plants. Ectomy-corrhizal seedlings contained a lower concentration of 134Cs than non-inoculated seedlings. Among plant parts, the amount of 134Cs was significantly lower in needles and lateral roots of ectomycorrhizal seedlings compared with non-inoculated seedlings. Among fungal and seedling tissues, highest X-ray net counts of 133Cs were measured in fungal hyphae of ectomy-corrhizal mantles. X-Ray net counts of 133Cs in lateral roots of ectomycorrhizal and non-inoculated plants were similar, but 5 to 10 times higher than in main roots and needles, suggesting an accumulation of 133Cs in lateral roots and slow translocation to other plant parts. In contrast, X-ray net counts of K indicated that K was readily mobilized from lateral roots to main roots and needles. Elemental mapping showed a relatively homoge-neous distribution of 133Cs within the root.
Keywords: gamma spectrometry, growth pouch, Hebeloma crustuliniforme, low-temperature scanning electron micros-copy, Picea abies, X-ray microanalysis.
Introduction
Radiocesium (137Cs) is a major radionuclide that has been deposited on the Earth’s surface as fallout from nuclear weap-ons testing during the 1950s and 1960s and has entered the biogeochemical cycles. The main sources of 134Cs and 137Cs in Europe, however, are depositions from the Chernobyl nuclear reactor accident in 1986. The amount of radioactive Cs in Swiss forest soils differs widely (from 0 to 1900 Bq kg−1) from
area to area and even from site to site (Riesen et al. 1995). Most of the Cs isotopes in contaminated forest soils are fixed in the top layers; however, because Cs isotopes may be bound in organic matter complexes, adsorbed by clay particles, or pre-cipitated on surfaces of micaceous minerals, the concentration of Cs in the soil solution is low and migration in the soil profile is slow (Myttenaere et al. 1993).
Although Cs ions in the soil solution can be readily taken up by plant roots, this uptake is competitive with that of K, resulting in markedly reduced content of Cs in plants grown in the presence of high concentrations of K (Coughtrey and Thorne 1983). Thus, the Cs/K ratio may be a key factor in determining Cs uptake by plant roots. Moreover, it is possible that plants are able to discriminate between K and Cs, because of the larger atomic weight of Cs (Coughtrey and Thorne 1983). It has been assumed that roots mobilize Cs ions pas-sively as a consequence of the mobilization and uptake of K from clay particles (Schaller et al. 1990). If this assumption is correct, then plants grown in K-impoverished soil should con-tain more Cs than plants grown in a K-enriched soil.
The Cs content of forest soils is largely a reflection of biological activity in the soil layers (Schaller et al. 1990, Guillitte et al. 1994). Fungi, especially basidiomycetes which are capable of forming ectomycorrhizae, are efficient accumu-lators of radiocesium (Dighton and Horrill 1988, Olsen et al. 1990, Römmelt et al. 1990, Haselwandter and Berreck 1994). Schaller et al. (1990) suggested that Cs transport into the plant is facilitated by ectomycorrhizal fungi that accumulate Cs during microbial decomposition of the litter. However, it is not known to what extent high Cs contents within fungal mycelia are transferred to the host plants (Shaw and Bell 1994).
We investigated the effects of ectomycorrhizae and various Cs/K ratios on the uptake of 134Cs by Norway spruce seedlings raised in a growth pouch plant--fungus system (Brunner and Scheidegger 1992, 1995). In addition, gamma-spectrometry and cryo-scanning X-ray microanalysis (Stelzer and Lehmann 1993) were used to study the distribution and localization of Cs in various plant parts.
