Effects of copper exposure upon nitrogen metabolism in tissue
cultured
Vitis
6
inifera
Nuria Llorens, Lluis Arola, Cinta Blade´, Alberto Mas *
Unitat d’Enologia — Centre de Refere`ncia en Tecnologia dels Aliments (UE-CeRTA),Departament de Bioquı´mica i Biotecnologia,
Facultat d’Enologia de Tarragona,Uni6ersitat Ro6ira i Virgili,Ramo´n y Cajal,70,43005Tarragona,Spain
Received 3 March 2000; received in revised form 18 August 2000; accepted 4 September 2000
Abstract
The present study analyses the effects of copper treatment on nitrogen metabolism in a closed system. Sauvignon grapevines were cultured in agar and exposed to copper levels ranging from 0.07 (control) to 5mg Cu g−1medium. Ammonium, nitrate,
nitrite, individual and total amino acids and protein contents were determined in root, and leaves. Enzyme activities of nitrate and nitrite reductases, glutamine synthetase, glutamate synthase and glutamate dehydrogenase were also determined. Copper exposure produces a dramatic change in nitrogen metabolism, with a reduction of total nitrogen, which reflects the reduction on nitrate and free amino acid contents in both root and leaves. The assimilation of nitrate the main nitrogen source in the medium, requires nitrate reductase, which is reduced to negligible activity as response to copper exposure. Primary nitrogen metabolism is also reduced in leaves, although to a lesser extent than in roots, which may explain the differences between the two organs in response to copper exposure. An alternative system for assimilation of nitrogen through glutamate dehydrogenase in roots is proposed, while higher levels of ammonium and glutamine may fullfil the needs of organic nitrogen in the leaves. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Copper; Nitrate reductase; Nitrite reductase; Glutamate dehydrogenase; Glutamate; Glutamine
www.elsevier.com/locate/plantsci
1. Introduction
Heavy metal pollution in the environment is continuously growing because of increased metal use in mining, industry and agriculture. The use of copper is frequent in Mediterranean agriculture as a fungicide, especially grapevine crops which are treated with copper in the form of copper sulphate or oxychloride. Due to this historic practice, the levels of copper in the soil are high in many grape growing regions reaching from 100 to 1500 mg k−1 [1]. Copper is taken up by the plants
accord-ing to soil pH [2] and may be found in grapes [3]. Although copper is an essential element, large exposures can negatively affect plant growth and productivity. Copper causes a broad range of
dele-terious effects such as inhibition of photosynthesis and pigment synthesis, damage to plasma mem-brane and functional changes and other metabolic disturbances, both in field plants [4 – 6] and in vitro grown plants [7,8].
Nitrogen metabolism is affected by heavy metal exposure, especially nitrogen availability and up-take [9,10], and nitrogen status and metabolism in grapevines strongly affect grape production and quality [11,12]. The effects of copper have been connected to the source of nitrogen, being more noticeable when plants are grown on nitrate [13]. The aim of the present study is to find out the effects of copper exposure on grapevine nitrogen metabolism in a closed system (in vitro explants). Major organic (proteins and amino acids) and inorganic (nitrate and ammonium) nitrogen com-ponent concentrations were measured in leaves and roots. Also the activities of the main
regula-* Corresponding author. Tel.: +34-977-250000; fax: + 34-977-250347.
E-mail address:[email protected] (A. Mas).
tory enzymes of nitrogen assimilation and metabolism were determined.
2. Methods
2.1. Plants
Meristems of Vitis 6inifera (cv. Sauvignon) grapevines were cultured in agar containing modified Murashige and Skoog [14] media and illuminated 14 h daylight (2000 lux) at 25°C in a growth chamber. Copper was added as a copper sulphate solution after 4 weeks of growth when the plants were developed. Concentrations were 0.07 (control, no copper added), 1, 2, 5 and 5 mg
Cu g−1 medium). For each copper concentration,
groups of 24 plantlets were grown for two addi-tional months in the presence of copper. Harvest was always done after 3 months of culture.
