Summary Northern red oak (Quercus rubra L.) seedlings and trees differ in their response to ozone. Previous work reported reductions in net photosynthesis, carboxylation effi-ciency and quantum yield of mature tree leaves, whereas seed-ling processes were unaffected by the same ozone exposure. To further characterize differences in ozone response between seedlings and mature trees, we examined carbon partitioning and allocation in 32-year-old trees and 4-year-old seedlings of northern red oak after exposure to subambient (seasonal SUM00 dose (sum of all hourly ozone exposures) = 31 ppm-h), ambient (SUM00 dose = 85 ppm-h) and twice ambient (SUM00 dose = 151 ppm-h) ozone concentrations for three growing seasons. For mature trees, ozone exposure decreased foliar starch partitioning, increased starch partitioning in branches and increased 14C retention in leaves. In contrast, starch partitioning in leaves and branches, and foliar 14C reten-tion in seedlings were unaffected by ozone exposure, but soluble carbohydrate concentrations in coarse and fine roots of seedlings were reduced. Differences in carbohydrate demand between seedlings and mature trees may underlie the higher leaf ozone uptake rates and greater physiological response to ozone in mature northern red oak trees compared with seed-lings.
Keywords: Quercus rubra, carbon metabolism, scaling.
Introduction
Ozone stress may reduce carbon uptake, alter carbon partition-ing to different chemical forms and change the allocation or flow of carbon among plant organs (Dickson and Isebrands 1993, Laurence et al. 1994). For example, Friend and Tomlin-son (1992) detected a reduction in carbohydrate partitioning to starch and an increase in partitioning to repair compounds in needles of Pinus taeda L. seedlings exposed to ozone. Photo-synthesis, speed of phloem transport and carbon transport to roots were also reduced in Pinus taeda seedlings as a result of ozone exposure (Spence et al. 1990). Pinus echinata Mill. seedlings responded to ozone by reducing the partitioning of carbon to sucrose and increasing the retention of carbon in
needles (Paynter et al. 1992). Changes in carbon allocation or partitioning in response to ozone stress may reflect plant injury or compensatory mechanisms that maintain aboveground growth (Matyssek et al. 1995).
Our understanding of the influence of ozone on carbon metabolism in trees is based largely on seedling research; however, recent work indicates that seedlings may not be satisfactory surrogates for mature trees. For instance, Grulke and Miller (1994) reported greater foliar photosynthetic sensi-tivity to ozone in two-year-old Sequoidendron giganteum Bucholz. seedlings than in 12-year-old rooted cuttings or 125-year-old trees. Exposure to twice ambient concentrations of ozone induced a 50% reduction in light-saturated photosynthe-sis and declines in carboxylation efficiency and quantum yield of leaves of mature northern red oak trees (Quercus rubra L.), whereas leaf physiology of seedlings was comparatively unaf-fected (Samuelson and Edwards 1993, Hanson et al. 1994). Differences in recurrent flushing, canopy microclimate, nutri-ent and water relations, ozone uptake and carbon dynamics between trees of different age and size may contribute to variation in ozone sensitivity between seedlings and mature trees, although canopy microclimate (Samuelson 1994) and leaf water relations (Hanson et al. 1994) did not account for the greater physiological sensitivity to ozone of mature northern red oak trees compared to seedlings. Higher leaf conductance throughout three growing seasons resulted in greater ozone uptake by leaves of mature trees. On an individual leaf basis and in the twice ambient concentration, mature tree leaves received a seasonal internal ozone dose that was approxi-mately 30% greater than the internal dose estimated for seed-ling leaves (Hanson et al. 1994). On a total tree basis, the cumulative ozone dose was much larger for mature trees than for seedlings because total projected leaf area was approxi-mately 620 times greater in mature tree canopies (Samuelson and Edwards 1993). Despite greater ozone uptake and signifi-cant reductions in foliar physiological processes of mature northern red oak trees compared with seedlings, ozone did not reduce aboveground growth (Samuelson et al. 1996). Changes in the allocation or partitioning of carbon in mature tree tissues possibly alleviated or postponed reductions in growth.