Influence of ectomycorrhization and cesium/potassium ratio on uptake
and localization of cesium in Norway spruce seedlings
IVANO BRUNNER,
1BEAT FREY
1and THOMAS K. RIESEN
21
Swiss Federal Institute for Forest, Snow and Landscape Research (WSL), CH-8903 Birmensdorf, Switzerland
2
Paul Scherrer Institute (PSI), CH-5232 Villigen, Switzerland
Received August 8, 1995
Materials and methods
Plant and fungal material
Seeds of Norway spruce (Picea abies (L.) Karst.) were col-lected from a single tree growing near Tägerwilen in the canton of Thurgau, Switzerland. Seeds were surface sterilized in 30% H2O2 for 20 min. Mycelia of the ectomycorrhizal fungal partner Hebeloma crustuliniforme (Bull. ex St. Amans) Quél. were isolated from a fruiting body collected in 1990 in a Norway spruce forest near Plaffeien in the canton of Freiburg, Switzerland. Small tramal pieces were cut from the cap under sterile conditions and cultured in petri dishes on modified Melin-Norkrans nutrient agar (Marx and Bryan 1975). Grow-ing mycelia, which were subcultured at regular intervals, were identified as No. 6.2 of the culture collection of the Swiss Federal Institute for Forest, Snow and Landscape Research, Birmensdorf, Switzerland.
Growth of seedlings and fungal inoculation
Fifty seven autoclaved polyamide/polypropylene pouches (Cellpack), measuring 13 × 16 cm, and divided longitudinally into two chambers that were lined with two activated charcoal filter papers (5 × 9 cm), were moistened with 5 ml per chamber of modified Melin-Norkrans nutrient solution without glucose (Brunner and Scheidegger 1995). Four surface-sterilized Nor-way spruce seeds were inserted in each chamber of the growth pouches. Pouches were suspended on glass rods and randomly distributed among four plastic buckets. The buckets were placed in a growth chamber at 20 °C, 70% relative humidity and a 16-h photoperiod (PAR = 100 µmol m−2 s−1). After seed germination, seedlings were reduced to one per pouch cham-ber to give a total of 114 plants.
After 10 weeks, when lateral roots had developed, another 5 ml of modified Melin-Norkrans nutrient solution containing 5 g l−1 glucose was added to each pouch chamber. To obtain ectomycorrhizal plants, approximately 60 seedlings were in-oculated with mycelia of H.crustuliniforme by placing four to six agar squares (4 × 4 mm) within 3 mm of the lateral roots of each seedling. The remaining seedlings were not inoculated with fungal mycelia. The pH of the nutrient solutions was 6.1 to 6.2 after autoclaving, and was 6.5 to 6.8 after two months, irrespective of fungal inoculation (Brunner and Scheidegger 1995). One strip of foam (1 × 1 × 3 cm) was placed beside each root to provide air space. Sterile distilled water was added as needed. Plants that became visibly contaminated were dis-carded.
Application and analysis of radioactive 134Cs
Nine weeks after inoculation, 40 plants each of the inoculated and the non-inoculated treatments were selected, and the nu-trient solutions in the pouches were washed out twice with 10 ml of sterile water. Two ml of sterile water was then added to the bottom of the pouches (without contacting the charcoal paper) to provide the roots reaching the bottom of the pouches with water during the experiment. After two days, Cs (133Cs + 134Cs) and K were applied continuously as CsCl and KH
2PO4 to the activated charcoal filter paper in daily portions of
50--250 µl to keep the charcoal paper moist. The Cs and K were added to maintain Cs/K ratios of 100, 10, 1, or 0.1 for each of 10 plants in the inoculated and the non-inoculated treatments. The concentration of Cs was kept constant, whereas the K concentration was varied from 367 µM to 367 mM. Over the 23-day experimental period, a total of 2.65 ml of a 36.7 mM CsCl solution containing 270 µl radioactive 134Cs was applied, resulting in a total of 26,474 Bq per plant with a 133Cs/134Cs (stable/radioactive) ratio of 23,791/1.
One day after the last application of Cs and K, plants were harvested, washed three times with 0.1 N HCl, separated into needles, stems, main roots (diameter 1--2 mm), and lateral roots (diameter < 1 mm). Roots grown on the activated char-coal filter paper were freed of adhering charchar-coal paper with a pair of tweezers. Plant parts were dried at 100 °C for 3 days, weighed, and dissolved in 2.5 ml of 65% HNO3 and 1.5 ml of 30% H2O2 in a high pressure microwave (Milestone MLS 1200 Mega, Microwave Laboratory Systems) for 10 min at 250 W, 6 min at 500 W, 2 min at 0 W, 6 min at 450 W, and 5 min of ventilation. Radioactivity was measured with a gamma-spec-trometer equipped with a Ge-detector connected to a mul-tichannel analyzer. The dry weight data were subjected to a one-factor analysis of variance to test for inoculation. The Cs/K ratio was analyzed only as a second treatment factor and did not influence growth over the 23-day exposure period. All other dependent data were subjected to a two-factor analysis of variance to test for the variable factors inoculation and Cs/K ratio.
