Summary The terminal (1-year-old) shoot of quiescent, two-year-old balsam fir (Abies balsamea (L.) Mill.) seedlings was ringed with lanolin containing 0, 1 or 10 mg g−1 Ethrel, an ethylene-generating compound, and cultured for 6 weeks under environmental conditions favorable for growth. Bud break and the elongation of the current-year terminal shoot were moni-tored, and the subjacent previous-year terminal shoot that had been treated with Ethrel was harvested to measure stem radial growth by microscopy, shoot ethylene evolution by gas chro-matography, and cambial region indole-3-acetic acid (IAA) concentration by combined gas chromatography--mass spec-trometry. Compared with the lanolin controls, Ethrel at 1 and 10 mg g−1 did not affect bud break or longitudinal growth, but stimulated tracheid production and bark increment up to about 2-fold at the application site, though not above or below it. In addition, the 1 and 10 mg g−1 Ethrel treatments increased the cambial region IAA concentration about 3-fold and the evolu-tion of ethylene at least 40-fold at the applicaevolu-tion site, com-pared with unwounded portions of both treated and control shoots. The 10 mg g−1 Ethrel treatment also stimulated ethylene evolution about 10-fold, both above and below the application site. However, this stimulation was not associated with an elevation in cambial region IAA concentration. Similarly, the lanolin control treatment increased ethylene evolution at the application site about 10-fold, without affecting the cambial region IAA concentration. Our results suggest that the local-ized stimulation of radial growth in woody shoots ringed with Ethrel is mediated by an increase in IAA concentration, which in turn is induced by a threshold, abnormally high concentra-tion of Ethrel-derived ethylene.
Keywords: balsam fir, bark, 2-chloroethylphosphonic acid, cortex, ethylene, tracheid, vascular cambium, xylem.
Introduction
Radial stem growth of both conifers and woody angiosperms is stimulated locally by ringing with 2-chloroethylphosphonic acid (Ethrel), an ethylene generator (Little and Savidge 1987, Savidge 1988, Eklund and Little 1995). The stimulation is manifested in both increased cambial activity, as measured by xylem production, and a greater thickness of bark, due primar-ily to an increase in the width of the cortex. The Ethrel effect
is assumed to be mediated by ethylene, but it is not known whether ethylene acts directly. A direct effect of ethylene on cambial activity is supported by the finding that evolution of ethylene by the stem is higher when the cambium is growing than when the cambium is dormant (Eklund 1990, 1993b, Ingemarsson et al. 1991b). Telewski (1990) observed that, in Pinus taeda L., stem diameter was positively correlated with ethylene evolution per unit cambial surface area in seedlings subjected to mechanical stress, and negatively correlated with ethylene evolution per unit fresh weight in unstressed seed-lings. Furthermore, considerable evidence suggests that ethyl-ene plays a role in the control of xylem differentiation, both by inducing the activity of enzymes involved in lignification (Miller et al. 1984, 1985, Abeles et al. 1989, Hennion et al. 1992) and by affecting polysaccharide deposition during wall formation (Eklund 1991, Ingemarsson et al. 1991a). Alterna-tively, Ethrel-derived ethylene may indirectly affect cambial growth by interacting with other endogenous phytohormones, in particular indole-3-acetic acid (IAA), a well established promoter of xylem production (Little and Pharis 1995).
Evidence of an ethylene--IAA interaction is provided by two findings: first, that exogenous IAA promotes the conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene by cam-bial region tissue excised from Abies balsamea (L.) Mill. stems (Savidge 1988); and, second, that Ethrel stimulates tracheid production in A. balsamea cuttings only when applied below a source of endogenous or exogenous IAA (Eklund and Little 1995). Eklund and Little (1995) found that Ethrel promotes IAA-induced cambial growth if applied in a ring of lanolin near the midpoint of the cuttings, but not when fed in aqueous solution through the basal cut surface. Consequently, they hypothesized that laterally applied Ethrel induces an abnor-mally high ethylene concentration locally, which indirectly promotes radial growth by causing IAA to accumulate. How-ever, whether ringing a woody stem with Ethrel actually in-creases the cambial region concentration of IAA has not been investigated, which fact prompted the work reported here.
