The journal homepage www.jpacr.ub.ac.id p-ISSN : 2302 – 4690 | e-ISSN : 2541 – 0733
Naturally Abundance Vanillin as Starting Material to
Synthesizing
4-(4-Hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1
H
)-one
Masruri MASRURI,* and Yuga Adi Pranata
Chemistry Department, Faculty of Mathematics and Natural Sciences, Brawijaya University, Jl. Veteran 65145 Malang, Indonesia
*
Corresponding author Tel./Fax.: +62 341 575878 / +62 341 554403, email : [email protected]
Received 5 May 2015; Revised 22 December 2015; Accepted 22 December 2015
ABSTRACT
Indonesia is the second biggest producer of natural vanillin. Traditionally it was isolated from the bean of vanilla (Vanilla planifolia Andrews). This paper reports on applying vanillin as starting material for synthesizing a biologically important chemical structure 3,4-dihydropyrimidinone. The reaction was undertaken in one step following multi component reaction (MCR). Products determination was undergone using FTIR and UV-Vis spectrophotometry, and also liquid chromatography-mass spectrometry (LCMS). After purification under flash column chromatography in ethyl acetate-hexane, it was found a white solid of 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one in 67% yield with a few amount of an unreacted vanillin.
Key word: Vanilla planifonia, multi component reaction, dihydropyrimidinone
INTRODUCTION
Vanillin is an aldehyde compound, also known as 4-hydroxy-3-methoxybenzaldehyde. It was isolated naturally from the beans of vanilla plant (Vanilla planifonia A.) [1,2]. Indonesia has been becoming the second largest producer of natural vanillin after Madagascar [3,4]. The total domestic production was mostly sent to overseas mainly for US market. It was also reported about 37 metric ton per month of US vanillin import from Indonesia. This total import reached to 195 MT during January-November 2008 [5]. According to the Directorate General of Plantation, Department of Agriculture Indonesia, total area of vanilla plantation was 25,429 hectares until 2008 [6], and this has been growing widely until recent decades. However this increasing total production did not contribute significantly for improving a domestic income [7]. Thus diversifying the potency and application rather than direct export as raw material of the local natural vanillin to gain more values and economic benefits become an important strategy.
under reflux stirring (Figure 1) [10]. Some examples of molecules have been prepared including evaluation of their biological activity such as antifungal, antibacterial [11,12,13,14,15,16,17,18], antiinflammation [11], and antitumor [18] have been reported recently. This paper discloses a recent application of vanillin, isolated from Indonesian
natural source of Vanilla planifoli Andrews as starting material for synthesis
dihydropyrimidinone alkaloid.
Figure 1. Schematic of Biginelli reaction
EXPERIMENT
Chemicals and instrumentation
Chemicals for this research were used as received from the manufacturer or as mentioned. Vanillin gave from local producer (98% purity), acetone (Merck), urea (Gresik Petrochemical Ltd.), magnesium sulfate anhydrate (Merck), ethyl acetate (Smart Lab, re-distillated), n-hexane (Smart Lab, re-re-distillated), ethanol (Smart Lab), pre-coated of TLC silica gel F254 (Merck), silica gel 60 (Merck).
Instrumentation operated for analysis such as gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010S), infrared spectrophotometry (Shimadzu Shimadzu FTIR-8400S), UV-Vis Spectrophotometry (UV-Vis Shimadzu 1601), LCMS/MS (LC conditions; column xxxx, solvent 1.0% formic acid in methanol isocratic, pump pressure 10.0 bar using Accela 1250 pump type. While MS using TSQ MS with 9.0 min total run time, scan event 1, +c SIM Q1MS), analytical balance (Mettler Toledo).