Application and analysis of stable 133Cs
Nine weeks after inoculation, 10 plants each from the inocu-lated and the non-inocuinocu-lated treatments were selected and treated as described above for the treatment with a Cs/K ratio of 100, except that 133Cs was substituted for radioactive 134Cs. One day after the last application, plant material was washed in deionized water and dried with blotting-paper. Needles, main roots, and lateral roots were cut into pieces approxi-mately 5--10 mm in length with a razor blade. The samples were set vertically in holes on aluminum stubs and held in place with cryo-adhesive (Scheidegger and Brunner 1993) and frozen in liquid nitrogen, or mounted in water between copper platelets (Brunner and Scheidegger 1995) and frozen in liquid propane. For cryo-scanning electron microscopy, the frozen specimens were transferred to the preparation chamber (Balz-ers SCU 020) of a scanning electron microscope (Philips 515), partially freeze-dried for 10 min at −80 °C in a high vacuum at < 2 × 10−4 Pa(Müller et al. 1991), freeze-fractured with a rotating microtome at −90 °C, and immediately sputter-coated with platinum (5 nm). The specimens were then transferred to the cold stage of the scanning electron microscope with the temperature kept below −120 °C.
of 18 kV with a beam current of 80 µA and a working distance of 12 mm. The scan raster was a 4 µm2 square with a maximum magnification of 10,000. In total, eight non-inoculated and eight ectomycorrhizal plants were analyzed. For each plant, one sample each of needles, main roots, and lateral roots was taken. Within the lateral roots of the ectomycorrhizal plants, only ectomycorrhizae were considered. Four spectra per cell type within the various plant parts were acquired for analysis with the live-time set for 120 s and the dead-time for approxi-mately 20%. Spectra were processed to calculate net counts of Cs and K using the Voyager software package (Noran Instru-ments Inc., Middleton, WI) which included an automatic peak identification system. The values were not converted to con-centrations because of the problems associated with obtaining quantitative results from bulk-frozen hydrated samples (Van Steveninck and Van Steveninck 1991).
Elemental mapping of the distribution of Cs in lateral roots was done by energy window mapping using the Voyager soft-ware package. X-Rays characteristic for Cs were collected at Lα = 4.29 keV across the cryosection area of interest. The spatial resolution was 128 × 128 pixels for X-rays with a dwell time of 0.1 s per pixel.
Results
Root colonization by the ectomycorrhizal fungus
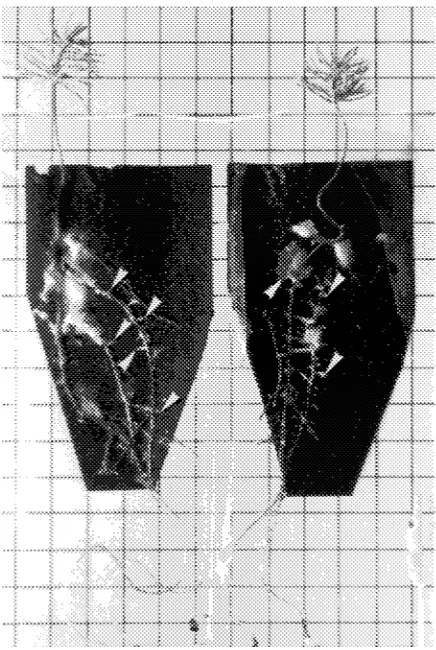
Within 9 weeks of fungal inoculation, mycelial colonization of the roots on the activated charcoal filter paper by the ectomy-corrhizal fungus was approximately 100% and resulted in abundant formation of ectomycorrhizae (Figure 1). The root systems below the charcoal paper were not colonized by my-celia because these roots were submerged.