Materials and methods
Plant material, Ethrel application, and culture conditions
Two-year-old balsam fir (Abies balsamea) seedlings, about
Laterally applied Ethrel causes local increases in radial growth and
indole-3-acetic acid concentration in
Abies balsamea
shoots
LEIF EKLUND
1and C. H. ANTHONY LITTLE
21 Department of Engineering and Natural Sciences, Växjö University, S-351 95 Växjö, Sweden 2
Natural Resources Canada, Canadian Forest Service, P.O. Box 4000, Fredericton, New Brunswick E3B 5P7, Canada
Received April 3, 1995
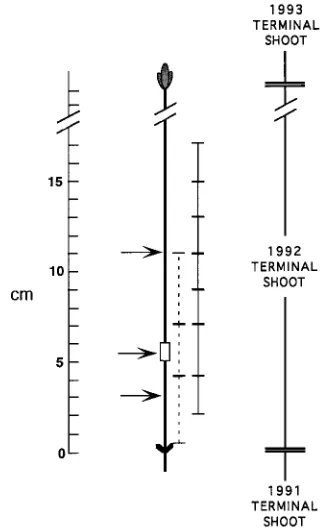
30-cm tall, were lifted from a nursery in Fredericton, New Brunswick, Canada, in November 1992, when they were in the quiescence stage of dormancy. After storage at −5 °C until January 1993, they were thawed, planted in 3-dm3 pots con-taining peat and perlite (2/1, v/v) and placed in a greenhouse. The greenhouse had a day/night temperature of 22/15 °C, a photon flux density of 600 µmol m−2 s−1 on sunny days, and a photoperiod extended to 16 h with 400-W high-pressure so-dium lamps (Sylvania) yielding a photon flux density at plant height of 30 µmol m−2 s−1. The day after planting, three groups of 10 seedlings were selected such that the average length of the 1992 terminal shoot (denoted 1-year-old shoot) was the same for each group. The axillary buds on each 1992 terminal shoot were removed with a scalpel and the shoots were ringed with lanolin containing 0, 1 or 10 mg Ethrel g−1, which was applied in a 1-cm wide band around the stem circumference, i.e., laterally, after removing the needles and periderm with a scalpel (Figure 1). About 0.5 g of lanolin was applied per seedling, after which the application site was covered with aluminum foil. The seedlings were cultured for up to 6 weeks, during which period they were watered and fertilized, as re-quired, to preventdrought and mineral deficiency. Ethylene evolution from the 1992 terminal shoot and bud break in the apical whorl on the 1992 terminal shoot were monitored. A bud was scored broken when needlespierced the sheath of bud scales. At the end of the 6-week experimental period, when current-year shoot elongation had ceased but early wood tra-cheids were still being formed, the length of the current-year
terminal shoot (denoted 1993 terminalshoot) was recorded, and the 1992 terminal shoot was harvested to measure radial growth, ethyleneevolution and IAA concentration.
Measurement of radial growth
Radial growth was measured both as bark radial width and as the number of tracheids per radial file produced by the vascular cambium during the experimental period (denoted 1993 tra-cheid number). The measurements were made at eight equidis-tant points around the circumference of transverse, handcut sections obtained at the midpoint of the Ethrel application site, as well as 5 cm above and 2 cm below the application site (Figure 1). Thesections were stained with an aqueous solution of phloroglucinol in 20%hydrochloric acid and mounted in glycerol. The number of 1993 tracheids per radial file was counted starting from the last-formed tracheid in the late wood of the 1992 annual ring. The measurement included only cells that reacted with the stain, i.e., cells in which lignification had begun.
