R
ecognition of microorganisms by the plant cell depends on the generation of elicitors by the pathogen1. These can be non-race-specific elicitors, such as fungal or plant cell wall fragments released during the infection process, which elicit a defense response that helps to minimize disease. Race-specific elicitors are molecules that are encoded by avirulence (Avr) genes in the pathogen2. Resistance involves the specific recognition of
the invading pathogen by a dominant or semi-dominant plant re-sistance gene product (R gene). This type of interaction is termed gene for gene, where for each gene that confers resistance in the host there is a corresponding gene in the pathogen that confers its virulence3
. The cloning of plant resistance (R) genes from host– pathogen model systems has facilitated a leap in understanding the molecular basis of host resistance. The proposed model sug-gests that the direct or indirect interaction of the AVR and R poly-peptides triggers resistance4
. Based on sequence analysis of known
R genes, it has been hypothesized that R gene products encode
re-ceptors capable of binding AVR products as ligands5
. Expression of these gene products in susceptible plants resulted in specific resistance3
, demonstrating that even susceptible plants possess the underlying biochemical machinery required for defense. Thus, the difference between resistance and susceptibility appears to lie in the proper recognition of the AVR products.
The amino acid sequences of R gene products have homology to a number of structural protein–protein interaction domains, suggesting that they play a role as signaling intermediates in a
reaction cascade2. In addition, it appears that multiple products,
some of which may be closely linked to the original R gene, are required for the manifestation of resistance. One example of this involves the functional interaction between the tomato Pto and Prf gene products for expression of resistance to
Pseudo-monas syringae pv. tomato. The Pto gene product is a serine/
threonine kinase6, whereas the Prf gene product contains
leucine-rich regions (LRRs), nucleotide binding sites (NBS) and leucine zippers (LZ) domains7. It is likely that other R genes, such as those
of the Cf family, may interact with other gene products to confer resistance. This notion is also supported by biochemical evi-dence. For example, binding of the AVR9 peptide to plant mem-branes could not be correlated with the presence of the Cf9 gene8,
suggesting that the R gene product might not be exclusively in-volved in recognition, but also in the coordination of membrane signaling.
Signaling
Immediately downstream of the initial elicitor–receptor recog-nition, the activation of ion fluxes and the production of H2O2are
the initial responses detected in plant cells. Biochemical evidence suggests that these processes, which occur prior to the transcrip-tional activation of defense-related genes, appear to be mediated through the regulation of plasma membrane-bound enzymes. These include changes in Ca2+-ATPase (Ref. 9) and H+-ATPase (Ref.
10) activities, the activation of plasma membrane-bound ion chan-nels11,12and the induction of a plasma
mem-brane-bound NADPH oxidase13,14
. These biophysical and biochemical processes can induce changes in the electrical potential difference (membrane potential) and pH gradient across the host plasma membrane that may affect the activity of ion channels and other ion transporters. Changes in the plasma membrane-bound oxidase electron transport chain will mediate the production of H2O2and active oxygen species (termed
the oxidative burst), which may affect the attacking pathogen and the host cell at the site of infection.
A number of signal transduction path-ways have been proposed to mediate these early responses in host cells, ensuring an elicitor-induced response that is quanti-tatively appropriate, correctly timed and highly coordinated with other activities of the host plant cells. These pathways might
Early signal transduction pathways in
plant–pathogen interactions
Eduardo Blumwald, Gilad S. Aharon and Bernard C-H. Lam
Disease resistance depends on the ability of the plant to recognize a pathogen early in the infection process. Molecules that indicate the presence of the pathogen (elicitors) activate host receptors and these rapidly generate an internal signal that triggers early defense responses. Several transduction pathways that relay the initial recognition signal through a series of cytosolic and membrane-delimited pathways have been proposed. Activation of these signal transduction pathways ensures an elicitor-induced response that is quantitative, timely and coordinated with other activities of the host cells.