Uptake and distribution of 134Cs
Ectomycorrhization had no significant effect on dry weights of whole plants or plant parts during the experimental period (Table 1). However, concentrations of 134Cs in total plants were
significantly reduced by fungal inoculation, whereas 134Cs concentrations increased significantly with increasing Cs/K ratio (Table 1). Needles and lateral roots exhibited the greatest increase in 134Cs concentration with increasing Cs/K ratio, Figure 1. Two, 5-month-old Norway spruce seedlings in a growth pouch system showing roots on activated charcoal filter papers thor-oughly colonized by mycelia of the ectomycorrhizal fungus Hebeloma crustuliniforme and with ectomycorrhizae (arrowheads). Grid = 1 cm.
Table 1. Effect of ectomycorrhization on dry weight and effects of ectomycorrhization and Cs/K ratio on the concentration of 134Cs of total plants and plant parts. Values are means of 10 replicates. For the one- and two-factor analyses of variance: NS = not significant; * = significant at P ≤ 0.05; ** = significant at P ≤ 0.01; *** = significant at P ≤ 0.001; **** = significant at P ≤ 0.0001.
Cs/K ratio Non-inoculated Ectomycorrhizal Probability
0.1 1 10 100 0.1 1 10 100 Fungus Cs/K
Dry weight (mg)
Total plant 88.7 86.1 109.3 94.3 82.4 87.0 92.5 95.6 NS
--Needles 48.3 49.4 60.0 51.8 44.8 43.6 49.3 54.4 NS
--Stem 5.6 4.9 5.9 6.5 6.7 5.2 5.2 6.6 NS
--Main roots 9.7 8.6 10.1 9.1 6.7 10.0 8.3 8.4 NS
--Lateral roots 25.1 23.2 33.3 26.9 24.2 28.2 29.7 26.2 NS
--Concentration of 134Cs (Bq.mg−1)
Total plant 21.8 32.3 31.1 43.2 23.4 24.9 27.8 31.0 * ***
Needles 13.6 18.5 20.6 28.1 12.0 13.3 16.4 18.9 ** **
Stem 42.4 40.2 32.6 53.4 44.5 31.6 46.0 52.3 NS NS
Main roots 37.5 41.3 40.7 47.0 45.2 46.5 32.1 49.3 NS NS
whereas 134Cs concentrations of stems and main roots were not significantly influenced by the Cs/K treatments (Table 1). At a Cs/K ratio of 0.1, ectomycorrhization had almost no effect on the concentration of 134Cs in total plants and needles, whereas at a Cs/K ratio of 100, ectomycorrhization led to a significant reduction in the concentration of 134Cs in total plants and needles (Table 1). At a Cs/K ratio of 0.1, lateral roots of non-inoculated plants had a lower concentration of 134Cs than lateral roots of ectomycorrhizal plants, whereas at Cs/K ratios of ≥ 1 the concentration of 134Cs was higher in ectomycorrhizal plants than in non-inoculated plants and increased with in-creasing Cs/K ratio (Table 1).
Between 7.2 and 14.4% of the applied 134Cs was taken up by the plants (Table 2). At a Cs/K ratio of 0.1 and independently of fungal inoculation, 7.2--7.3% of the applied 134Cs was taken up by the plants. In non-inoculated plants, uptake of 134Cs was doubled by increasing the Cs/K ratio from 0.1 to 100, whereas in ectomycorrhizal plants uptake was only enhanced by a factor of 1.5 (Table 2). Uptake of 134Cs by stems and main roots was not affected by any of the treatments. In contrast, uptake of 134Cs by needles and lateral roots was significantly reduced by the inoculation treatment but it increased significantly with increasing Cs/K ratios (Table 2).
There was a linear dependency of 134Cs uptake by plants for log-transformed Cs/K ratios, with ectomycorrhizal plants ab-sorbing significantly less 134Cs than nonmycorrhizal plants (Figure 2). Ectomycorrhization led to a 21 to 27% decrease in Cs uptake in treatments in which the Cs concentration was equal to or higher than the K concentration in the application solution.
