T
he sesquiterpene abscisic acid (ABA) was first described in the mid-1960s and, like other phytohormones, appears to influence an array of both physiological and developmental events1,2. However, in contrast to other plant hormones, en-dogenous concentrations of ABA can rise and fall dramatically in response to either environmental or developmental cues. For ex-ample, leaf ABA concentrations can increase 10- to 50-fold within a few hours of the onset of a water deficit1
. Subsequent rewatering will return the concentrations to normal over the same time period. In seeds, after embryonic pattern formation is com-plete, ABA concentrations rise while the embryo establishes dor-mancy and acquires storage reserves3
. This spectrum of changes in ABA concentrations led to attempts to understand ABA action by either applying the compound onto tissues and evaluating its effects or measuring its endogenous level in specific tissues and correlating this with physiological responses4
. These approaches have shown a correlation between ABA and particular processes, but they have not provided any clear mechanistic evidence for the role of ABA. In addition, the interpretation of application experi-ments is influenced by questions of uptake, sequestration and metabolism of the substance applied, and studies aimed at corre-lating hormone levels with function often make assumptions about tissue or cell types based solely on gross anatomical obser-vations that may not reflect subcellular organization4
.
The power of the genetic approach ABA synthesis
Clues are now starting to emerge as to how the ABA signal is transduced in higher plants, mainly through approaches using molecular genetics. ABA is dispensable to plants, and therefore endogenous levels can be genetically altered without causing seri-ous deleteriseri-ous effects under lab conditions. Mutants deficient in ABA biosynthesis in a variety of plant species also lose control of stomatal aperture in leaves and whole plant acclimation to en-vironmental stresses such as drought, salt and cold5
. An allelic series of ABA auxotrophic mutations in Arabidopsis has demonstrated that seed dormancy correlates directly with the amount of ABA synthesized by the embryo6
. Because many of the signaling path-ways that have been studied are dosage dependent, the concen-tration-dependent effect of ABA on embryo dormancy suggests that the ABA specifies dormancy through some form of intracellular signaling.
ABA signal transduction
A working hypothesis is that ABA acts through a standard signal transduction pathway in which the binding of the hormone to a receptor elicits a transduction cascade. According to this hypothe-sis, the identification of specific genes or gene families that are
expressed in response to ABA would probably represent the end point of the signaling pathway. Experiments that focus on ABA-dependent gene expression are mostly designed to identify proteins that respond to the ABA after the signal has been transduced7
. By contrast, genetic analysis has been an invaluable tool in the iden-tification of early ABA signaling components, because genetic screens can be based on the phenotypes of well characterized ABA-biosynthesis mutants. Ideally, one can reasonably expect that mu-tations in genes that disrupt any one of the positively acting components of a signal transduction pathway should have similar phenotypes to those of the biosynthetic auxotroph. In turn, it is possible to distinguish between response and auxotrophic mutants because the former is not rescued by exogenous application of the hormone.
Phenotypic analysis of mutations that alter ABA signal trans-duction should provide some indication as to what part of the pathway has been affected. For example, mutations in an ABA receptor or in a transduction component should result in pleio-tropic phenotypes, whereas mutations in a downstream component might be limited to more specific responses. However, a possible caveat to this logic can be made from work on signaling in fungal and animal systems. In these systems, signaling pathways are highly intersected so that there are as many inputs as there are out-puts8
. Recent genetic screens using transgenic Arabidopsisplants containing reporter gene constructs that respond to ABA, drought and cold suggest that ABA-dependent and ABA-independent path-ways interact and converge to activate genes involved in stress responses9
. Therefore, although traditional ABA-response mutant screens were designed to isolate defects in specific ABA pro-cesses, the degree of pleiotropy of phenotypes seen in some of these mutants might reflect communication between signaling pathways. If this is true, the lack of pathway specificity puts a greater burden on geneticists to establish whether an ABA-response mutant will provide insights into the process being investigated.