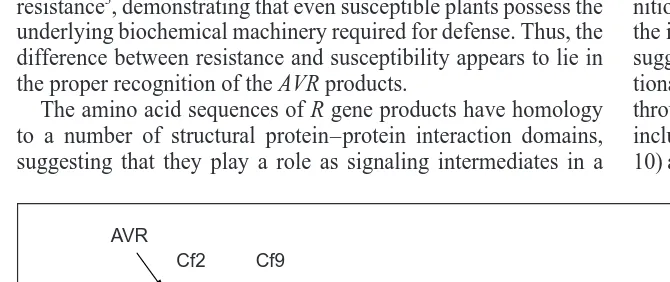
Fig. 1. Putative mechanism for the involvement of membrane-associated G proteins in host–
pathogen interactions. Receptor proteins, encoded by the Cf genes, are predicted to consist predominantly of leucine-rich repeats (LRR). Binding of the avirulence (AVR) peptide to the receptor results in the activation of a G protein-coupled receptor (7TMS), probably via extra-cellular protein–protein interactions through the conserved leucine-rich repeats. The ques-tion mark indicates a putative membrane-localized intermediate in the signaling pathway.
? Leucine-rich repeats
Defense responses
GTP
GTP GDP
7TMS NH2
NH2
COOH COOH Cf9 Cf2 AVR
Leucine-rich repeats
comprise G proteins (to relay the initial elicitor–receptor recog-nition through a series of membrane-delimited pathways), changes in cytosolic Ca2+
concentrations and protein kinases/phosphatases, that affect the activity of key enzymes.
G proteins
G proteins act as molecular signal transducers whose active or in-active states depend on the binding of GTP or GDP, respectively. The G proteins include two major subfamilies, the heterotrimeric G proteins and the small G proteins. Whereas the heterotrimeric G proteins contain a, band gsubunits, the small G proteins ap-pear to be similar to free asubunits, operating without the bg heterodimer. Generally, it is the asubunit of the heterotrimeric G protein that has the receptor-binding region and possesses a guanosine nucleotide binding site and GTPase activity15
. Both classes of G proteins use the GTP/GDP cycle as a molecular switch for signal transduction. Interaction of the G protein with the activated receptor promotes the exchange of GDP, bound to the asubunit, for GTP and the subsequent dissociation of the a–GTP complex from the bgheterodimer.
A variety of evidence suggests a role for heterotrimeric G pro-teins in the plant defense response pathway. Most of the research has been based on the use of non-hydrolyzable GTP analogs16,17
, toxins such as CTX (Ref. 18), mastoparan11,16,19, and recombinant
G proteins20
. Although much of the evidence is correlative, some interesting hypotheses can be proposed. For example, it could be argued that the activation of defense responses by nonhydrolyz-able GTP analogs is a consequence of receptor activation caused by the interaction of these analogs with the NBS domain of the R gene product. However, this possibility is unlikely given the low affinity of the NBS for the nucleotides, which is several orders of magnitude lower than that of the G proteins20. A more
plau-sible explanation is that G proteins are activated downstream of elicitor–receptor recognition. The Cf family of receptors consists of extracellular LRRs. Although the N-terminal LRRs appear to be different, the C-terminal LRRs are conserved among the dif-ferent genes cloned to date. It is conceivable that the conserved LRRs are involved in extracellular protein interactions while the N-terminal LRRs are involved in ligand binding. In such a model, rather than the receptor protein interacting with an additional mol-ecule intracellularly, it might interact with a G protein-coupled receptor (Fig. 1). Data showing the direct activation of a plasma membrane Ca2+
channel by a recombinant a-subunit suggests that the activation of defense responses could be G protein mediated through plasma membrane-delimited pathways20
.
Protein kinases
The phosphorylation of proteins, probably initiated by the recep-tor, is thought to relay the defense signal to different downstream effectors. In some cases, the receptor contains a kinase domain that may trigger the phosphorylation cascade, whereas in others a secondary messenger such as Ca2+may trigger the protein kinases.
The R gene class containing rice Xa-21 (Ref. 21) and wheat Lr10 (Ref. 22) encodes receptor-like kinases with an extracellular domain, a membrane-spanning domain and a cytosolic serine/threonine kinase domain. It might be expected that following perception of a signal from the pathogen (such as an elicitor), the kinase domain of these proteins would trigger a cascade of phosphorylation. Unfor-tunately, no downstream-interacting proteins for these receptor-like kinases have been identified to date. The tomato Pto gene is a unique class of R gene that encodes a cytosolic protein with serine/ threonine kinase activity. Several proteins that interact with the Pto kinase have been characterized23
. The Pti1 (‘Pto-interacting 1’) gene encodes a serine/threonine kinase that is probably a downstream
substrate of Pto; three other proteins, encoded by the genes Pti4,
Pti5 and Pti6, were also found to interact with the Pto kinase.