The distribution of 134Cs in different plant parts is given in Table 2. Most of the 134Cs was detected in lateral roots (38.3 to 48.3%) and needles (25.9 to 36.0%), and only 5.6 to 19.4% of the total 134Cs in the plants was located in stems and main roots. Although the Cs/K ratio treatments had no effect on the distri-bution of 134Cs in needles of nonmycorrhizal plants, 134Cs in needles of ectomycorrhizal plants increased with increasing Cs/K ratio. The Cs/K ratio treatments had no significant effects
on the distribution of 134Cs in stems and lateral roots (Table 2). Averaged across the Cs/K ratio treatments, ectomycorrhization significantly decreased 134Cs distribution in needles (30 versus 33.6%), significantly increased the amount of 134Cs in stems (10.9 versus 7.7%), and had no effect on the amounts of 134Cs in main roots and lateral roots (14.3 versus 13.9% and 44.8 versus 44.8%, respectively).
Localization of 133Cs
The X-ray net counts revealed that the largest amounts of 133Cs were in fungal hyphae of the ectomycorrhizal mantle (Table 3), whereas less 133Cs was evident in the Hartig net. The amounts of 133Cs in the cortex and stele of lateral roots were similar and independent of fungal inoculation. Cells of main roots and needles contained 5 to 10 times less 133Cs than cells of lateral roots. No differences in the distribution of 133Cs were evident between epidermal and mesophyll cells of needles, or between apoplastic and symplastic cells of the cortex and stele
(Ta-Table 2. Effects of ectomycorrhization and Cs/K ratio on uptake (percent recovered from the application solution) and distribution of recovered 134Cs in total plants and plant parts. Values are means of 10 replicates. For the two-factor analysis of variance: NS = not significant; * = significant
at P ≤ 0.05; ** = significant at P ≤ 0.01; *** = significant at P ≤ 0.001; **** = significant at P ≤ 0.0001.
Cs/K ratio Non-inoculated Ectomycorrhizal Probability
0.1 1 10 100 0.1 1 10 100 Fungus Cs/K
Uptake (%)
Total plant 7.3 10.4 12.5 14.4 7.2 8.1 9.5 11.1 ** ****
Needles 2.5 3.4 4.5 5.0 2.0 2.2 3.0 3.9 ** ****
Stem 0.8 0.9 0.7 1.4 1.1 0.6 1.0 1.4 NS NS
Main roots 1.5 1.4 1.4 1.7 1.1 1.6 0.9 1.6 NS NS
Lateral roots 2.5 4.7 5.9 6.4 3.1 3.8 4.5 4.3 *** ****
Distribution (%)
Needles 33.4 31.7 36.0 33.2 28.2 25.9 31.6 34.5 * *
Stem 8.9 7.1 5.6 9.2 13.5 7.3 10.4 12.4 * NS
Main roots 19.4 12.9 11.4 12.0 14.4 19.1 10.0 13.8 NS *
Lateral roots 38.3 48.3 47.0 45.4 43.9 47.8 48.1 39.3 NS NS
ble 3). The distribution pattern of K was more or less the inverse of that of Cs. Low amounts of K and high amounts of Cs were present in mantle and Hartig net hyphae and in cortical cells of lateral roots. High amounts of K and low amounts of Cs were present in epidermal and mesophyll cells of needles. However, large amounts of both K and Cs were found in the stele of the lateral roots, and low amounts of both elements were measured in all types of cells of main roots (Table 3).
The elemental mapping of Cs in a longitudinal fracture of a frozen-hydrated ectomycorrhiza is shown in Figure 3. The map shows that Cs was distributed relatively homogeneously within the root fracture, but with a tendency for higher net counts in the fungal mantle than in the Hartig net or in the epidermal and cortical cells.
Discussion
Ectomycorrhizal fungi did not increase seedling biomass dur-ing the first two months after inoculation. Similar observations have been made by Brunner and Scheidegger (1992, 1995) who showed that although ectomycorrhizal development oc-curs during the first two months after inoculation, the increase in nutrient uptake that accompanies ectomycorrhization does not result in an increase of plant biomass for at least two months. Uptake of 134Cs by Norway spruce seedlings was significantly affected by the K concentration in the application solution. Lowering the K concentration by a factor of 1000 by increasing the Cs/K ratio from 0.1 to 100 enhanced the uptake of Cs by a factor of two in non-inoculated seedlings and by a factor of 1.5 in ectomycorrhizal seedlings. The mechanism by Table 3. X-Ray net counts of Cs and K in various cell types of freeze-fractured plant parts (lateral roots, main roots, needles) of non-inoculated and ectomycorrhizal plants. Within the lateral roots of the ectomycorrhizal plants, only ectomycorrhizae were considered for analysis. Values are means ± SD of eight non-inoculated and eight ectomycorrhizal samples and of four spectra of each cell type. ND = not detected.