2.2. Samples
Roots and leaves from in vitro plantlets were processed after thorough cleaning with MilliQ wa-ter. Roots and leaves were homogenized by grind-ing in a mortar after freezgrind-ing in liquid nitrogen. For enzyme activities determination, samples were homogenized and were concentrated by ultrafiltra-tion (10 kDa porus size) according to Harada’s method [15].
2.3. Copper analyses
The copper concentrations were determined by AAS (Hitachi Z-8200) after wet nitric/ hydrochlo-ric (3:1) acid digestion.
2.4. Nitrogen analyses
Total nitrogen was determined by an elemental analyzer (EA-1108, Fysons Inst.). The nitrate and ammonium contents were quantified using an en-zymatic method (Boehringer Mannheim). Total amino acids were measured by ninhydrin test us-ing glycine as a standard. The protein content was estimated by the Bradford [16] method using bovine serum albumin (BSA) as standard. Individ-ual amino and imino acids were determined by OPA/MCE and FMOC derivatizations respec-tively using the HPLC-Gilson (Villiers le Bel, France) ASTED™ System method.
2.5. Determination of enzyme acti6ities
The activities of nitrate reductase, nitrite reduc-tase, glutamate synthase Ferredoxin dependent, glutamine synthetase and glutamate dehydroge-nase were determined according to Dey et al. [17], and that of glutamate synthase NADH dependent according to Harada et al. [15].
Statistical analysis were done by the Student t-test at significance level set at P50.05.
3. Results and discussion
Plants are affected by copper exposure by re-duction of growth, which is significant at 5 mg Cu
g−1 (Table 1). The levels of copper increase both
in leaves and roots but to higher concentrations in roots suggesting an immobilising mechanism in this organ, as previously reported using the same experimental model [8]. This immobilising mecha-nism, which produces very high copper concentra-tions in roots, may be the reason for a stronger effect on this organ than on others, such as on leaves.
The total content of nitrogen in roots decreases severely while no variation can be seen in leaves. The result on the whole plant is a reduction of the overall nitrogen as reported by Siedlecka [10]. The nitrate concentration in roots decreases in propor-tion to the increase of copper, while no changes are observed in leaves. In fact, inhibition of nitrate uptake induced by copper has been reported in Chlorella [9], where it may be due both to a direct effect on transport and upon and inhibition of synthesis of the ATP, needed for nitrate uptake [18].
Ammonium levels do not show significant changes, except the increase in roots at 2.5 mg Cu
g−1 levels. However, ammonium levels never
reach a concentration which may produce necrosis [11].
Total amino acids are severely affected by cop-per, showing a dramatic decrease both in roots and leaves, yet a recovery of the control levels is seen at 5 mg Cu g−1 in leaves. However the
the main amino acids involved in nitrogen assimi-lation (glutamate and glutamine) in roots, but not in leaves. In leaves, the concentration of glutamate decreases as well, but the levels of glutamine in-crease significantly. Similar results to those of glutamate are observed in other amino acid levels (results not shown), except for asparagine and arginine, with changes similar to those of glu-tamine. As glutamine, asparagine and arginine are the main organic carriers of nitrogen in plants [19], the dramatic decrease in roots may represent the reduction of nitrogen assimilation and the limited uptake of inorganic nitrogen, as well as an in-crease in translocation to leaves, which will secure the minimum levels of organic nitrogen. No changes were observed in protein concentrations as previously reported [8].