Synthesis procedure
A dried of a 100 mL round-bottom flask was added vanillin (304.29 mg; 2.00 mmol), urea (120.11 g, 2.00 mmol), and acetone (2.00 mL, 27.24 mmol). A drop of glacial acetic acid was added. This mixture was stirred at 60 oC until reaction complete by monitoring in TLC. The product mixture was extracted with ethyl acetate, and dried under magnesium sulfate anhydrate. Then, the product was concentrated using rotary evaporator in vacuum. The crude product was further purified using flash column chromatography with silica as stationary phase and n-hexane/ethyl acetate as solvent. After TLC monitoring, the product fraction was added magnesium sulfate anhydrate and concentrated in vacuum to afford a pure white solid of 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H )-one in 67% yield. Analysis using UV-Vis spectrophotometer found max in 214.60, 232.40,
284.20, 308.20, and 350.40 nm (solution in ethanol). FTIR (KBr plate, cm-1) 3435.31, 3358.77, 3218.97, 1670.30, 1623.36; The GCMS analysis using Agilent developed method on Agilent manual for analysis alkaloid (injector temperature 200 oC, column temperature 210 oC isoterm for 30 min) did not give both of chromatogram and mass spectra data. LCMS/MS (Low resolution) found a single peak at 4.55 min with molecular weight 235.00 (100% intensity). Theoretical calculation for C12H14N2O3 is 234.10. 1H-NMR (400 MHz,
MHz, methanol-d) 17.5; 56.0; 56.1; 101.1; 112.4; 115.4; 118.4; 134.2; 136.9; 146.7; 147.3; 150.2.
RESULT AND DISCUSSION
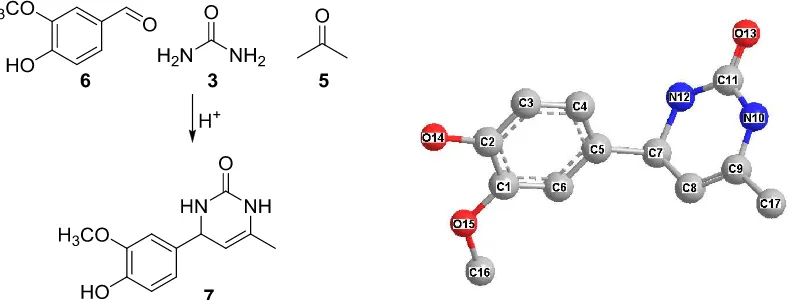
Synthesis of 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one,
7
The reaction was started by mixing of vanillin, urea and acetone in equivalent molar ratio (Figure 2). This mixture was not completely dissolved, and a few drop of ethanol was added. The reaction was maintained under reflux condition. Monitoring the reaction product was performed using spotting an aliquot sample on TLC plate. Appearing of new spot was an indication the reaction occur. First experiment was accomplished in 8 h. After the reaction was stopped and concentrated in vacuum, direct separation on flash chromatography afforded product in low yield. The un-reacted vanillin was isolated in significant amount as white solid (Table 1). Second experiment was performed by increasing of acetone quantity. It replaced the usage of ethanol as in previous reaction. It was not only homogenized the reaction mixture but also accelerate the reaction time to a half. Beside that, the reaction temperature was also much lower.
Figure 2. Schematic reaction to yield 7 (left) and ball-stick model structure (right, hydrogen atom was not shown for clarity).