Cell type Lateral roots Main roots Needles
Cs K Cs K Cs K
Non-inoculated
Cortex (Symplasm) 1604 ± 424 573 ± 131 213 ± 102 942 ± 256 -- --Cortex (Apoplasm) 1367 ± 322 555 ± 84 ND 271 ± 107 -- --Stele (Symplasm) 1398 ± 245 2044 ± 483 155 ± 76 612 ± 318 -- --Stele (Apoplasm) 1276 ± 403 1308 ± 478 ND 165 ± 38 --
--Epidermis (Symplasm) -- -- -- -- 211 ± 208 1272 ± 590
Mesophyll (Symplasm) -- -- -- -- 287 ± 144 1953 ± 771
Ectomycorrhizal
Mantle hyphae (Symplasm) 3511 ± 817 670 ± 210 -- -- -- --Hartig net hyphae (Symplasm) 1964 ± 521 903 ± 341 -- -- -- --Cortex (Symplasm) 1327 ± 315 349 ± 127 241 ± 69 879 ± 244 -- --Cortex (Apoplasm) 1547 ± 432 843 ± 236 ND 175 ± 86 -- --Stele (Symplasm) 1033 ± 341 1450 ± 211 128 ± 45 427 ± 187 -- --Stele (Apoplasm) 1270 ± 298 2068 ± 467 ND 211 ± 103 --
--Epidermis (Symplasm) -- -- -- -- 280 ± 212 2381 ± 403
Mesophyll (Symplasm) -- -- -- -- 293 ± 256 2567 ± 831
which plants and fungi discriminate between Cs and K is not known nor is the identity of the uptake mechanism (Shaw and Bell 1994).
Under conditions similar to those used in our study, Hasel-wandter and Berreck (1994) found significantly less 137Cs in shoots of endomycorrhizal Festuca than in nonmycorrhizal controls. Clint and Dighton (1992) measured short-term up-take of 137Cs by Calluna and found that the apparent influx of 137Cs into mycorrhizal plants was less than into nonmycorrhi-zal controls. In a study using the growth pouch system, Riesen and Brunner (unpublished data) measured less 134Cs in ec-tomycorrhizal Picea seedlings under low ammonium condi-tions than under high ammonium condicondi-tions. In contrast to these studies, increased amounts of 137Cs in endomycorrhizal Melilotus and Paspalum were observed by Rogers and Wil-liams (1986) and McGraw et al. (1979), respectively. In addi-tion, Entry et al. (1994) observed a significantly higher uptake of 90Sr in two ectomycorrhizal Pinus species than in non-in-oculated controls. Although it is not known whether mycorrhi-zae generally reduce plant uptake of radionuclides (Haselwandter and Berreck 1994), it is evident that harvest time, fungal and plant species, and the test systems used can all influence radionuclide accumulation in plants (Hasel-wandter and Berreck 1994).
Based on our observation of lower uptake of Cs by ectomy-corrhizal plants compared to non-inoculated controls, we con-clude that Cs is trapped by the hyphae of the fungal mantles and by the extramatrical mycelium. Our finding of high net counts of Cs in the hyphae of the fungal mantles supports the hypothesis that fungi have the potential to accumulate large amounts of radionuclides and heavy metals (Colpaert and Van Assche 1987, Galli et al. 1994, Haselwandter and Berreck 1994, Haselwandter et al. 1994). Furthermore, the potential is higher for ectomycorrhizal fungi than for saprophytic fungi (Römmelt et al. 1990). In the present study, however, hyphae of the extramatrical mycelium, growing throughout the char-coal paper, could not be recovered and measured for their Cs concentration, making the Cs balance impossible. Although the mechanism by which Cs accumulates in filamentous fungi is unknown, binding to cell walls components, polyphosphate granules, or sulfhydryl compounds can probably be excluded because Cs has similar characteristics to those of K (Galli et al. 1994, Haselwandter and Berreck 1994). In fungal fruiting bodies of the ectomycorrhizal species Xerocomus, Cs is bound in large amounts to the pigments of the cap (Aumann et al. 1989).