The specificity of ABA signal transduction
Mutational analysis of ABA signaling has been confined to a few species in which mutants can be easily identified10
. In Arabidopsis, genetic screens to distinguish genes involved in ABA responses usually entail identifying seeds that germinate on exogenous con-centrations of ABA that normally inhibit wild-type germination. The popularity of screening for defective ABA responses at the level of seed germination reflects the relative ease with which screens and selections can be performed on sufficient numbers of mutagenized individuals. To date, five distinct mutations, desig-nated abi(‘ABA insensitive’), have been identified11,12
. The abi1 and abi2mutations are semi-dominant and appear to alter many vegetative and seed ABA-regulated functions. Seeds defective at
Genetic analysis of ABA signal
transduction pathways
Dario Bonetta and Peter McCourt
these loci show reduced dormancy and mature plants wilt exces-sively under mild water stress because of a loss of stomatal regu-lation. The other abimutations are recessive and their phenotypes appear to be restricted to late seed development. Recently, a sec-ond class of mutations that causes seeds to show an enhanced response to ABA (era) at the level of seed germination has also been identified13
. The eramutations are recessive, indicating that the genes involved might encode negative regulators of ABA action. Although molecular analysis of many of these ABA mu-tants is incomplete, their phenotypes imply that there are at least two partially redundant pathways for ABA signaling: one specific for seed development and the other for both seed and vegetative responses.
Is ABI3 insensitive or just indifferent?
The genetic studies with abi and era mutations are compelling because some of the response-mutant phenotypes overlap those of an ABA-auxotrophic mutant. As expected, the majority of abi
mutants have varying degrees of diminished dormancy, whereas
era1 mutants have extended dormancy. However, full under-standing of a complex process such as seed development requires analysis of many mutant alleles at a given locus, so that relation-ships between gene activity and the resulting phenotypes can be established. Developmental phenotypes resulting from different alleles of the same locus often vary widely depending on the severity of the allele in question. For example, aside from reduced dormancy and seed ABA sensitivity, early phenotypic comparison showed little difference between a weak allele ofabi3 (abi3-1) and a wild-type allele at the level of seed development11
. The lim-ited phenotypes of abi3-1 mutants suggest that the ABI3 gene product is restricted to seed ABA signaling. However, null alleles of abi3result in highly non-dormant, underdeveloped seeds that are desiccation intolerant14,15
. Processes such as the breakdown of chlorophyll, accumulation of storage reserves and premature acti-vation of the germination program, all processes not normally observed in ABA auxotrophs or Abi1and abi2mutants, suggest that ABI3 might have functions broader than just ABA signal
transduction. It is interesting that severe alleles of abi3 share many phenotypic similarities with the viviparous(vp1) mutation of maize and that molecular characterization of these two genes has shown that they encode evolutionarily conserved seed-specific transcriptional activators16
. Thus, the role of ABI3 and VP1 in seed development appears to be conserved between monocot and dicot species.
Evidence that ABI3specifies seed developmental programs is that its ectopic expression causes seed-specific mRNA transcript accumulation in leaves in response to ABA (Ref. 17). However, these ABI3-dependent expression patterns require a functional ABI1 protein, because they are lost in an abi1 mutant back-ground18
. These results indicate that ABI3 is sufficient to change ABA responsiveness in vegetative tissues and that ABI1 is re-quired for these ABI3-regulated processes. However, clear as-signment of genetic relationships based on ectopic expression studies can be difficult because the situation is artificial. For ex-ample, loss of stomatal control in abi1mutants is suppressed by Fig. 1.Genetic model of interactions of ABI3 with ABI1, FUS3
and LEC1. ABI3 might be a shared component of developmental and ABA signaling pathways. FUS3 and LEC1 regulate ABI3 protein accumulation, suggesting that a developmental or environ-mental signal is tranduced via LEC1 and FUS3 to mediate ABI3-regulated seed maturation. At the same time, a developmental or environmental signal can also influence ABI3 indirectly by affect-ing endogenous ABA concentration. Because ABI3 ectopic expression can suppressabi1mutant phenotypes, the transduction of this ABA signal would be through an ABI1-mediated pathway. It is also possible that ABI3 can directly regulate physiological responses (broken arrow), because ABI3 can suppress ABI1-mediated stomatal closure.