Better understanding of nitrogen metabolism can be achieved after analysing the activities of the main enzymes involved (Table 2). Although this analysis in vitro of the enzyme activities does not mean the actual activities in vivo, it reflects the potential and, thus, the capacity to use different sustrates. The two inorganic nitrogen reducing
enzymes, nitrate and nitrite reductases are dramat-ically reduced both in leaves and roots, reaching negligible amounts. Although other metabolic pathways may exist, the regular pathway for nitro-gen assimilation is supposed to start from nitrate, more abundant in soils and culture media, and through the two reductases and the glutamine synthetase-glutamate synthase (GS-GOGAT) cycle to be incorporated into glutamate. So, it must be assumed that the ability of the plant to use nitrate is reduced or even eliminated, although in mutant studies a small fraction of nitrate reductase activ-ity is sufficient for growth. However, an alterna-tive pathway for nitrogen use, i.e. the use of ammonium or the recycling of organic nitrogen should be the main nitrogen source, may also exist. Ammonium is rather abundant, at least in leaves, as well as glutamine, and it can be hypoth-esized that the limited difference in growth ob-served at these copper levels is due to this compensatory mechanism. In leaves, though the activities of the other enzymes are also reduced, the reduction is limited to 30%, which might be enough to maintain the minimum nitrogen
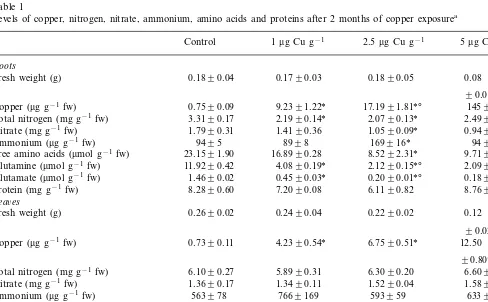
Table 1
Levels of copper, nitrogen, nitrate, ammonium, amino acids and proteins after 2 months of copper exposurea
5mg Cu g−1
2.5mg Cu g−1
1mg Cu g−1
Control
Roots
0.1890.04 0.1790.03 0.1890.05 0.08 Fresh weight (g)
90.01*oc
0.7590.09
Copper (mg g−1 fw) 9.2391.22* 17.1991.81*° 14598*oc
2.4990.26* 2.0790.13*
2.1990.14* 3.3190.17
Total nitrogen (mg g−1fw)
1.7990.31 1.4190.36
Nitrate (mg g−1fw) 1.0590.09* 0.9490.10*
169916* 9498 Ammonium (mg g−1fw) 9495 8998
16.8990.28
23.1591.90 8.5292.31* 9.7191.17* Free amino acids (mmol g−1fw)
4.0890.19*
11.9290.42 2.1290.15*° 2.0990.18*o
Glutamine (mmol g−1fw)
0.1890.01*o
0.2090.01*° 0.4590.03*
Glutamate (mmol g−1fw) 1.4690.02
Protein (mg g−1fw) 8.2890.60 7.2090.08 6.1190.82 8.7691.02
Lea6es
0.2690.02
Fresh weight (g) 0.2490.04 0.2290.02 0.12
90.02*oc
0.7390.11 4.2390.54*
Copper (mg g−1 fw) 6.7590.51* 12.50
90.80*oc
Total nitrogen (mg g−1fw) 6.1090.27 5.8990.31 6.3090.20 6.6090.26
1.3690.17 1.3490.11
Nitrate (mg g−1fw) 1.5290.04 1.5890.11
Ammonium (mg g−1fw) 563978 7669169 593959 6339130 19.7193.34*
29.6591.71 28.7693.91oc
Free amino acids (mmol g−1fw) 18.9190.51*
3.1590.18* 2.7690.02*
2.8090.09* 2.0990.27
Glutamine (mmol g−1fw)
2.2190.28 1.0290.07*
Glutamate (mmol g−1fw) 0.9990.10* 1.3890.10*
Protein (mg g−1fw) 11.8991.11 13.7891.43 12.0091.80 9.8091.04
a* Different from control;odifferent from 1
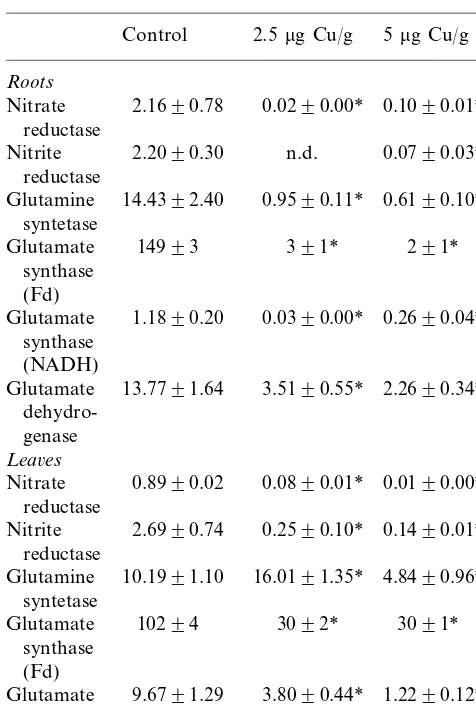
Table 2
Enzyme activities (expressed as nmols min−1 mg protein−1)
of major primary nitrogen enzymes after 2 months of copper exposurea