We used an X-ray microanalysis method to determine the distribution of Cs in frozen-hydrated, freeze-fractured roots and needles of an ectomycorrhizal plant. We were able to demonstrate the localization of stable Cs in various plant tissues and to compare patterns of 133Cs distribution between cells of the cortex and stele, the inner and outer cortex and the apoplasm and symplasm. Analysis of Cs within the root-frac-tures revealed a relatively homogeneous distribution, which is similar to that of Sr (Frey et al. unpublished data) but different from that of La which is mainly localized in apoplasts of cortical cells and Hartig net hyphae, but is absent from sym-plasts (Scheidegger and Brunner 1995). X-Ray microanalysis
showed that the distribution patterns of Cs in the various plant tissues were more or less the inverse of those of K. Thus, high amounts of Cs and low amounts of K were found in the outer parts of roots, whereas high amounts of K and low amounts of Cs were found in the inner parts of roots. Although Cs and K are similar in mobility and uptake, the different distribution patterns indicate a strong preference by the fungus for K uptake over Cs uptake. The mechanisms that regulate the transfer of K and Cs ions between the ectomycorrhizal fungus and its host are unknown (Haselwandter et al. 1994).
There are technical difficulties associated with quantifica-tion of ion concentraquantifica-tions in bulk-frozen hydrated and freeze-fractured plant tissues including the uncertain etching process, the uneven fracture planes, and the lack of available standards (Stelzer et al. 1988, Van Steveninck and Van Steveninck 1991). However, cryosectioning and freeze-drying under controlled conditions in an EM provides a more reliable method (Zierold 1988).
Acknowledgments
This work was supported by the Swiss Federal Nuclear Safety Inspec-torate and the Federal Office of Energy. We thank A. Wolf for technical assistance, C. Scheidegger and D. Rigling for critical reading, and S. Ortloff for correcting the English text.
References
Aumann, D.C., G. Clooth, B. Steffan and W. Steglich. 1989. Kom-plexierung von Caesium-137 durch die Hutfarbstoffe des Maronen-röhrlings (Xerocomus badius). Angew. Chemie 101:495--496. Brunner, I. and C. Scheidegger. 1992. Ontogeny of synthesized Picea
abies (L.) Karst.-Hebeloma crustuliniforme (Bull. ex St Amans) Quél. ectomycorrhizas. New Phytol. 120:359--369.
Brunner, I. and C. Scheidegger. 1995. Effects of high nitrogen concen-trations on ectomycorrhizal structure and plant growth of Picea abies (L.) Karst. seedlings. New Phytol. 129:83--95.
Clint, G.M. and J. Dighton. 1992. Uptake and accumulation of radio-caesium by mycorrhizal and non-mycorrhizal heather plants. New Phytol. 121:555--561.
Colpaert, J.V. and J.A. Van Assche. 1987. Heavy metal tolerance in some ectomycorrhizal fungi. Funct. Ecol. 1:415--421.
Coughtrey, P.J. and M.C. Thorne. 1983. Radionuclide distribution and transport in terrestrial and aquatic ecosystems. A.A. Balkema, Rot-terdam, 496 p.
Dighton, J. and A.D. Horrill. 1988. Radiocaesium accumulation in the mycorrhizal fungus Lactarius rufus and Inocybe longicystis, in upland Britain, following the Chernobyl accident. Trans. Brit. My-col. Soc. 91:335--337.
Entry, J.A., P.T. Rygiewicz and W.H. Emmingham. 1994. 90Sr uptake by Pinus ponderosa and Pinus radiata seedlings inoculated with ectomycorrhizal fungi. Environ. Pollut. 86:201--206.
Galli, U., H. Schüepp and C. Brunold. 1994. Heavy metal binding by mycorrhizal fungi. Physiol. Plant. 92:364--368.
Haselwandter, K. and M. Berreck. 1994. Accumulation of radionu-clides in fungi. In Metal Ions in Fungi. Eds. G. Winkelmann and D. Winge. Marcel Dekker, New York, pp 259--278.