Each of the three proteins was shown to be a transcription factor that binds to the promoter region of a large number of pathogenesis-related (PR) genes24
. In parsley, recognition of a nonspecific elici-tor by the host cell also triggers a signaling pathway mediated by a mitogen-activated protein (MAP) kinase25
. This elicitor-responsive MAP kinase (ERM kinase) is likely to be involved in transcrip-tional activation as the kinase translocates into the nucleus upon elicitor treatment25.
Fungal elicitors induce changes in the phosphorylation status of proteins in tomato cells in suspension culture26, and these changes
correlate with an increase in cytosolic free Ca2+
concentrations. The dephosphorylation of the plasma membrane H+-ATPase was
evident soon after treatment with elicitors from incompatible races of Cladosporium fulvum26. Distinct peaks of protein kinase C
(PKC)-like and Ca2+
/calmodulin-dependent protein kinase activi-ties were observed 40 min and 90 min, respectively, after treat-ment, and this increase in cytosolic kinase activity was associated with the rephosphorylation of the plasma membrane H+-ATPase.
Among the plant calcium-dependent protein kinases, calmodulin-like domain protein kinases (CDPKs) have attracted great atten-tion27
. Although CDPKs have been implicated in general stress responses28, specific evidence for their participation in signal
trans-duction pathways during plant–pathogen interaction is still lacking. However, increasing evidence suggests that the elevated cytosolic calcium concentrations is a common consequence of pathogen per-ception11,24,29,30, and it is likely that CDPKs are involved in these
signaling processes.
The biophysical and biochemical response Plasma membrane H+-ATPase
The plasma membrane H+
-ATPase is essential for the growth of cells. Proton extrusion provides the electrochemical gradient of protons across the plasma membrane that drives the different H+
-coupled (antiport and symport) and membrane potential--coupled (uniport) transport mechanisms for the uptake and extrusion of solutes. In addition, the membrane potential regulates a number of plasma membrane-bound ion channels, and acidification of the extracellular medium regulates the physical and biochemical properties of the cell wall. Changes in the host plasma membrane H+-ATPase activity (with the associated changes in ion fluxes across
the plasma membrane) are among the earliest events associated with elicitation. In some cases, treatment with elicitors results in an inhibition of H+
-ATPase activity and a concomitant depolarization of the plasma membrane potential10,31. In other cases, treatment with
elicitors results in activation of plasma membrane H+
-ATPase, with consequent acidification of the extracellular milieu and hyper-polarization of the membrane potential10
. In tomato, activation of host plasma membrane H+-ATPase by elicitors from incompatible
races of C. fulvum appears to be mediated by a heterotrimeric G protein that activates a membrane-bound phosphatase to induce the dephosphorylation of the H+
-pump17,26
. It has been proposed that the differential effect of elicitors on the plasma membrane H+
-ATPase and the resultant acidification or alkalinization of the extra-cellular medium is in response to the difference between specific and nonspecific elicitors32
.
Calcium homeostasis
Many cellular processes, including plant responses to pathogens, are regulated by changes in cytosolic Ca2+
concentrations, where free Ca2+can serve to transduce a particular stimulus to target
pro-teins that guide the cellular response. Many of the biochemical re-sponses associated with the defense mechanisms directly correlate
343
with an increase in cytosolic free Ca2+concentration.
Measure-ments of external Ca2+
with ion-selective electrodes and of Ca2+
fluxes using radiometric techniques have revealed a large and tran-sient Ca2+
influx with concomitant acidification of the extracellu-lar medium. This suggests a correlation between fungal elicitor activity, hyperpolarization of the host cell plasma membrane, and Ca2+influx1. The activation of plasma membrane Ca2+channels by
specific11
and nonspecific elicitors12
provides a direct demonstration of a pathway by which cytosolic free Ca2+concentrations increase
to levels that can initiate various defense responses, including the production of active oxygen species, callose and phytoalexins. Stimulation of Ca2+
influx, without a corresponding control of the Ca2+efflux is unlikely. The host cell might face two disadvantages:
• An inability to sustain the high cytosolic Ca2+
levels that are responsible for subsequent biochemical changes
• The inefficient utilization of energy for maintaining the func-tion of the plasma membrane-bound Ca2+-ATPase.