ABA Developmental or environmental signal
FUS3 and LEC1
ABI3
Seed maturation ABI1
Physiological responses
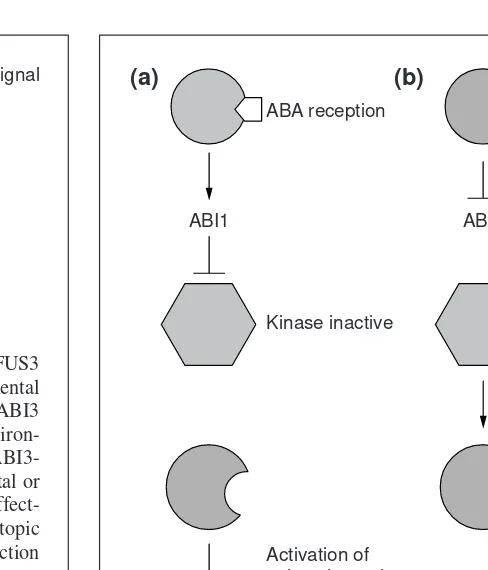
Fig. 2.Speculative model of the involvement of ABI1 in slow anion channel regulation in guard cells. By performing patch-clamp experiments on Arabidopsisguard cells, it is possible to show that ABA is involved in the control of slow anion channels and that this regulation is mediated by the ABI1 protein phos-phatase. The inability of Abi1mutants to close their stomata in the presence of ABA is partially rescued by a kinase inhibitor, which suggests that a protein kinase works downstream of the ABI1 phosphatase. (a) In the presence of ABA, the ABI1 wild-type pro-tein either directly or indirectly inactivates the kinase so that it cannot catalyze the phosphorylation of a downstream element that could be the channel itself or some protein needed for channel inactivation. Alternatively, the ABI1 phosphatase could directly dephosphorylate the kinase substrate. Either of these scenarios will result in anion-channel activation and stomatal closure. (b) In the absence of ABA, ABI1 is inactive and can no longer inhibit the kinase. The kinase phosphorylates the downstream compo-nent, which results in inactivation of the channel.
(a)
ABA reception
Kinase inactive Kinase active
Activation of anion channel
Stomata closed
Stomata open ABI1
(b)
ABI1
ectopic expression of ABI3 (Ref. 18). If ABI3 is a transcription factor, this result is surprising because guard cell closure in response to the application of ABA is too rapid to involve gene expression. Poss-ibly, these studies and the expansive role of ABI3 beyond ABA-established seed dormancy might mean that this gene is actually a global regulator of cell fate rather than an ABA signaling molecule. If ABI3 has an ‘instructive’ role, the re-sponding cells should develop along one (embryonic) pathway in the presence of the ABI3 signal, and along another (veg-etative) pathway in its absence. Alterna-tively, if ABI3 is merely ‘permissive’, then the cells would be dependent on an ABI3 signal to complete their matur-ation. For example, if embryonic cells were more sensitive to ABA than other cells in the plant, conversion of cells to-wards a more embryonic fate might make them more sensitive to ABA. Ectopic expression of ABI3 suggests that it has an instructive role, because the domain of embryo-specific gene expression is ex-panded. If ABI3 is instructive then it would not be directly involved in ABA sig-nal transduction but would ‘select’ cells to become sensitive to the hormone. Thus, loss of ABI3 function results in cells that are unable to respond to ABA appropriately.