Table 1. Reaction condition to result product 7
Experiment Reagent Solvent Temperature Time
1 Vanillin (2.0 mmol), urea (1.0
equiv.), acetone (1.0 equiv.), acetic acid
ethanol 70 oC 8 h
2* Vanillin (2.0 mmol), urea (1.0
equiv.), acetone (13.6 equiv.), acetic acid
no solvent 55 oC 4 h
Note: *Heating in water batch
The schematic reaction is displayed as in Figure 2 (left). An equivalent amount of vanillin 6 and urea 3 reacts with acetone 5. The resulted product is dihydropyrimidinone
structure 7, and was identified as
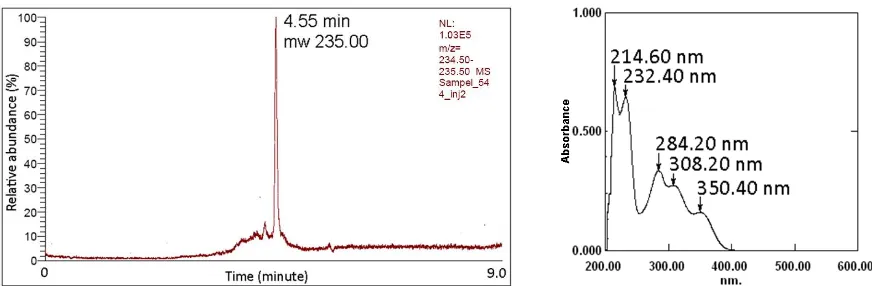
Figure 3. LCMS chromatogram and molecular weight of 7 (left), and its UV spectra (right)
Figure 4. FTIR spectra of product 7
Analysis the molecular structure of 7 was performed using several spectroscopy techniques. Analysis using ultra violet spectrophotometer gave UV absorption spectra with
five a maximum wavelength (Figure 3). These spectra characteristic for
3,4-dihydropyrimidinone structure with two N-H amines adjacent to carbonyl groups and an aromatics ring. In addition, infrared spectra recorded significant signal for all the functional groups comprises in the product (Figure 4). Stretching vibration for amine groups appear as double band in 3435.31 and 3358.77 cm-1. Next to it is absorption band for =C-H aromatic or double bond and detected at 3218.97 cm-1. The absorption band for stretching vibration of carbonyl amide group was recorded in 1670.30 and 1623.36 cm-1, and also peak 1456.16 cm-1 for methyl group vibration. Furthermore, analysis of the mass spectra using LCMS/MS instrumentation gave a single and clear chromatogram peak at 4.55 min. This peak has molecular weight 235.00 that correspond to molecular mass of the ion from protonated molecule, [M+H]+. Molecular formula of product 7 is C12H14N2O3 and has theoretical mass
234.10 atomic unit. This result proved the presence of the isolated product 7 as 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one.
Proposed reaction mechanism
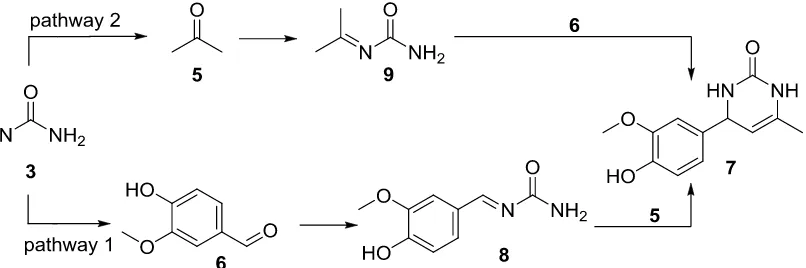
formation. In the mean time, the other side of N-urea attaches to carbonyl group from ethyl-acetoacetate and provides an intermediate structure, called as ureide, which is then constructs a cyclic form and further displace a hydrate producing a dihydropyrimidinone [19]. Recently, Alvim et al. also reported solventless mechanism of the Biginelli reaction [20]. Mechanism was favored following iminium formation as key important step. This adduct is clearly also discovered on MS kinetic study. For this course, iminium intermediate could be afforded by two possible pathways (Figure 5).
Figure 5. Proposed two possible reaction pathways
First pathway provides the iminium from urea 3 with vanillin 6, and intermediate iminium 8 was afforded. This intermediate theoretically is stabilized by aromatic ring as well as carbamate groups on urea. And the product 7 was easily be formed. On the other side, route 2 was initiated with smaller substrate but less reactive than vanillin. The adjacents -hydrogen on iminium intermediate 9 stabilize its structure by enamine formation. This route ends up provides dihydropyrimidinone 7. However, stabilization of aromatic ring on iminium
9 was predicted more intent to be favored.
CONCLUSION
An pyrimidinone alkaloid has been synthesized as 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one using naturally abundant vanillin as a yellowish solid in 67% yield.