Measurement of ethylene evolution
Ethylene evolution was measured in a 3-cm segment including the Ethrel application site, as well as in 2-cm segments located above and below the application site (Figure 1). Immediately after harvest, the lanolin was wiped from the application site and the segments placed individually in 8.5-ml glass vials sealed with a rubber stopper that was known not to release ethylene. After incubation at room temperature for 30 min, a period chosen to avoid the production of stress ethylene (Yamanaka 1986, Morgan et al. 1990, L. Eklund unpublished results), a 1-ml sample of air was drawn from the head space with a gas-tight syringe and injected into a Varian 3400 gas chromatograph (Varian Canada, Ltd., Mississauga, Ontario) equipped with a flame ionization detector and a 2-m stainless steel column packed with 80 mesh Al2O3. The N2 carrier gas flowed at 45 ml min−1 and the temperatures of the injector, column and detector were 200, 65 and 250 °C, respectively, giving a retention time for ethylene of 1.5 min. Ethylene concentration was calculated on a fresh weight basis using a 1 ppm ethylene standard (Valley Oxygen, Fredericton, Can-ada) after measuring and subtracting the volume of the shoot segment from the vial volume and determining the segment’s fresh weight.
Measurement of IAA
The IAA concentration in cambial region tissue was measured in a 3-cm segment containing the Ethrel application site, as well as in 4-cm segments located immediately above and below the application site (Figure 1). For each segment, the bark was peeled and both exposed surfaces were scraped with a scalpel, providing differentiating xylem and cambium plus phloem tissues, which were accumulated on ice. The tissue scrapings for each segment were pooled, weighed, immersed in ice-cold 70% methanol containing 0.01 M butylated hy-droxytoluene, and stirred vigorously. The interval between excising the segment and placing the scrapings in 70% metha-nol was about 1 min. After adding [13C6]-IAA to serve as an
internal standard for quantitation of endogenous IAA, the samples were stored at −80 °C.
After pooling the tissues for a particular segment from three trees, IAA was purified and measured as described by Savidge (1990), exceptthat the methylation step was omitted. The IAA concentration was determined bycombined gas chromatogra-phy--mass spectrometry (Hewlet-Packard Canada, Ltd., Mis-sissauga, Ontario, Models 5890 and 5970, respectively) selected ion monitoring of the trimethylsilyl derivatives ofIAA and [13C6]IAA, focusing on the fragments m/z 202 and 208, respectively. TheIAA concentration was calculated from the ratio of the peak areas, after correcting for mass detector response. It was expressed as a concentration on the basis of both freshweight and dry weight, the latter determined after oven-drying the extracted sample at 70 °Cfor 24 h.
Statistical analysis
Analysis of variance was applied to each data set, with tree as a randomreplication. The significance (P≤ 0.05) of the differ-ence between means wasdetermined with Fisher’s Least Sig-nificant Difference test.
Results
In seedlings treated with lanolin containing 0 mg Ethrel g−1 (denoted lanolin-treated control), bud break occurred about two weeks after placement in the greenhouse and subsequent shoot and needle elongation progressed normally. At the end
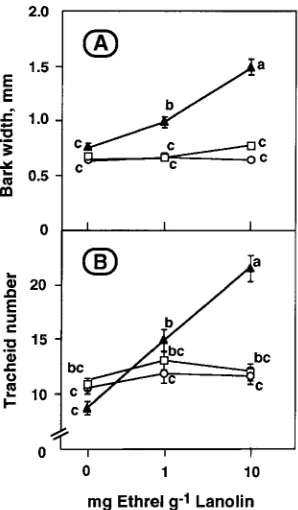
of the 6-week experimental period, bark radial growth and tracheid number in these seedlings did not vary along the length of the 1992 terminal shoot (Figure 2). The application of 1 or 10 mg Ethrel g−1 lanolin did not affect the rate or degree of bud break, the elongation of the 1993 terminal shoot (data not shown) or radial growth above and below the application site (Figure 2). However, the width of bark and the number of tracheids produced at the application site were increased by as much as 2-fold, the extent of the increase being positively related to Ethrel concentration. The increase in bark width was evident in the cortex.
At the end of the 6-week experimental period, ethylene evolution along the length of the 1992 terminal shoot in the lanolin-treated control seedlings was constant and similar to that in untreated seedlings (data not shown), except for about a 10-fold increase at the lanolin application site (Figure 3). This localized increase is attributed to wound ethylene (Abeles et al. 1992) produced as a consequence of removing the need-les and periderm. The 1 and 10 mg g−1 Ethrel treatments stimulated ethylene evolution at the application site by about 4- and 8-fold, respectively, compared with the lanolin-treated control, and about 40 and 80-fold, respectively, compared with the unwounded portions of lanolin-treated control shoots. The 10 mg g−1 Ethrel treatment also increased ethylene evolution about 10-fold both above and below the application site. Meas-urements made earlier in the experimental period indicated that Ethrel-stimulated ethylene evolution was evident one day after treatment and that the degree of stimulation measured subsequently did not vary significantly (data not shown).