Carbon partitioning and allocation in northern red oak seedlings and
mature trees in response to ozone
L. J. SAMUELSON
1and J. M. KELLY
21
School of Forestry, M. White Smith Hall, Auburn University, AL 36849-5418, USA 2
Department of Forestry, Iowa State University, Ames, Iowa 50011-1021, USA
Received September 20, 1995
To obtain a more detailed understanding of differences in ozone response between seedlings and mature trees, we exam-ined the influence of ozone on carbon metabolism in 32-year-old northern red oak trees and 4-year-32-year-old seedlings. Carbon partitioning in leaf, stem and root tissues, and foliar 14C reten-tion in leaves were examined after three years of exposure to a subambient, ambient or twice ambient concentration of ozone.
Materials and methods
Ozone treatments
Subambient (charcoal-filtered), ambient, and twice ambient ozone concentrations were delivered on a 24-h basis to nine mature northern red oak trees each enclosed in a separate open-top tree chamber (4.6 × 10.8 m) and nine open-top seedling chambers (3.0 × 2.4 m) that housed up to 30 seedlings within a chamber. Chambers and ozone treatments were ar-ranged in three blocks. Ozone treatments were administered continuously from mid-April through early October during the 1992, 1993 and 1994 growing seasons.
An ozone analyzer (TECO Model 49, Franklin, MA) and computer-controlled monitoring and injection system were used to monitor ozone concentrations and deliver the twice ambient ozone concentration. Ozone concentrations were measured at 7-min intervals within the mid-canopy of each tree chamber and center of each seedling chamber. The ozone fumigation chambers, and ozone delivery and monitoring sys-tem have been described previously (Samuelson and Edwards 1993). Variations in microclimate and ozone concentration within and among chambers were characterized (Samuelson 1994).
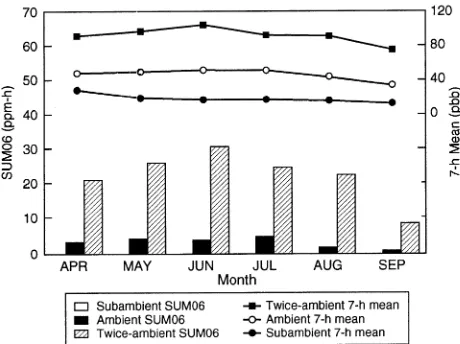
Average 7-h (1100--1700 h) ozone concentrations and SUM06 (sum of all hourly average ozone concentrations equal to or above 60 ppb) exposure indices for the 1994 growing season are presented in Figure 1. Averaged over three growing seasons, seasonal SUM00 (sum of all hourly average ozone
concentrations) exposures for the subambient, ambient and twice ambient ozone concentrations were 31, 85 and 151 ppm-h, respectively. Ozone exposure indices for the three growing seasons were previously summarized (Samuelson et al. 1996).
Cultural history
The 32-year-old mature northern red oak trees were estab-lished in a seed orchard in the mid-1960s by grafting scions of different genetic lineage onto common root stock planted at a 6.5 × 6.5 m spacing on the Tennessee Valley Authority Reser-vation in Norris, TN.
Four-year-old, half-sib seedlings were grown from acorns collected from different trees within the seed orchard. When the seedlings were two years old they were transplanted to 24-l pots containing soil from the study site (Staser series: fine-loamy, mixed, thermic Cumulic Hapludoll). Before the third season of ozone treatment, seedlings were transplanted to 33-l pots containing soil from the same source as described pre-viously. Pots were set in styrofoam within each chamber to minimize direct solar heating of the soil.
Seedlings and trees were watered to maintain soil field capacity. Water status of soil within tree chambers and soil within seedling pots was monitored at a depth of 15 cm by time domain reflectometry (Trace System I, Soil Moisture Equip-ment Corp., Santa Barbara, CA).
Because of the destructive nature of the sampling proce-dures and potential variation in branch and leaf growth among trees, carbon partitioning and allocation were measured late in September. Buds on seedling and mature tree branches were set by October 1 and leaves of seedlings and mature trees senesced late in October.