Haselwandter, K., C. Leyval and F.E. Sanders. 1994. Impact of arbus-cular mycorrhizal fungi on plant uptake of heavy metals and ra-dionuclides from soil. In Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Eds. S. Giani-nazzi and H. Schüepp. Birkhäuser, Basel, pp 179--189.
Marx, D.H. and W.C. Bryan. 1975. Growth and ectomycorrhizal development of loblolly pine seedlings in fumigated soil infested with the fungal symbiont Pisolithus tinctorius. For. Sci. 21:245--254.
McGraw, A.C., J.F. Gamble and N.C. Schenk. 1979. Vesicular-arbus-cular mycorrhizal uptake of cesium-134 in two tropical pasture grass species. Phytopathology 69:1038--1041.
Müller, T., G. Guggenheim, M. Düggelin and C. Scheidegger. 1991. Freeze-fracturing for conventional and field emission low-tempera-ture scanning electron microscopy: the scanning cryo unit SCU 020. J. Microsc. 161:73--83.
Myttenaere, C., L. Sombre, Y. Thiry, S. De Brouwer and C. Ronneau. 1993. Cycling of radiocesium in forest ecosystems. J. Radioecol. 1:7--14.
Olsen, R.A., E. Joner and L.R. Bakken. 1990. Soil fungi and the fate of radiocesium in the soil ecosystem. In Transfer of Radionuclides in Natural and Semi-Natural Environments. Eds. G. Desmet, P. Nassimbeni and M. Belli. Elsevier Applied Science, London, pp 657--663.
Riesen, T.K., S. Zimmermann and P. Blaser. 1995. Verteilung von Cäsium-137 in Schweizerischen Waldböden. In Umweltradioak-tivität und Strahlendosen in der Schweiz 1994. Eds. H. Völkle and M. Gobet. Bundesamt für Gesundheitswesen, Bern, pp 214--218. Rogers, R.D. and S.E. Williams. 1986. Vesicular-arbuscular
mycor-rhiza: influence on plant uptake of cesium and cobalt. Soil. Biol. Biochem. 18:371--376.
Römmelt, R., L. Hiersche, G. Schaller and E. Wirth. 1990. Influence of soil fungi (Basidiomycetes) on the migration of Cs134 and 137 and Sr90 in coniferous forest soils. In Transfer of Radionuclides in Natural and Semi-Natural Environments. Eds. G. Desmet, P. Nas-simbeni and M. Belli. Elsevier Applied Science, London, pp 152--160.
Schaller, G., C. Leising, R. Krestel and E. Wirth. 1990. Cäsium- und Kalium-Aufnahme durch Pflanzen aus Böden. Bundesamt für Strahlenschutz, Institut für Strahlenhygiene-Berichte 146:1--26. Scheidegger, C. and I. Brunner. 1993. Freeze-fracturing for low tem-perature scanning electron microscopy of Hartig net in synthesized
Picea abies (L.) Karst.--Hebeloma crustuliniforme (Bull. ex St. Amans) Quél. and --Tricholoma vaccinum (Pers.:Fr.) Kummer ec-tomycorrhizas. New Phytol. 123:123--132.
Scheidegger, C. and I. Brunner. 1995. Electron microscopy of ectomy-corrhiza: Methods, applications and findings. In Mycorrhiza. Eds. A. Varma and B. Hock. Springer, Berlin, pp 205--228.
Shaw, G. and J.N.B. Bell. 1994. Plants and radionuclides. In Plants and the Chemical Elements. Ed. E.F. Farago. VCH Verlagsgesellschaft, Weinheim, pp 179--220.
Stelzer, R. and H. Lehmann. 1993. Recent developments in electron microscopical techniques for studying ion localization in plant cells. Plant Soil 155/156:33--43.
Stelzer, R., J. Kuo and H.W. Koyro. 1988. Substitution of Na+ by K+ in tissues and root vacuoles of barley (Hordeum vulgare L. cv. Aramir). J. Plant. Physiol. 132:671--677.
Van Steveninck, R.F.M. and M.E. Van Steveninck. 1991. Micronana-lysis. In Electron Microscopy of Plant Cells. Eds. J.L. Hall and C. Hawes. Academic Press, London, pp 415--455.