It has been shown recently that almost immediately after the treat-ment of tomato cells with incompatible elicitors of C. fulvum, activation of plasma membrane Ca2+
channels leads to a parallel inhibition of a plasma membrane Ca2+-ATPase (Ref. 9). These
re-sults suggest a finely coordinated elicitor-dependent increase in cytosolic Ca2+in the cell that then acts as a signal for the activation
of downstream biochemical pathways (Fig. 2). Although there is increasing evidence for a role for Ca2+
signaling in this process, a fundamental question remains unanswered: how does the cell use these calcium signals to control downstream targets and the acti-vation of a number of Ca2+
-dependent pro-tein kinases (i.e. CDPKs, PKC and Ca2+/
CaM-dependent protein kinase)? A model recently proposed by De Koninck and Schulman33
may explain the concerted ac-tion of these kinases. They demonstrated that CaM KII kinases were primed by Ca2+
bursts of a given frequency and maintained their activity with Ca2+
signals of substan-tially lower frequency. This may allow the cells to distinguish betweem specific cal-cium signals (intracellular calcal-cium spikes) and nonspecific changes in steady-state cytosolic Ca2+ concentrations. Plant cells
could also reinforce intracellular calcium signals (due to the influx of Ca2+from the
extracellular space) by coupling these Ca2+
bursts with Ca2+release from vacuoles34.
NADPH oxidase
Among plant defense responses to patho-gen attack, the rapid production of hydropatho-gen peroxide (H2O2) and its probable precursor,
superoxide (O2
2), plays an important role.
The release of these active oxygen species (AOS) in the oxidative burst may affect the attacking pathogen and the host cells at the infection site, thereby limiting the spread of the pathogen35
. Evidence supporting the role of a plasma membrane-bound NADPH oxidase in the oxidative burst and the simi-larity between the NADPH oxidase in plant cells and phagocytic animal cells is emerg-ing. In phagocyte cells, the oxidase elec-tron transport chain, located in the plasma membrane, comprises a cytochrome b heterodimer (gp91-phox and p22-phox). It is nonfunctional until at least three proteins, p47-phox, p67-phox and Rac (a monomeric G protein), move from the cytosol to dock on cytochrome b. The docking process involves the interaction of Src-homology-3 domains (SH3) on p47-phox (activated via a PKC-mediated phosphorylation) with p22-phox. After the docking process, the electron transporting component is able to transfer electrons from NADPH to oxygen36
. Elicitor in-duction of the oxidative burst in soybean, parsley and Arabidopsis is inhibited by diphenylene iodonium, an inhibitor of mammalian NADPH oxidase13,35,37. Antibodies raised against human
neutro-phil p47-phox and p67-phox cross-react with proteins of the same molecular mass in extracts from soybean, cotton, Arabidopsis and tomato13,14,37
, and genes coding for plant homologs of neutrophil NADPH oxidase gp91-phox have recently been cloned38,39. As
in phagocytes, immunocytochemistry has shown that treatment of tomato cells with race-specific elicitors induces translocation of p47-phox-, p67-phox- and rac-like proteins from the cytosol to the plasma membrane36. The assembly process also involved the
phosphorylation of these proteins36
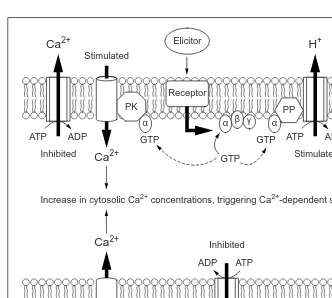
. The similarity between the mode of action of NADPH oxidase in tomato and in phagocytes suggests that plants and animals share common elements in this signal transduction pathway. Nevertheless, biochemical evidence Fig. 2. Signal events leading to the increase in cytosolic Ca2+concentrations. The
bind-ing of elicitors to receptors at the host plasma membrane triggers the activation of heterotrimeric G proteins. These, in turn, transduce the signal by activating phosphatases (PP) that stimulate the plasma membrane H+-ATPase. The concomitant hyperpolarization of the membrane potential induces the opening of a Ca2+channel. Further activation of the channel by the heterotrimeric G protein, modulated by a membrane-bound protein kinase (PK), together with inhibition of the Ca2+-ATPase, results in a transient increase in cyto-solic Ca2+ concentrations. Alternatively, inhibition of plasma membrane H+
-ATPase by nonspecific elicitors, with the concomitant depolarization of the membrane potential, induces the opening of a depolarized-activated Ca2+channel that also results in a transient increase in cytosolic Ca2+concentrations. Broken lines indicate activation of heterotrimeric G proteins.