Another possibility is that ABI3 is a shared component in both ABA signal transduction and seed maturation, thereby allowing coordinate regulation of ABA-dependent and ABA-independent processes (Fig. 1). A coordinate model of ABI3 action is sup-ported by the demonstration that maize VP1participates in both the activation of seed maturation-specific programs and the re-pression of germinative programs19
. Thus, mutually exclusive de-velopmental programs in maize seed development would be regulated by the ABI3homolog. Alternatively, if ABI3 is directly involved in ABA signaling, its molecular identity and seed speci-ficity imply that it is just one of the possible outputs of a branched pathway. Support for this latter possibility comes from recent experiments in which tobacco seed ABA concentrations were dramatically reduced by the transgenic expression of an ABA-specific antibody that sequesters free ABA (Ref. 20). These seeds were phenotypically similar to severe abi3null mutants, suggest-ing that the available ABA auxotrophic mutations in Arabidopsis
are still leaky enough to allow seed maturation to proceed un-hindered. However, ultrastructural analysis of immunomodulated transgenic seed showed alteration in cotyledon development that was more reminiscent offus3and lec1mutants of Arabidopsis21–24
. The FUS3and LEC1genes encode proteins that are important in specifying cotyledon identity, since loss of these functions re-sults in ‘leaf-like’ development of the cotyledons. Originally, these genes were not considered to have a direct role in ABA signal transduction, because the fus3and lec1mutant seeds retained nor-mal sensitivity to exogenously applied ABA (Refs 21 and 22). Recent molecular studies, however, indicate that different combi-nations ofabi3, fus3and lec1alleles can impact on seed ABA sen-sitivity, with FUS3 and LEC1 regulating the abundance of ABI3 protein25
. The action of ABI3 might be modulated by both ABA and developmental regulators such as FUS3 and LEC1. Because
FUS3 and LEC1 are essential components of cotyledon identity, their interaction with ABI3, an ABA-associated late-embryogenesis regulator, may allow the coordinate control of ABA-related seed functions with morphological development.
To phosphorylate or not to phosphorylate?
Although interpretation of the role of ABI3 in ABA signaling is unclear, the molecular characterization of ABI1 and ABI2 sug-gests that protein phosphorylation and dephosphorylation are involved in ABA action26–28
. Both genes encode homologous pro-tein type 2C phosphatases, suggesting that their functions are par-tially redundant. This overlapping functionality has been suggested to explain why only dominant mutations in these two genes can be identified in ABA-insensitive genetic screens. Moreover, dephos-phorylation assays of abi1 mutant protein show decreased enzyme activity, suggesting that this mutation might act in a dominant negative manner29
. In this case, the abi1 mutant protein would interfere with a signaling complex of proteins that might include the ABI2 protein. However, loss-of-function alleles do not exist for ABI1 and ABI2, making modeling the function of these pro-teins in ABA signaling difficult.
Nevertheless, the recent achievements of patch clamping Arabid-opsisguard cells have opened up the possibility of applying the technology of electrophysiology directly to mutant cells altered in their ABA response30
. These studies indicate that both ABI1 and ABI2 function in stomata by facilitating the ABA-induced acti-vation of both slow anion channels and stomatal closing. Further-more, protein kinase inhibitors can partially rescue the defects in ABA signaling of abi1but not of abi2. The simplest interpretation of these results is that a protein kinase acts as a negative regulator of stomatal closing and that ABI1 down-regulates this kinase by acting either on the kinase itself or on the kinase substrate (Fig. 2).
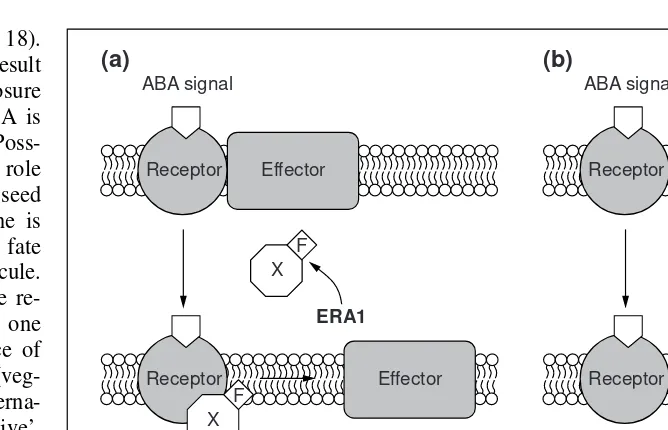
Fig. 3.Possible role for farnesylation in ABA-mediated signaling. Mutations in the ERA1
gene of Arabidopsisresult in loss of farnesylation activity and increased ABA sensitivity in the seed. Loss-of-function mutations result in increased sensitivity, suggesting that a negative regulator of ABA signal transduction needs to be farnesylated to function. (a) In the wild-type, after perception of the ABA signal by a hypothetical receptor, the signal is transduced via a membrane-bound effector down the response pathway. At some point after perception of the ABA signal, a farnesylated protein (X) inserts itself into the membrane, interfering with effector function. This results in an attenuation of the ABA response. (b) In the era1
mutant, X is not farnesylated and therefore does not localize to the membrane. The lack of interference of effector function prevents attenuation of the ABA response.