Carbon allocation experiment
One mid-stem branch on three seedlings within each of the nine seedling chambers and two mid-canopy branches on each of the nine mature trees were exposed to 25 µCi 14C for 30 min on September 1, 1994 at approximately midday (1000--1500 h). Branches were exposed to 14C by block so that any influence of sampling time would be averaged across all treat-ments and ages. Approximately the same size branch (61 cm in length) was treated on each seedling and tree. Each branch was enclosed within a mylar sleeve and lactic acid was injected into a cup containing 14C-labeled sodium carbonate residue attached inside the mylar sleeve (Edwards et al. 1992). To quantify initial leaf 14C fixation, sleeves were removed after a 30-min exposure period and samples were immediately taken from the 14C-exposed leaves. One sample, consisting of 10 leaf discs from 10 separate leaves, was collected from each branch. Discs were frozen in liquid nitrogen and dried to a constant weight at 70 °C .
At midday on the fifth and seventh day following initial exposure, one foliar sample from each 14C exposed branch was collected as described previously. Discs were taken from the same leaves and the same general locations as the initial samples. Discs were frozen in liquid nitrogen and dried. Figure 1. Monthly SUM06 indices and 7-h means for subambient,
The amount of 14C in ground leaf tissue was measured by liquid scintillation spectroscopy and expressed as dpm (1dpm = 0.0167 Bq) per g of tissue dry weight. Carbon retention was expressed as a percentage of initial 14C fixation.
Carbon partitioning experiment
From September 23 to 25, 1994, four seedlings were randomly chosen and harvested from each chamber between 1200 and 1500 h. One sample of foliar, stem, branch, and fine (≤ 2 mm) and coarse root (> 2 mm) tissue made up of representative subsamples was collected from each of the four seedlings in each of the nine seedling chambers. Seedlings and trees pro-duced only one flush.
Three foliage, branch, and fine root samples were collected from each of the nine mature trees. Leaves and current-year branches were collected from a mid-canopy position on the south-facing aspect of each tree. Fine root samples were col-lected by digging three 0.093 m2 pits approximately 30 cm in depth at equally spaced intervals 1.25 m from the bole of each tree.
All samples were frozen in liquid nitrogen, and stored in plastic bags on ice until transported to a freezer. Procedures for tissue extractions and quantification of carbohydrates by high-performance anion-exchange chromatography have been de-scribed by Wilson et al. (1995).
Statistical analysis
Seedling and tree responses were averaged by chamber and analyzed separately because mean square errors were dissimi-lar in magnitude between seedlings and trees (Samuelson and Edwards 1993). Regression analyses were used to determine significant linear or quadratic ozone effects on the concentra-tion of soluble carbohydrates (SC = glucose + sucrose + fructose), starch and total nonstructural carbohydrates (TNC), and percent retention of 14C in leaves. Because the SUM00 index produced a better fit to northern red oak response data
then the SUM06 index (Hanson et al. 1994, Samuelson et al. 1996), ozone treatments were defined in the analyses by the SUM00 index accumulated over three seasons of exposure. The analyses of percent 14C retention were performed on arcsine transformed values to satisfy heterogeneity of variance assumptions. Ozone treatment effects were considered signifi-cant at P ≤ 0.10.
Results and discussion
Seedling responses
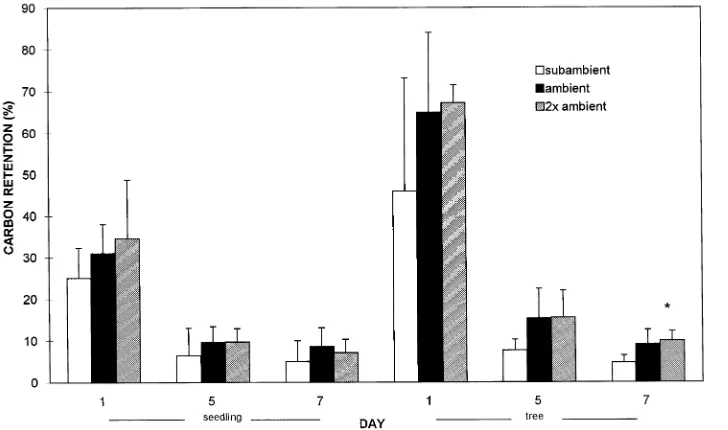
On average, 70% of recently fixed 14C in seedling leaves was respired or translocated within 24 h (Figure 2). Dickson et al. (1990) report that approximately 25% of fixed 14C was translo-cated from fully expanded but immature leaves of northern red oak seedlings within 24 h. In our study, carbon translocation was examined later in the growing season when leaves were fully mature and roots likely acted as a strong sink for carbon (Ursino and Paul 1973, Kuhns and Gjerstad 1988, Adams et al. 1990).