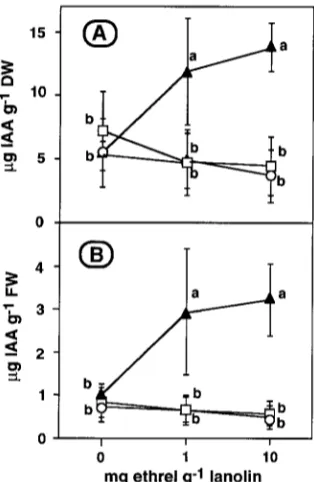
On both a dry and a fresh-weight basis, the concentration of IAA in the cambial region tissues of the lanolin-treated control seedlings was the same at the application site as above and below it (Figure 4), indicating that the wounding associated with the removal of the needles and periderm did not affect IAA concentration. In contrast, both Ethrel treatments raised the IAA concentration at the application site about 3-fold.
Figure 2. Bark radial width (A) and tracheid number (B) in the 1992 terminal shoot measured above (s), at (m) and below (h) the point
where 0, 1 or 10 mg Ethrel g−1 lanolin was applied laterally in a ring for 6 weeks. Mean ± SE, n =10. Means accompanied by the same letter are not significantly different at P ≤ 0.05.
Figure 3. Ethylene evolution from segments of the 1992 terminal shoot obtained above, at and below the point where 0 (s), 1 (m) or 10 (h)
mg Ethrel g−1 lanolin was applied laterally in a ring for 6 weeks. The thick horizontal bar indicates the Ethrel application point. Mean ± SE,
However, neither treatment altered the IAA concentration above or below the application site.
Discussion
Lateral application of Ethrel in lanolin stimulated ethylene evolution from the 1-year-old terminal shoot of Abies bal-samea seedlings (Figure 3), as observed in comparable experi-ments with attached shoots of Malus domestica Borkh. cv Winesap (Robitaille and Leopold 1974) and Pinus densiflora Siebold et Zucc. (Yamamoto and Kozlowski 1987) and cut-tings of Abies balsamea (Eklund and Little 1995). The Ethrel-induced stimulation occurred throughout the experimental period and was much greater at the application site than above or below it. Our finding that the 10 mg g−1 Ethrel concentration increased ethylene evolution acropetally and basipetally (Fig-ure 3) indicates that Ethrel, and probably ethylene as well (Eklund and Little 1995), passed through the cortex at the application site into the phloem and xylem, where it was transported (Foster et al. 1992, Eklund 1993a) to the cambial region both locally and at a distance. Thus, increased ethylene evolution from a shoot segment presumably reflected elevation of the ethylene concentration in that segment’s cambial region (see also Eklund and Little 1995), although the exact relation-ship needs to be determined.
The lateral application of Ethrel also stimulated both tra-cheid production and bark increment (Figure 2), as reported
previously for shoots of Abies balsamea and several other woody species (Little and Savidge 1987, Savidge 1988, Little and Eklund 1995). Radial growth was enhanced at the applica-tion site of both 1 and 10 mg g−1 Ethrel, where concomitantly there was about a 3-fold increase in IAA concentration and a 40- and 80-fold increase in ethylene evolution, respectively, compared with unwounded portions of lanolin-treated control shoots (Figures 2, 3 and 4). However, there was no radial growth enhancement above or below the sites of application of 1 or 10 mg g−1 Ethrel, or at the application site of lanolin without Ethrel, where the IAA concentration was unchanged and where ethylene evolution was stimulated no more than about 10-fold either by Ethrel or by wounding.