(a)
ABA signal
Receptor Effector
Receptor
Attenuation of ABA response
Effector X
ERA1
F
X F
(b)
ABA signal
Receptor Effector
Receptor
No attenuation of ABA response
Effector X
X
The abi2-mutant guard cells are not rescued by kinase inhibitors; and inhibitors alone are insufficient to produce anion-channel activation and stomatal closure. These two results suggest that a second pathway might be involved in ABA-mediated stomatal closure in Arabidopsis. However, the substrates for neither ABI1 nor ABI2 are known and consequently they could have very dif-ferent roles in ABA signaling.
Putting on the brakes
Aside from the phosphorylation status of the cell, the recent mol-ecular characterization of one of the eraloci (era1) indicates that protein farnesylation could be another modulator of ABA signal-ing in higher plants13
. The ERA1gene encodes the b-subunit of a heterodimeric farnesyl transferase, an enzyme that catalyzes the attachment of a 15-carbon farnesyl lipid to specific proteins. Al-though little is known about the biological function of this activ-ity in plants, this covalent lipid modification plays a critical role in the subcellular localization and function of eukaryotic signaling molecules such as trimeric G-proteins and small GTP-binding proteins of the RAS superfamily in yeast and mammalian cells31
. If the molecular function of farnesylation is analogous in plants, then the increased sensitivity to ABA observed in the era1 farne-sylation-deficient mutants suggests that a negative regulator of ABA signaling might have to be membrane-localized to function (Fig. 3). However, as with the interpretation of ABI3 function, ERA1 might modify proteins that are only peripherally associated with ABA signaling. The ABA and dormancy defects of era1 mu-tants could be a reflection of a developmental program that only loosely cross-connects with ABA-induced responses that rein-force the same processes. Hopefully, once the targets of ERA1 are identified, differentiation of these models can be resolved.
It is possible that a greater number of checks and balances exist in the seed because the commitment to germinate, unlike many developmental decisions in higher plants, cannot be reversed or reiterated. Thus, the role of ABA in the establishment of seed dor-mancy might be buffered by other developmental pathways. Ostensibly, all the proteins identified by ABA sensitivity mutant screens are potential candidates as signaling components. Based on the molecular identity of these proteins it is likely that either a phosphatase or a farnesyltransferase would be located further upstream in a signaling pathway than would a transcription factor.
Back to the future: more genetic screens
Many genes in signaling pathways can function as components of a binary switch. The state of this switch is defined mutationally by either the dominant, gain-of-function allele, which fixes the switch to a high level or constitutively active state, or alternatively by the null, loss-of-function allele, which fixes the switch to a low level or inactive state32
. In perceiving a signal, a gene could either positively or negatively regulate another; thus, by defining the alternative states of the whole pathway, it is possible to divide genes into either positive or negative regulators of that pathway. Because of the lack of appropriate mutations, we do not yet have a clear understanding of how the ABA signal is transmitted or perceived. Although traditional genetic techniques identify the loss-of-function mutation of a gene with relative ease, the gain-of-function mutation can be more difficult to obtain. The value of gain-of-function mutations is that they can uncover the function of non-essential or functionally redundant genes that would be missed because they have no loss-of-function phenotype. Molecu-lar techniques have made it possible to simulate these mutations by overexpression of cloned genes, creating gain-of-function sce-narios to juxtapose the loss-of-function situations. However, as with ectopic ABI3 expression, the interpretation of misexpression
mutant phenotypes requires careful consideration, because tissue specificity is lost and misexpression is normally constitutive over the life of the plant.