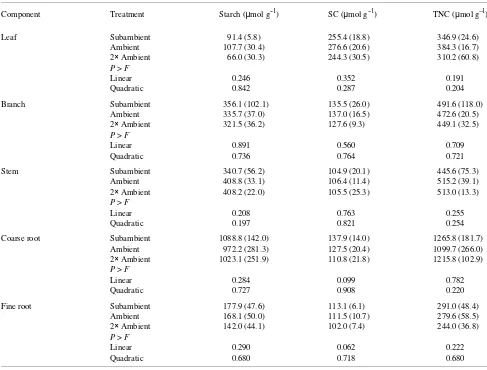
Carbon allocation patterns in Pinus ponderosa Dougl. ex Laws. (Temple et al. 1993), Pseudotsuga menziesii (Mirb.) Franco (Gorissen et al. 1991) and Pinus taeda (Spence et al. 1990, Friend and Tomlinson 1992) seedlings were sensitive to ozone exposure. In contrast, carbon retention in leaves of northern red oak seedlings was unaffected by ozone treatment (Figure 2). Although ozone had no significant influence on carbon retention in leaves, linear reductions in soluble carbo-hydrate concentrations in coarse and fine root tissues indicated an influence of ozone on carbon partitioning in seedling roots (Table 1). Ultimately, lower soluble carbohydrate concentra-tions in roots were not accompanied by reducconcentra-tions in root growth or changes in root versus shoot biomass after three seasons of ozone exposure (Samuelson et al. 1996).
Figure 2. Foliar retention (percent of initial fixation) of 14C in leaves 1, 5, or 7 days
Mature tree responses
Twice ambient concentrations of ozone reduced leaf net pho-tosynthesis of mature trees on average by 50% during the three seasons of ozone exposure (Hanson et al. 1994, Wullschleger et al. 1996). These declines in carbon assimilation affected foliar carbon retention and carbohydrate partitioning in leaves and branches of mature trees. A trend (P = 0.139) toward a linear increase in foliar carbon retention was observed 5 days after exposure to 14C, and at 7 days a significant (P = 0.060) linear increase in foliar carbon retention was detected in re-sponse to ozone treatment (Figure 2). Retention values in leaves exposed to ambient ozone concentrations were similar to foliar 14C retention values reported for lower canopy leaves of mature Quercus alba L. trees (McLaughlin and McConathy 1979).