Previously, we observed that Ethrel increased tracheid pro-duction in Abies balsamea cuttings if applied in a lanolin ring near the midpoint, but not when fed basally or apically through a cut surface, even though all three methods of application markedly promoted ethylene evolution (Eklund and Little 1995). It was also found that exogenous IAA concentration, ethylene evolution and tracheid production were not consis-tently related, and neither Co2+, which decreases ethylene evolution, nor Ag+, an inhibitor of ethylene action, inhibited IAA-induced tracheid production (Eklund and Little 1995). Other research has shown that raising the cambial region IAA concentration with exogenous IAA or gibberellin A4/7 pro-motes tracheid production (Sundberg and Little 1990, Wang et al. 1992), and that bark radial width, particularly cortex incre-ment, is increased in the immediate vicinity of an exogenous IAA source (Little et al. 1990), where the internal IAA concen-tration is presumably elevated. We conclude that ringing with Ethrel promotes radial growth in shoots by elevating their IAA concentration, which occurs when the Ethrel-derived ethylene concentration rises above a threshold value. This threshold is assumed to be abnormally high, because it apparently exceeds even the elevated ethylene concentration that is induced in a shoot after it has been wounded.
Ethrel-generated ethylene may increase the IAA concentra-tion in the cambial region by either stimulating IAA biosyn-thesis, reducing IAA conjugation or catabolism, or inhibiting IAA transport. A stimulatory effect of ethylene on IAA biosyn-thesis seems unlikely, because Ethrel application did not affect the growth of the current-year shoots, the major source of IAA for the cambial region of one-year-old shoots (Sundberg and Little 1987, 1990), nor did it raise the IAA concentration above the application site (Figure 4). Furthermore, ethylene treat-ment has been observed to increase, rather than decrease, IAA conjugation and catabolism (Sagee et al. 1990). However, an inhibitory effect of ethylene on basipetal IAA transport is well documented, at least in the stems of herbaceous species and the midrib of leaves (Suttle 1988). Thus, we speculate that when laterally applied Ethrel increases the ethylene concentration above a threshold value, the basipetal IAA movement that occurs in the cambial region (Little and Pharis 1995) is inhib-ited, thereby increasing the IAA concentration locally. If so, the inhibitory effect is presumably small and cumulative, be-cause the IAA concentration below the Ethrel application site was not reduced (Figure 4). In addition, the finding that the 10
mg g−1 Ethrel treatment, compared with the 1 mg g−1 treat-ment, increased the evolution of ethylene, but not the concen-tration of IAA, at the application site (Figures 3 and 4), suggests that there is a second, higher threshold concentration of ethylene, above which not only IAA transport is inhibited, but also IAA conjugation or catabolism, or both, are enhanced. However, whether laterally applied Ethrel affects IAA move-ment and metabolism in shoots of woody species remains to be demonstrated.
Acknowledgments
We thank Dr. R.A. Savidge for the use of a GC--MS and Rhône-Poulenc Canada Ltd. for a gift of Ethrel. Financial support for L. Eklundwas provided by the Swedish Council for Forestry and Agri-cultural Research,Stockholm, Sweden.
References
Abeles, F.B., C.L. Biles and L.J. Dunn. 1989. Hormonal regulation and distribution of peroxidase isoenzymes in the Cucurbitaceae. Plant Physiol. 91:1609--1612.
Abeles, F.B., P.W. Morgan and M.E. Saltveit. 1992. Ethylene in plant biology. 2nd Edn. Academic Press, San Diego, California, 414 p. Eklund, L. 1990. Endogenous levels of oxygen, carbon dioxide and
ethylene in stems of Norway spruce trees during one growing season. Trees 4:150--154.
Eklund, L. 1991. Relations between indoleacetic acid, calcium ions and ethylene in the regulation of growth and cell wall composition in Picea abies. J. Exp. Bot. 42:785--789.
Eklund, L. 1993a. Movement and possible metabolism of ethylene in dormant Piceaabies. Plant Growth Regul. 12:37--41.
Eklund, L. 1993b. Seasonal variations of O2,CO2, and ethylene in oak
and maple stems. Can. J. For. Res. 23:2608--2610.