Glutamine 0.9590.11* 0.6190.10* syntetase
Glutamate 0.0390.00* synthase
(NADH)
2.2690.34* 13.7791.64
Glutamate 3.5190.55*
dehydro-Glutamine 10.1991.10 16.0191.35* 4.8490.96*c
syntetase
a* Different from control;cdifferent from 2.5
mg Cu g−1.
Significance level set atP50.05; n.d.: not detected.
role in keeping the necessary recycling of ammo-nium. NADH-glutamate dehydrogenase in grapes is present in the form of seven isoenzymes that show similar anabolic and catabolic activities [22]. If the direction of the activity depends, as pro-posed, on the levels of ammonium (aminating sense, [23]) or nitrate (deaminating, [24]); in our case, the reduction of nitrate levels and the maintenance of those of ammonium will mark a tendency for the aminating activity, while in con-trol plants the deaminating activity may be dominant.
These results show that nitrogen metabolism is strongly affected by copper exposure in grapevines. The dramatic changes in nitrogen metabolism affect especially the root system, where both the levels of total nitrogen, nitrate, free amino acids (and specially glutamine and glutamic acid) and the activities of most of the primary nitrogen enzymes are heavily reduced. An alterna-tive system for nitrogen assimilation in roots through NADH-glutamate dehydrogenase and glutamine synthetase may be in use to keep the root function. In leaves, although there is also a reduction in most of the parameters studied, the reduction is never as strong as in roots, and spe-cially the concentration of ammonium is normally high enough, and that of glutamine is higher than in the control one which may be the main system to fill the leaves needs for organic nitrogen. How-ever it should be considered that leaves might be more sensitive to the reduction of GS-GOGAT cycle due to their relation to photorespiration.
Acknowledgements
This work was supported by ALI92-0483 and ALI 96-0497 Grants from the Direccio´n General de Investigacio´n Cientı´fica y Te´cnica (DGICYT).
References
[1] L.M. Flores-Velez, J. Ducaroir, A.M. Jaunet, M. Robert, Study of the distribution of copper in an acid sandy vineyard soil by three different methods, Eur. J. Soil Sci. 47 (1996) 523 – 532.
[2] L.A. Brun, J. Maillet, J. Richarte, P. Hermann, J.C. Remy, Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils, Env. Pollut. 102 (1998) 151 – 161.
metabolism to keep the plant alive, although with growth reduction.
[3] V. Angelova, A. Ivanov, D. Bralkov, Heavy metal distri-bution in grapes ofc6sBolgar and Mavrud grown in an industrially polluted region, Wein Wissenchaft Wies-baden 53 (1998) 27 – 31.
[4] T. Lanaras, M. Moustakas, L. Symeonidis, S. Diaman-toglou, S. Karataglis, Plant metal content, growth re-sponses and some phtosynthetic measurement on field-cultivated wheat growing on ore bodies enriched in Cu, Physiol. Plant 88 (1993) 307 – 314.
[5] C.H.R. de Vos, W.M.T. Bookum, R. Vooijs, H. Schat, L.J. De Kok, Effect of copper on fatty acid composition and peroxidation of lipids in the roots of copper tolerant and sensitive Silene cucubalus, Plant Physiol. Biochem. 31 (1993) 151 – 158.