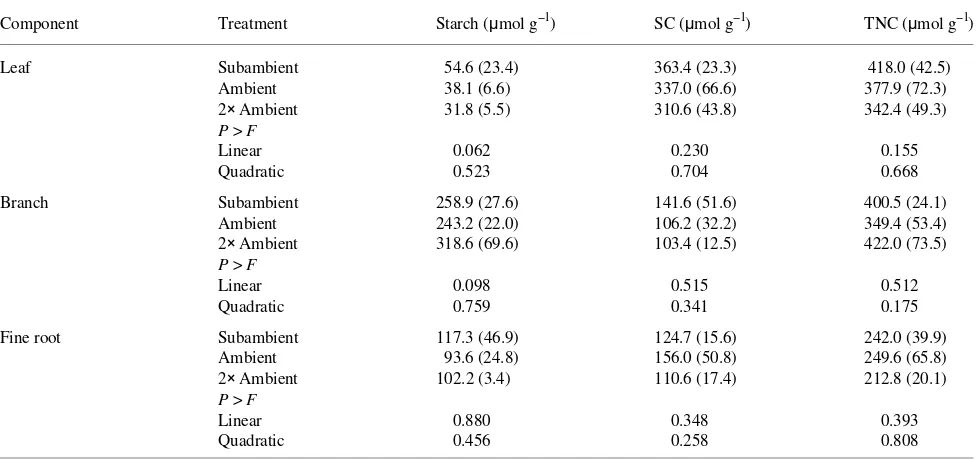
Reduced foliar starch and increased branch starch concen-trations indicated an influence of ozone on the partitioning of carbon in branch and leaf tissues (Table 2). Similarly, Smeulders et al. (1996) reported concurrent reductions in pho-tosynthesis, translocation of assimilates from needles to
branches, and foliar starch concentrations in 25-year-old Pseudotsuga menziesii branches exposed to ozone. Likewise, increasing ozone concentrations reduced foliar starch concen-trations in Pinus taeda seedlings (Meier et al. 1990, Friend et al. 1992), hybrid Populus cuttings (Buckner and Ballach 1992), and Pinus echinata seedlings (Paynter et al. 1991). Decreased starch partitioning may result from impaired photo-synthesis, increased partitioning to repair compounds (Friend and Tomlinson 1992), impeded starch hydrolysis (Hanson and Stewart 1970), or elevated respirational demands (Skarby et al. 1987). Because Wullschleger et al. (1996) observed no influ-ence of ozone on construction or maintenance respiration in mature tree leaves during the third year of exposure, elevated foliar respiration was not likely the cause of reduced starch partitioning in leaves.
Carbon retention in shoots and shifts in carbon partitioning from storage carbohydrates such as starch to soluble carbohy-drates and carbon compounds involved in repair are believed to be compensatory responses to maintain photosynthetic rates in plants stressed by ozone exposure (Pell et al. 1994). The Table 1. Starch, soluble carbohydrate (SC) and total nonstructural carbohydrate (TNC) concentrations in four-year-old northern red oak seedlings exposed to a subambient, ambient or twice ambient ozone concentration. Carbohydrate concentrations were measured on September 23, 1994 during the third season of ozone exposure. Standard deviations are indicated in parentheses (n = 3).
Component Treatment Starch (µmol g--1) SC (µmol g--1) TNC (µmol g--1)
Leaf Subambient 91.4 (5.8) 255.4 (18.8) 346.9 (24.6)
Ambient 107.7 (30.4) 276.6 (20.6) 384.3 (16.7)
2× Ambient 66.0 (30.3) 244.3 (30.5) 310.2 (60.8)
P > F
Linear 0.246 0.352 0.191
Quadratic 0.842 0.287 0.204
Branch Subambient 356.1 (102.1) 135.5 (26.0) 491.6 (118.0)
Ambient 335.7 (37.0) 137.0 (16.5) 472.6 (20.5)
2× Ambient 321.5 (36.2) 127.6 (9.3) 449.1 (32.5)
P > F
Linear 0.891 0.560 0.709
Quadratic 0.736 0.764 0.721
Stem Subambient 340.7 (56.2) 104.9 (20.1) 445.6 (75.3)
Ambient 408.8 (33.1) 106.4 (11.4) 515.2 (39.1)
2× Ambient 408.2 (22.0) 105.5 (25.3) 513.0 (13.3)
P > F
Linear 0.208 0.763 0.255
Quadratic 0.197 0.821 0.254
Coarse root Subambient 1088.8 (142.0) 137.9 (14.0) 1265.8 (181.7)
Ambient 972.2 (281.3) 127.5 (20.4) 1099.7 (266.0)
2× Ambient 1023.1 (251.9) 110.8 (21.8) 1215.8 (102.9)
P > F
Linear 0.284 0.099 0.782
Quadratic 0.727 0.908 0.220
Fine root Subambient 177.9 (47.6) 113.1 (6.1) 291.0 (48.4)
Ambient 168.1 (50.0) 111.5 (10.7) 279.6 (58.5)
2× Ambient 142.0 (44.1) 102.0 (7.4) 244.0 (36.8)
P > F
Linear 0.290 0.062 0.222
inability of mature northern red oak trees to maintain photo-synthetic rates when exposed to higher ozone concentrations indicates that, at the foliar level, trees were unable to compen-sate fully for ozone damage to photosynthetic processes. How-ever, at the whole-plant level, no reductions in foliar biomass and stem diameter growth below and within the crown were observed after three seasons of ozone treatment (Samuelson et al. 1996). Thus, some degree of compensation likely occurred because carbohydrate pools rather than aboveground growth were affected by ozone. Although not studied during the third season, reductions in fine root biomass of mature trees exposed to twice ambient ozone concentrations during the second growing season (Kelting et al. 1995) suggest that belowground processes may be sensitive to changes in carbon metabolism.