ACKNOWLEDGMENT
Authors thanks to Mr. Kaliawan (State Polytechnique of Malang) for performing LCMS/MS analysis. All authors have an equal contribution; MSR writing and evaluating the data meanwhile YAP collecting data.
REFERENCES
[1] Javier De La Cruz Medina, Guadalupe C. Rodriguez Jimenes, Hugo S. Garcia, Thelma Lucia Rosado Zarrabal, Miguel Angel Garcia Alvarado, and Victor Jose Robles Olvera, 2009, Vanilla: Post-harvested operation, Food and Agriculture Organization, INPho-Post-harvest Copendium, pages 1-50.
[3] Gilles Berthoumieux, Vanilla production in Madagascar, Comoro, and Uganda,
Conference Proceeding at the IFEAT International Conference, Cape Town, South Africa, 27 November to 1 December 2006: ‘The Industry in Sub-Saharan Africa and the Indian Ocean Islands’, pages 63-74;
[4] M. Anandaraj, J. Rema, B. Sasikumar, R. Suseela Bhai, P. Rajeev, and R. Dinesh, 2005, Vanilla (Extended Pamphlet, Indian Institute of Spice Research, India, page 1-6. [5] Champon Vanilla, Vanilla imports/exports & market update, 2009, accessed from
www.vanillabean.com on 25 March 2014.
[6] Meynarti S. D. I., Laba U. and Endang H., 2010, Accessed from www.balittri.litbang.deptan.go.id in 25 April 2014.
[7] Unang Mansur, Buletin Teknik Pertanian, 2009, 14, 2, 76-79. [8] A. Domling, Chem. Rev., 2006, 106, 17-89.
[9] Biginelli, P. Gazz. Chim. Ital.1893,23, 360–413
[10] Ryabukhin, Sergey V., Andrey S. Plaskon A, B, Semen S. Bondarenk, Eugeniy N. Ostapchuk, Oleksandr O. Grygorenko, Oleg V. Shishkin, Andrey A. Tolmachev,
Tetrahedron, 2010, 51, 4229–4232
[11] Anjna Bhatewara, Srinivasa Rao Jetti, Tanuja Kadre, Pradeep paliwal, and Shubha Jain,
Int. J. Med. Chem., 2013, ID 197612, 5 (DOI: http;/dx.doi.org/10.1155/2013/197612) [12] Natvar A. Sojitra, Rajesh K. Patel, Ritu B. Dixit, and Bharat C. Dixit, Org. Chem. Curr.
Res., 2013, 2 (2), 1-6.
[13] Ragini Gupta, Anshu Jain, Rahul Joshi, and Meenakshi Jain, Bull. Korean Chem. Soc.,
2011, 32 (3), 899-904.
[14] Saeed Balalaie, Hamid Moghimi, Morteza Bararjanian, Frank Rominger, Hamid Reza Bijanzadeh, and Masoumeh Sheikhahmadi, J. Heterocyclic Chem., 2013, 50 (6), 1304-1312.
[15] Atmika Paudel, Keiichi Kaneko, Ayako Watanabe, Matsunaga Shigeki, Kanai Motomu, Hiroshi Hamamoto, and Kazuhisa Sekimizu, J. Antibiot., 2013, 66, 663-667.
[16] Majid Ghashang, Syed Sheik Mansoor, and Krishnamoorthy Aswin, J. Adv. Res., 2014, 5, 209-218.
[17] Michael Brands, Reiner Endermann, Reinhold Gahlmann, Jochen Kruger, and Siegfried Raddatz, Bioorg. Med. Chem. Lett.,2003, 13, 241-245.
[18] Mastoura M. Edrees, Thoraya A. Farghaly, Fatma A. A. El-Hag, and Mohamed M. Abdalla, Eur. J. Med. Chem., 2010, 45, 5702-5707.
[19] C. Oliver Kappe, J. Org. Chem.,1997, 62, 7201.