GTP GTP GTP
ADP ATP
ADP ATP Inhibited
Inhibited
Increase in cytosolic Ca2+ concentrations, triggering Ca2+-dependent signaling
Stimulated Stimulated
Stimulated
Ca2+
Ca2+
Ca2+
α α
α β γ
ADP Cytosol
Cytosol ATP
H+
H+
Elicitor
Receptor
supports the involvement of different protein kinases in the acti-vation of p47-phox and p67-phox in phagocytes (PKC) and tomato cells (possibly a CDPK; Ref. 14). These differences may reflect the unique requirements for spatial and temporal distribution in the plant cell and differences in the developmental and environ-mental signals to which plants must respond.
Future perspectives
Immediately downstream of pathogen recognition processes, sig-nal transduction pathways activate key plasma membrane-bound enzymes (Fig. 3). Evidence suggests that these pathways may in-volve the activation of heterotrimeric G proteins and membrane-bound phosphatases that subsequently mediate the activation of plasma membrane H+
-ATPase and Ca2+
channels. The increase in cytosolic Ca2+concentration initiates the activation of a number of
protein kinases that induce a series of defense responses (e.g. the oxidative burst and callose synthesis) and are responsible for the
temporal and spatial resolution of the defense response. Further research is needed to characterize the different signaling compo-nents downstream of the initial recognition events. The molecular characterization of the different protein kinases involved in the signal transduction pathways and the identification of their spe-cific targets will help to clarify the cellular network required for activation of the defense response.
References
1 Dixon, R.A., Harrison, M.J. and Lamb, C.J. (1994) Early events in the
activation of plant defense responses, Annu. Rev. Phytopathol. 32, 479–501
2 De Wit, P.J.G.M. (1998) Pathogen avirulence and plant resistance: a key role
for recognition, Trends Plant Sci. 12, 452–458
3 Bent, A.F. (1996) Plant disease resistance genes: function meets structure,
Plant Cell 8, 1757–1771
4 Dangl, J.F. (1995) Pièce de Resistance: novel classes of plant disease
resistance genes, Cell 80, 363–366
5 Jones, D.A. et al. (1994) Isolation of the tomato Cf-9 gene for resistance to
Cladosporium fulvum by transposon tagging, Science 266, 789–793
6 Loh, Y.T. and Martin, G.B. (1995) The Pto bacterial resistance gene and the
FenI insecticide sensitivity gene encode functional protein kinases with serine/threonine specificity, Plant Physiol. 108, 1735–1739
7 Salmeron, J.M. et al. (1996) Tomato Prf is a member of leucine-rich repeat
class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster, Cell 86, 123–133
8 Kooman-Gersmann, M. et al. (1996) A high-affinity binding site for the
AVR9 peptide elicitor of Cladosporium fulvum is present on plasma membranes of tomato and other solanaceous plants, Plant Cell 8, 929–938
9 Lam, C.H.B. et al. Effect of race-specific elicitors of Cladosporium fulvum on
the tomato plasma membrane Ca2+-ATPase, Physiol. Mol. Plant Pathol. (in
press)