Tree versus seedling responses
Ozone exposure reduced leaf photosynthesis (Samuelson and Edwards 1993, Hanson et al. 1994, Wullschleger et al. 1996) and foliar starch partitioning, and increased carbon retention in leaves and starch partitioning in branches of mature trees. In contrast, seedlings responded to the same external ozone expo-sure with little reduction in leaf photosynthesis (Samuelson and Edwards 1993, Hanson et al. 1994, Wullschleger et al. 1996), diminished soluble carbohydrate concentrations in roots and no significant changes in carbon retention, and foliar and branch carbohydrate partitioning. Seedling biomass, di-ameter and height were not reduced after three seasons of ozone exposure (Samuelson et al. 1996).
During two growing seasons in a subambient ozone concen-tration, net photosynthesis and leaf conductance were more than twofold higher in mature trees than in seedlings (Hanson et al. 1994). Foliar nitrogen concentrations were also higher in
mature trees than in seedlings, although no differences in soil nutrient concentrations between seedlings and mature trees were detected (Samuelson et al. 1996). The cause of greater leaf conductance in larger northern red oak trees is central to our understanding of differences in ozone uptake, dose and subsequent response in northern red oak. We propose that an ozone-induced increase in demand for carbohydrates may un-derlie the higher foliar gas exchange rates in mature northern red oak trees. Differences in foliar retention of carbon and partitioning of starch between seedlings and mature trees ex-posed to subambient ozone concentrations suggest that carbon demands are proportionately greater and foliar carbon reserves are smaller in larger northern red oak trees. For example, foliar retention of carbon was approximately 45% greater in mature trees than in seedlings one day after initial exposure to 14C (Figure 2). Yet, carbon retention was similar between seedlings and trees five and seven days after exposure, indicating a greater loss of carbon to respiration or transport in tree leaves than in seedling leaves. In addition, foliar starch concentra-tions were 40% lower in trees than in seedlings (Tables 1 and 2). Although these differences were not tested statistically, greater reduction in initial 14C fixed and lower starch partition-ing in leaves of mature trees compared with seedlpartition-ings suggest a greater demand for recently fixed carbon and less partition-ing of carbon into storage in leaves of mature trees. Mature tree characteristics such as a large respiratory mass, flowering and fruiting, and complex root and canopy systems may increase the number and strength of carbon sinks. In this study, mature trees produced acorns in each of the three growing seasons.
In summary, changes in carbon allocation and partitioning in branches and leaves of mature northern oak trees indicate the existence of compensatory responses that may postpone Table 2. Starch, soluble carbohydrate (SC) and total nonstructural carbohydrate (TNC) concentrations in 32-year-old northern red oak trees exposed to a subambient, ambient or twice ambient ozone concentration. Carbohydrate concentrations were measured on September 23, 1994 during the third season of ozone exposure. Standard deviations are indicated in parentheses (n = 3).