Expanding the current collection of mutants by traditional gen-etic screens will definitely help to increase our knowledge of ABA signaling. Moreover, since mutants already exist, it should be poss-ible to design genetic screens to address the problems of pleiotropy. One method that is widely used to identify new loci in a signaling pathway is a suppressor or enhancer screen33
. Either suppressor or enhancer mutations can identify genes located downstream of a known gene. This approach is based on the premise that such sec-ond-site mutations can cause imbalances in the flux of a pathway, either by reducing it or by exaggerating it, and as a consequence have a detectable phenotype. In the case of a phenotypically com-plex mutation such as abi3, specific phenotypes can be targeted for suppression or enhancement: for example, dormancy versus desiccation intolerance or ABA insensitivity. The success of such screens is, however, largely dependent on the initial mutant allele. There is no guarantee that mutations will be limited to the same signaling pathway or to parallel redundant pathways.
Apart from for the isolation of mutations that affect gene inter-actions, mutations identified by screens that do not utilize ABA directly have been mostly set aside. This is because of the likeli-hood that these screens do not identify mutations that directly affect ABA signaling. However, this standard is based on the as-sumption that ABA is an instructive signal. In many physiological experiments, ABA seems to act as a relay between the environ-ment and the plant2
. If this is true, then there would be no need to in-vent specific pathways for ABA signaling. The alternative is that ABA acts in a more permissive way to modulate multiple pathways, thereby either amplifying or dampening the strength of the signal in some essential developmental or physiological pathway. This may explain why ABA and perhaps other plant hormones seem to have a role in apparently distinct processes. Based on what is cur-rently known about ABA signaling, however, it is difficult to dis-tinguish between the two possibilities. For instance, the genes that have so far been cloned through ABA screens all encode proteins that are modulators of signal transduction pathways rather than the core components of a typical signaling pathway (e.g. a receptor).
Conclusions
Genetic analysis has limitations: some genes can act at more than one stage in development and growth, or can function in more than one cell or tissue type, making phenotypic analysis difficult. For example, the recent identification of a cyclic ADP-ribose in ABA signaling through microinjection experiments might not have been found through a traditional genetic screen34
. It is also possible that certain steps in the ABA pathway are genetically redundant, as with the ethylene receptor in Arabidopsis35
. Reces-sive, loss-of-function mutations in such genes will have no ob-vious phenotype. However, as hormone screens become more sophisticated, these problems will become surmountable. For example, suppression of eramutants that are already sensitized to ABA might uncover mutations that would normally only give weak insensitivity phenotypes. Although our understanding of ABA signaling in higher plants is still rudimentary, the collection of abiand eramutations that presently exist in Arabidopsiscan now serve as a foundation for many new genetic screens. Deci-phering the ABA signal transduction map is still a formidable challenge, but for plant geneticists the route is clear.
Acknowledgements
References
01Zeevaart, J.A.D. and Creelman, R.A. (1988) Metabolism and physiology of abscisic acid, Annu. Rev. Plant Physiol. Plant Mol. Biol.39, 439–473 02Chandler, P.M. and Robertson, M. (1994) Gene expression regulated by
abscisic acid and its relationship to stress tolerance, Annu. Rev. Plant Physiol. Plant Mol. Biol.45, 113–141
03Rock, C.D. and Quatrano, R.S. (1995) The role of hormones during seed development, inPlant Hormones: Physiology, Biochemistry and Molecular Biology (Davies, P.J., ed.), pp. 671–697, Kluwer
04Trewavas, A. (1981) How do plant growth substances act? Plant Cell Environ. 4, 203–228
05Shinozaki, K. and Yamaguchi-Shinozaki, K. (1996) Molecular responses to drought and cold stress, Curr. Opin. Biotech. 7, 161–167
06Karssen, C.M. et al. (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of
Arabidopsis thaliana(L.) Heynh, Planta 157, 158–165
07Yamaguchi-Shinozaki, K. and Shinozaki, K. (1997) Gene expression and signal transduction in water-stress response,Plant Physiol. 115, 327–334