10 Vera-Estrella, R. et al. (1994) Plant defense response to fungal pathogens. I.
Activation of host plasma membrane H+
-ATPase by elicitor-induced enzyme dephosphorylation, Plant Physiol. 104, 209–215
11 Gelli, A., Higgins, V.J. and Blumwald, E. (1997) Activation of plasma
membrane Ca2+-permeable channels by race-specific fungal elicitors,
Plant Physiol. 113, 269–279
12 Zimmermann, S. et al. (1997) Receptor-mediated activation of a Ca2+
-permeable ion channel involved in pathogen defense, Proc. Natl. Acad. Sci. U. S. A. 94, 2751–2755
13 Desikan, R. et al. (1996) Generation of active oxygen in elicited cells of
Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme, FEBS Lett. 383, 213–217
14 Xing, T., Higgins, V.J. and Blumwald, E. (1997) Race-specific elicitors of
Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells, Plant Cell 9, 249–259
15 Gilman, A.G. (1987) G proteins: transducers of receptor-generated signals,
Annu. Rev. Biochem. 56, 615–649
16 Vera-Estrella, R., Higgins, V.J. and Blumwald, E. (1994) Plant defense
response to fungal pathogens. II: G protein mediated changes in host plasma membrane redox reactions, Plant Physiol. 106, 97–102
17 Xing, T., Higgins, V.J. and Blumwald, E. (1997) Identification of G proteins
mediating fungal elicitor-induced dephosphorylation of host plasma membrane H+-ATPase, J. Exp. Bot. 48, 229–237
18 Beffa, A.F. et al. (1995) Cholera toxin elevates pathogen resistance and induces
pathogenesis-related gene expression in tobacco, EMBO J. 14, 5753–5761
19 Legendre, L., Heinstein, P.F. and Low, P.S. (1993) Evidence for participation
of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells, J. Biol. Chem. 267, 20140–20147
20 Aharon, G. et al. (1998) Regulation of a plant plasma membrane Ca2+-channel
by TG-a-1, a heterotrimeric G protein I subunit homologue, FEBS Lett. 424, 17–21
21 Song, W. (1995) A receptor-like protein kinase encoded by the rice disease
resistance gene Xa21, Science 270, 1804–1806
345
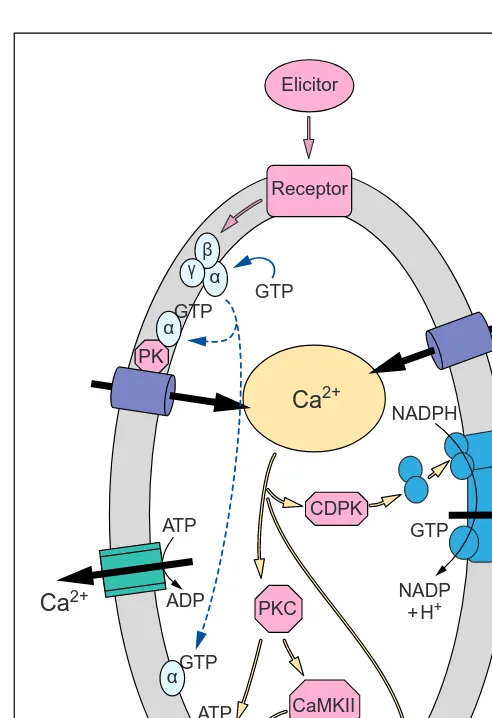
September 1998, Vol. 3, No. 9 Fig. 3. A hypothetical model of the early signal transduction
events in plant–pathogen interactions. Following the events in-volving G proteins that lead to an increase in cytosolic Ca2+ con-centrations, Ca2+ activates a calmodulin-like domain protein kinase (CDPK). This induces NADPH oxidase activity with the consequent production of active oxygen species (O2
–
) and H2O2.
The increase in cytosolic Ca2+concentrations will also induce b -1,4 glucan synthase and the production of callose. Following these initial events, Ca2+activates a protein kinase C (PKC) and a Ca2+/ CaM-dependent protein kinase that rephosphorylate the H+ -ATPase, returning the enzyme activity to control levels. Broken lines indicate activation of heterotrimeric G proteins.