Component Treatment Starch (µmol g--1) SC (µmol g--1) TNC (µmol g--1)
Leaf Subambient 54.6 (23.4) 363.4 (23.3) 418.0 (42.5)
Ambient 38.1 (6.6) 337.0 (66.6) 377.9 (72.3)
2× Ambient 31.8 (5.5) 310.6 (43.8) 342.4 (49.3)
P > F
Linear 0.062 0.230 0.155
Quadratic 0.523 0.704 0.668
Branch Subambient 258.9 (27.6) 141.6 (51.6) 400.5 (24.1)
Ambient 243.2 (22.0) 106.2 (32.2) 349.4 (53.4)
2× Ambient 318.6 (69.6) 103.4 (12.5) 422.0 (73.5)
P > F
Linear 0.098 0.515 0.512
Quadratic 0.759 0.341 0.175
Fine root Subambient 117.3 (46.9) 124.7 (15.6) 242.0 (39.9)
Ambient 93.6 (24.8) 156.0 (50.8) 249.6 (65.8)
2× Ambient 102.2 (3.4) 110.6 (17.4) 212.8 (20.1)
P > F
Linear 0.880 0.348 0.393
reductions in aboveground growth in response to ozone stress. The impact of these changes on root and canopy health over the long term is unknown, but it is likely that the response of northern red oak to future increases in ambient ozone concen-tration will depend on tree age and size.
Acknowledgments
Funding for this project was provided by the Tennessee Valley Author-ity and the Electric Power Research Institute. The authors thank the Environmental Protection Agency for carbohydrate analyses. M.H. Wolfe and P.A. Mays provided valuable assistance in data collection and analyses. The authors gratefully acknowledge the aid of E.G. O’Neill at Oak Ridge National Laboratory for help in methodology and use of equipment. Alabama Agricultural Experiment Station Jour-nal article No. 9--965189
References
Adams, M.B., N.T. Edwards and G.E. Taylor, Jr. 1990. Whole-plant 14C-photosynthate allocation in Pinus taeda: seasonal patterns at
ambient and elevated ozone concentrations. Can. J. For. Res. 20:152--158.
Buckner, J. and H.-J. Ballach. 1992. Alterations in carbohydrate con-centrations in leaves of Populus due to ambient air pollution. Physiol. Plant. 86:512--517.
Dickson, R.E. and J.G. Isebrands. 1993. Carbon allocation terminol-ogy: should it be more rational? Bull. Ecol. Soc. Am. 74:175--177. Dickson, R.E., J.G. Isebrands and P.T. Tomlinson. 1990. Distribution and metabolism of current photosynthate by single-flush northern red oak seedlings. Tree Physiol. 7:65--77.
Edwards, G.S., A.L. Friend, E.G. O’Neill and P.T. Tomlinson. 1992. Seasonal patterns of biomass accumulation and carbon allocation in Pinus taeda seedlings exposed to ozone, acidic precipitation, and reduced soil Mg. Can. J. For. Res. 22:640--646.
Friend, A.L. and P.T. Tomlinson. 1992. Mild ozone exposure alters 14C dynamics in foliage of Pinus taeda L. Tree Physiol. 11:215--227. Friend, A.L., P.T. Tomlinson, R.E. Dickson, E.G. O’Neill, N.T.
Ed-wards, and G.E. Taylor, Jr. 1992. Biochemical composition of loblolly pine reflects pollutant exposure. Tree Physiol. 11:35--47. Gorissen, A., G.C. Schelling and J.A. van Veen. 1991.
Concentration-dependent effects of ozone on translocation of assimilates in Douglas-fir. J. Environ. Qual. 20:169--173.
Grulke, N.E. and P.R. Miller.1994. Gas exchange characteristics from seedlings to mature trees in giant sequoia: implications for response to current and future concentrations of atmospheric ozone. Tree Physiol. 14:659--668.
Hanson, G.P. and W.S. Stewart. 1970. Photochemical oxidants: effect on starch hydrolysis in leaves. Science 168:1223--1224.
Hanson, P.J., L.J. Samuelson, S.D. Wullschleger, T.A. Tabberer and G.S. Edwards. 1994. Seasonal patterns of light-saturated photosyn-thesis and leaf conductance for mature and seedling Quercus rubra L. foliage: differential sensitivity to ozone exposure. Tree Physiol. 14:1351--1366.