[6] G. Ouzounidou, R. Lannoye, S. Karataglis, Photoacous-tic measurements of photosynthePhotoacous-tic activities in intact leaves under copper stress, Plant Sci. 89 (1993) 221 – 226. [7] P. Gori, S. Schiff, G. Santandrea, A. Bennici, Response of in vitro cultures of Nicotiana tabacumL. to copper stress and selection of plants from Cu-tolerant callus, Plant Cell Tissue Organ Cult. 53 (1998) 161 – 169. [8] A. Romeu, A. Mas, Effects of copper exposure in tissue
cultured Vitis 6inifera, J. Agr. Food Chem. 47 (1999) 2519 – 2522.
[9] P.K. Rai, N. Mallick, L.C. Rai, Physiological and bio-chemical studies on an acid-tolerant Chlorella 6ulgaris under copper stress, J. Gen. Appl. Microbiol. 39 (1993) 529 – 540.
[10] A. Siedlecka, Some aspects of interactions between heavy metals and plant mineral nutrients, Acta Soc. Bot. Poloniae 64 (1995) 265 – 272.
[11] M. Keller, W. Koblet, Stress-induced development of inflorescence necrosis and bunch-stem necrosis in Vitis
6iniferaL. in response to environmental and nutritional effects, Vitis 34 (1995) 145 – 150.
[12] S. Gu, P.B. Lombard, S.F. Price, Effect of shading and nitrogen source on growth, tissue ammonium and nitrate status, and inflorescence necrosis in Pinot noir grapevines, Am. J. Enol Vitic. 47 (1996) 173 – 180. [13] V. Kumar, D.V. Yadav, D.S. Yadaw, Effects of nitrogen
sources and copper levels on yield, nitrogen and copper contents of wheat (Triticum aesti6um L.), Plant Soil 26 (1990) 79 – 83.
[14] T. Murashige, F. Skoog, Revised medium for rapid growth and bioassay with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 473 – 479.
[15] M. Harada, S. Takio, S. Takami, Nitrogen assimilating enzymes in chlorophyllous cells of the liverwort,
Marchantia paleaceavar. diptera, grown in the dark, J. Plant Physiol. 141 (1993) 527 – 532.
[16] M.M. Bradford, A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254. [17] P.M. Dey, J.B. Harbone, Methods in plant biochem-istry, in: P.J. Lee (Ed.), Enzymes of Primary Metabolism, vol. 3, Academic Press, Inc. London, 1990. [18] N.M. Crawford, A.D.M. Glass, Molecular and physio-logical aspects of nitrate uptake by plants, Trends Plant Sci. 3 (1998) 389 – 395.
[19] B.J. Miflin, P.J. Lea, The biochemistry of plants, in: P.K. Stumpf, E.E. Conn (Eds.), Intermediary Nitrogen Metabolism, vol. 16, Academic Press Inc., San Diego, 1990.
[20] D.A. Wilkins, The measurement of tolerance to edaphic factors by means of root growth, New Phytol. 80 (1978) 623 – 633.
[21] I. Arduini, D.L. Godbold, A. Onnis, Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pinaster seedlings, Physiol. Plant 92 (1994) 675 – 680.
[22] K.A. Loulakakis, K.A. Roubelakis-Angelakis, The seven NAD(H)-glutamate dehydrogenase isoenzymes exhibit similar anabolic and catabolic activities, Physiol. Plant. 96 (1996) 29 – 35.
[23] K.A. Roubelakis-Angelakis, W.M. Kliewer, Nitrogen metabolism in grapevine, Hort. Rev. 14 (1992) 408 – 452. [24] M.P. Cordovilla, F. Ligero, C. Lluch, Growth and nitro-gen assimilation in nodules in response to nitrate levels in Vicia faba under salt stress, J. Exp. Bot. 47 (1996) 203 – 210.