ADP ADP
ATP ATP
GTP
GTP
Ca2+
H+
α
α β γ
Elicitor
Receptor
Glucan synthase
Callose PP
GTP
GTP
α
PK
PKC CDPK
NADP +H+ NADPH
CaMKII
O2– H2O2
O2
e–
22 Feuillet, C., Schachermayr, G. and Keller, B. (1997) Molecular cloning of a
new receptor-like kinase gene encoded at the Lr10 disease resistance locus of wheat, Plant J. 11, 45–52
23 Zhou, J. et al. (1995) The tomato gene Pti1 encodes a serine/threonine kinase
that is phosphorylated by Pto and is involved in the hypersensitive response, Cell 83, 925–935
24 Zhou, J., Tang, X. and Martin, G.B. (1997) The Pto kinase conferring
resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes, EMBO J. 16, 3207–3218
25 Ligterink, W. et al. (1997) Receptor-mediated activation of a MAP kinase in
pathogen defense of plants, Science 276, 2054–2057
26 Xing, T. Higgins, V.J. and Blumwald, E. (1996) Regulation of plant defense
response to fungal pathogens: two types of protein kinases in the reversible phosphorylation of the host plasma membrane H+-ATPase, Plant Cell 8, 555–564
27 Roberts, D.M. and Harmon, A.C. (1992) Calcium-modulated proteins: targets
of intracellular calcium signals in higher plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 375–414
28 Sheen, J. (1996) Ca2+-dependent protein kinase and stress signal transduction,
Science 274, 1900–1902
29 Tavernier, E. et al. (1995) Involvement of free calcium in action of cryptogein,
a proteinaceous elicitor of hypersensitive reaction in tobacco cells, Plant Physiol. 109, 1025–1031
30 Xu, H. and Heath, M.C. (1998) Role of calcium in signal transduction during
the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus, Plant Cell 10, 585–597
31 Atkinson, M.M. and Baker, C.J. (1989) Role of the plasmalemma H+-ATPase
in Pseudomonas syringae-induced K+
/H+
exchange in suspension-cultured tobacco cells, Plant Physiol. 91, 298–303
32 De Wit, P.J.G.M. (1995) Fungal avirulence genes and plant resistance genes:
unravelling the molecular basis of gene for gene interactions, Adv. Bot. Res. 21, 148–177
33 De Koninck, P. and Schulman, H. (1998) Sensitivity of CaM Kinase II to the
frequency of Ca2+
oscillations, Science 279, 227–230
34 Allen, G.J. and Sanders, D. (1997) Vacuolar ion channels, Adv. Bot. Res. 25,
217–252
35 Mehdy, M.C. (1994) Active oxygen species in plant defense against
pathogens, Plant Physiol. 105, 671–681
36 Segal, A.W. and Abo, A. (1993) The biochemical basis of the NADPH
oxidase of phagocytes, Trends Biochem. Sci. 18, 43–47
37 Dwyer, S.C. et al. (1996) Plant and human neutrophil oxidative burst
complexes contain immunologically related proteins, Biochim. Biophys. Acta 1289, 231–237
38 Groom, Q.J. et al. (1996) rbohA, a rice homologue of the mammalian gp91phox
respiratory burst oxidase gene, Plant J. 10, 515–522
39 Keller, T. et al. (1998) A plant homolog of the neutrophil NADPH oxidase
gp91phoxsubunit gene encodes a plasma membrane protein with Ca2+binding
motifs, Plant Cell 10, 255–266
C
yanobacteria (blue-green algae) are a diverse group of pro-karyotes. A common feature is their oxygenic photosyn-thesis, which is similar to that in algae and higher plants and is the most important biological mechanism for capturing solar energy. As sunlight is their energy source and water the reductant, they generate oxygen in the light. Energy and reductant generated by photosynthesis are usually used for carbon dioxide reduction. Some strains are strict photoautotrophs, whereas others can use exogenous carbon sources such as fructose and glucose.Nitrogen fixation occurs only in prokaryotes and one line of evidence for the common origin of the nitrogen fixation mecha-nism is the similar physical, chemical and biological characteris-tics of the nitrogen-fixing enzyme system in otherwise dissimilar organisms. Many cyanobacteria are able to reduce atmospheric
dinitrogen to ammonia. In some filamentous cyanobacteria nitro-gen-fixing heterocysts are formed. Heterocysts are terminally dif-ferentiated cells whose interior becomes anaerobic, mainly as a consequence of respiration, allowing the oxygen-sensitive process of nitrogen fixation to continue. Heterocysts are spaced at semi-regular intervals along the filament with approximately 7% of the cells differentiating into heterocysts in free-living Anabaena/Nostoc species. The regulation of dinitrogen fixation has been extensively studied in the heterocyst system of diazotrophic cyanobacteria1
.
Nitrogen fixation and heterocyst formation
During differentiation of a vegetative cell into a heterocyst, major structural and biochemical changes occur that affect nitrogen fix-ation. Upon nitrogen deprivation phycobiliproteins are broken Eduardo Blumwald*, Gilad S. Aharon and Bernard C-H. Lam are at the Dept of Botany, University of Toronto, 25 Willcocks St, Toronto, Ontario, Canada M5S 3B2.
*Author for correspondence (tel +1 416 978 2378;
fax +1 416 978 5878; e-mail [email protected]).