Kelting, D.L., J.A. Burge and G.S. Edwards. 1995. Ozone effects on red oak root dynamics: seedlings versus mature tree comparisons. For. Ecol. Manage. 79:197--206.
Kuhns, M.R. and D.H. Gjerstad. 1988. Photosynthate allocation in loblolly pine (Pinus taeda) seedlings as affected by moisture stress. Can. J. For. Res. 11:145--154.
Laurence, J.A., R.G. Amundson, A.L. Friend, E.J. Pell and P.J. Tem-ple. 1994. Allocation of carbon in plants under stress: an analysis of the ROPIS experiments. J. Environ. Qual. 23:412--417.
Matyssek, R., P. Reich, R. Oren and W.E. Winner. 1995. Response mechanisms of conifers to air pollutants. In Ecophysiology of Coniferous Forests. Eds. W.K. Smith and T.M. Hinckley, Academic Press, New York, pp 255--308.
McLaughlin, S.B. and R.K. McConathy. 1979. Temporal and spatial patterns of carbon allocation in the canopy of white oak. Can. J. Bot. 57:1407--1413.
Meier, S., L.F. Grand, M.M. Schoeneberger, R.A. Reinert and R.I. Bruck. 1990. Growth, ectomycorrhizae and nonstructural carbohy-drates of loblolly pine seedlings exposed to ozone and soil water deficit. Environ. Pollut. 64:11-- 27.
Paynter, V.A., J.C. Reardon and V.B. Shelburne. 1991. Carbohydrate changes in shortleaf pine (Pinus echinata) needles exposed to acid rain and ozone . Can. J. For. Res. 21:666--671.
Paynter, V.A., J.C. Reardon and V.B. Shelburne. 1992. Changing carbohydrate profiles in shortleaf pine (Pinus echinata) after pro-longed exposure to acid rain and ozone. Can. J. For. Res. 22:1556--1561.
Pell, E.J., P.J. Temple, A.L. Friend, H.A. Mooney and W.E. Winner. 1994. Compensation as a plant response to ozone and associated stresses: an analysis of ROPIS experiments. J. Environ. Qual. 23:429--436.
Samuelson, L.J. 1994. The role of microclimate in determining the sensitivity of Quercus rubra L. to ozone. New Phytol. 128:235--241. Samuelson, L.J. and G.S. Edwards. 1993. A comparison of sensitivity to ozone in seedlings and trees of Quercus rubra L. New Phytol. 125: 373--379.
Samuelson, L.J., J.M. Kelly, P.A. Mays and G.S. Edwards. 1996. Growth and nutrition of Quercus rubra L. seedlings and mature trees after three seasons of ozone exposure. Environ. Pollut. 191:317--332.
Skarby, L., E. Troeng and C.A. Bostrom. 1987. Ozone uptake and effects on transpiration, net photosynthesis, and dark respiration in Scots pine. For. Sci. 33:801--808.
Smeulders, S.M., A. Gorissen, N.N. Joosten and J.A. Veen. 1995. Effects of short-term ozone exposure on the carbon economy of mature and juvenile Douglas-firs (Pseudotsuga menziesii (Mirb.) Franco). New Phytol. 129:45-- 53.
Spence, R.D., E.J. Rykiel and P.J.H. Sharpe. 1990. Ozone alters carbon allocation in loblolly pine: assessment with carbon-11 label-ing. Environ. Pollut. 64:93--106.
Temple, P.J., G.H. Reichers, P.R. Miller and R.W. Lennox. 1993. Growth responses of ponderosa pine to long-term exposure to ozone, wet and dry acidic deposition, and drought. Can. J. For. Res. 23:59--66.
Ursino, D.J. and J. Paul. 1973. The long-term fate and distribution of 14C photoassimilated by young white pines in late summer. Can. J.
Bot. 51:683--687.
Wilson, R., A. Cataldo and C.P. Andersen. 1995. Determination of total nonstructural carbohydrates in tree species by high-perform-ance anion- exchange chromatography with pulsed ampecometric detection. Can. J. For. Res. 25:2022--2028.