08Broach, J.R. and Levine, A.J. (1997) Oncogenes and cell proliferation,Curr. Opin. Genet. Dev. 7, 1–6
09Ishitani, M. et al. (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid dependent and abscisic acid independent pathways, Plant Cell9, 1935–1949
10 Reid, J.B. and Howell, S.H. (1995) The function of hormones in plant growth and development, inPlant Hormones: Physiology, Biochemistry and Molecular Biology(Davies, P.J., ed.), pp. 671–697, Kluwer
11 Koornneef, M., Reuling, G. and Karssen, C.M. (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana,
Physiol. Plant.61, 377–383
12 Finkelstein, R.R. (1994) Mutations at two new ArabidopsisABA response loci are similar to the abi3mutations, Plant J.5, 765–771
13 Cutler, S. et al. (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis, Science273, 1239–1241
14 Ooms, J.J.J. et al. (1993) Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. A comparative study using abscisic acid-insensitive abi3mutants, Plant Physiol. 102, 1185–1191
15 Nambara, E. et al. (1995) A regulatory role for the ABI3gene in the establishment of embryo maturation in Arabidopsis thaliana, Development
121, 629–636
16 Giraudat, J. et al. (1992) Isolation of the Arabidopsis ABI3gene by positional cloning, Plant Cell4, 1251–1261
17 Parcy, F. et al. (1994) Regulation of gene expression programs during
Arabidopsisseed development: roles of the ABI3locus and of endogenous abscisic acid, Plant Cell6, 1567–1582
18 Parcy, F. and Giraudat, J. (1997) Interactions between the ABI1and the ectopically expressed ABI3genes in controlling abscisic acid responses in
Arabidopsisvegetative tissues, Plant J. 11, 693–702
19 Hoecker, U., Vasil, I.K. and McCarty, D.R. (1995) Integrated control of seed maturation and germination programs by activator and repressor functions of
Viviparous-1of maize, Genes Dev.9, 2459–2469
20 Phillips, J. et al. (1997) Seed-specific immunomodulation of abscisic acid activity induces a developmental switch, EMBO J. 16, 4489–4496
21 Meinke, D.W. (1992) A homeotic mutant of Arabidopsis thalianawith leafy cotyledons,Science258, 1647–1650
22 Keith, K. et al.(1994) fusca3: a heterochronic mutation affecting late embryo development in Arabidopsis, Plant Cell6, 589–600
23 Baumlein, H. et al. (1994) The FUS3gene of Arabidopsisis a regulator of gene expression during late embryogenesis, Plant J. 6, 379–387
24 West, M.A.L.et al. (1994) LEAFY COTYLEDON1is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis, Plant Cell6, 1731–1745
25 Parcy, F. et al. (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and
LEAFY COTYLEDON1loci act in concert to control multiple aspects of
Arabidopsisseed development,Plant Cell9, 1265–1277
26 Leung, J. et al. (1994) ArabidopsisABA-response gene ABI1: features of a calcium-modulated protein phosphatase, Science264, 1448–1452
27 Meyer, K., Leube, M.P. and Grill, E. (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana, Science264, 1452–1455
28 Leung, J., Merlot, S. and Giraudat, J. (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE(ABI2) and ABI1genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction, Plant Cell9, 759–771
29 Bertauche, N., Leung, J. and Giraudat, J. (1996) Protein phosphatase activity of abscisic acid insensitive 1(ABI1) protein from Arabidopsis thaliana,Eur. J. Biochem. 247, 193–200
30 Pei, Z-M. et al. (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsiswild-type and abi1and abi2mutants, Plant Cell.9, 409–423
31 Schafer, W.R. and Rine, J. (1992) Protein prenylation: genes, enzymes, targets and functions, Annu. Rev. Genet.30, 209–237
32 Sternberg, P.W. and Horvitz, R.H. (1984) The genetic control of cell lineage during nematode development, Annu. Rev. Genet.18, 489–524
33 Karim, F.D. et al.(1996) A screen for genes that function downstream of Ras1 during Drosophilaeye development, Genetics143, 315–329
34 Wu, Y. et al. (1997) Abscisic acid signaling through cyclic ADP-ribose in plants, Science 278, 2126–2130
35 Keiber, J.J. (1997) The ethylene response pathway in Arabidopsis, Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 277–298
Dario Bonetta and Peter McCourt*are at the Dept of Botany, University of Toronto, 25 Willcocks St,
Toronto, Canada M5S 3B2
*Author for correspondence
(tel +1 416 978 0523; fax +1 416 978 5878; e-mail [email protected]).
Forthcoming meetings
Are you organizing a meeting or workshop that you think should be included as a calendar entry in Trends in Plant Science ?