Eklund, L. and C.H.A. Little. 1995. Interaction between indole-3-ace-tic acid and ethylene in the control of tracheid production in de-tached shoots of Abies balsamea. Tree Physiol. 15:27--34. Foster, K.R., D.M. Reid and R.P. Pharis. 1992. Ethylene biosynthesis
and ethephon metabolism and transport in barley. Crop Sci. 32:1345--1352.
Hennion, S., C.H.A. Little and C. Hartmann. 1992. Activities of enzymes involved in lignification during the postharvest storage of etiolated asparagus spears. Physiol. Plant. 86:474--478.
Ingemarsson, B.S.M., L. Eklund, and L. Eliasson, 1991a. Ethylene effects on cambial activity and cell wall formation in hypocotyls of
Picea abies seedlings. Physiol. Plant. 82:219--224.
Ingemarsson, B.S.M., E. Lundqvist and L. Eliasson. 1991b. Seasonal variation in ethylene concentration in the wood of Pinus sylvestris
L. Tree Physiol. 8:273--279.
Little, C.H.A. and R.P. Pharis. 1995. Hormonal control of radial and longitudinal growth in the tree stem. In Plant Stems: Physiology and Functional Morphology. Ed. B.L. Gartner. Academic Press, San Diego, CA, pp 281--319.
Little, C.H.A. and R.A. Savidge. 1987. The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul. 6:137--169.
Little, C.H.A., B. Sundberg and A. Ericsson. 1990. Induction of acropetal 14C-photosynthate transport and radial growth by indole-3-acetic acid in Pinus sylvestris shoots. Tree Physiol. 6:177--189. Miller, A.R., W.L. Pengelly and L.W. Roberts. 1984. Induction of
xylem differentiation in Lactuca by ethylene. Plant Physiol. 75:1165--1166.
Miller, A.R., D.L. Crawford and L.W. Roberts. 1985. Lignification and xylogenesis in Lactuca pith explants cultured in vitro in the presence of auxin and cytokinin: a role for endogenous ethylene. J. Exp. Bot. 36:110--118.
Morgan, P.W., C.-J. He, J.A. De Greef and M.P. De Proft. 1990. Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol. 94:1616--1624.
Robitaille, H.A. and A.C. Leopold. 1974. Ethylene and the regulation of apple stem growth under stress. Physiol. Plant. 32:301--304. Sagee, O., J. Riov and R. Goren. 1990. Ethylene-enhanced catabolism
of [14C]indole-3-acetic acid to indole-3-carboxylic acid in citrus leaf tissues. Plant Physiol. 92:54--60.
Savidge, R.A. 1988. Auxin and ethylene regulation of diameter growth in trees. Tree Physiol. 4:401--414.
Savidge, R.A. 1990. Characterization of indol-3-ylacetic acid in devel-oping secondary xylem of 26 Canadian species by combined gas chromatography-mass spectrometry. Can. J. Bot 68:521--523. Sundberg, B. and C.H.A. Little. 1987. Effect of defoliation on tracheid
production and the level of indole-3-acetic acid in Abiesbalsamea
shoots. Physiol. Plant. 71:430--435.
Sundberg, B. and C.H.A. Little. 1990. Tracheid production in response to changes in the internal level of indole-3-acetic acid in 1-year-old shoots of Scots pine. Plant Physiol. 94:1721--1727.
Suttle, J.C. 1988. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epi-cotyls. Plant Physiol. 88:795--799.
Telewski, F.W. 1990. Growth, wood density, and ethylene production in response to mechanical perturbation in Pinus taeda. Can. J. For. Res. 20:1277--1282.
Wang, Q., C.H.A. Little, C. Sheng, P.C. Odén and R.P. Pharis. 1992. Effect of exogenous gibberellin A4/7 on tracheid production, longi-tudinal growth and the levels of indole-3-acetic acid and gibberel-lins A4, A7 and A9 in the terminal shoot of Pinussylvestris seedlings.
Physiol. Plant. 86:202--208.
Yamamoto, F. and T.T. Kozlowski. 1987. Effects of flooding, tilting of stems, and ethrel application on growth, stem anatomy and ethylene production of Pinus densiflora seedlings. J. Exp. Bot. 38:293--310. Yamanaka, K. 1986. Wound ethylene production by phloem and