L
Journal of Experimental Marine Biology and Ecology 242 (1999) 1–20
www.elsevier.nl / locate / jembe
The effects of polycyclic aromatic hydrocarbon
contamination and grazing on the abundance and
composition of microphytobenthos in salt marsh sediments
(Pass Fourchon, LA)
I. A microcosm experiment
a a ,* b ,1 c ,2
Amy Bennett , Thomas S. Bianchi , Jay C. Means , Kevin R. Carman
a
Institute for Earth and Ecosystem Sciences, Department of EEO Biology, Tulane University, New Orleans,
LA70118-5698, USA b
Department of Chemistry, Western Michigan University, Kalamazoo, MI 49008-3842, USA c
Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70808, USA Received 9 June 1998; received in revised form 28 May 1999; accepted 28 June 1999
Abstract
A microcosm experiment was conducted to examine the effects of polycyclic aromatic hydrocarbon (PAHs) contamination on the abundance and composition of microphytobenthos in surface (0–10 cm) sediments collected from a salt marsh in Pass Fourchon, LA. Plant pigment biomarkers were used to monitor changes in the microphytobenthos in sediments (over 60 days) with treatments containing high and low concentrations of PAHs. Grazing pressure on micro-phytobenthos by the periwinkle snail Littorina irrorata as well as its responses to PAH contamination were also examined.
Concentrations of total PAHs declined in sediments over the duration of the experiment in all microcosms. There was a pattern of increasing relative abundance of high-molecular-weight PAHs in High-PAH sediments (5.5–16 ppm). Microphytobenthic biomass, estimated by chlorophyll-a concentrations, did not significantly change in the treatments with Low-PAH sediments (0.04–0.33 ppm); however, biomass decreased significantly in all treatments after 4 days from 64mg to 2–8
21
mg g dry sed. . Concentrations of the carotenoids fucoxanthin (a biomarker for diatoms) and zeaxanthin (a biomarker for cyanobacteria) were consistent with chlorophyll-a, showing little change in Low-PAH treatments; sediments from the High-PAH treatments showed significantly
21
higher concentrations of fucoxanthin and zeaxanthin (1.8 and 1.2mg g dry sed. , respectively) than the Low-PAH treatments during the initial phase of the microcosm experiment. However,
*Corresponding author. Tel.: 11-504-862-8000 ext. 1557; fax: 11-504-862-8706.
E-mail address: [email protected] (T.S. Bianchi) 1
Tel.: 11-616-387-2923; fax: 11-616-387-2909; e-mail: [email protected] 2
Tel.: 11-504-388-1761; fax: 11-504-388-1763; e-mail: [email protected]
concentrations of fucoxanthin and zeaxanthin decreased dramatically after 4 days to 0.1–0.3mg g
21 21
dry sed. and 0.01–0.15 mg g dry sed. , respectively. Increased total phaeophorbide con-centrations (a biomarker for metazoan grazing) over the first 12 days of the experiment in treatments with and without L. irrorata additions indicated that meiobenthos may have been important in grazing-down microphytobenthos. High-Exposure snails (from High-PAH field sites) gained more weight during the initial phase of the experiment when microphytobenthic abundance was decreasing but eventually lost more weight than did Low-Exposure snails (from Low-PAH field sites) by the end of the experiment. Although there was considerable variability in the snail growth values, we speculate that the High-Exposure snails were more active in feeding during this experiment and, after the initial decline in microphytobenthos, became resource-limited throughout the remainder of the experiment. 1999 Elsevier Science B.V. All rights reserved.
Keywords: PAH; Plant pigments; Microphytobenthos; Littorina irrorata; Salt marsh
1. Introduction
Studies over the last several decades have shown that the accumulation of polycyclic aromatic hydrocarbons (PAHs) in sediments may have profound effects on benthic communities (Griffiths et al., 1981; Wyndham, 1985; Bauer and Capone, 1985a,b; Bunch, 1987; Bauer et al., 1988). In areas subject to petroleum pollution, benthic communities may be particularly susceptible to diesel fuel and its by-products because of the high PAH levels associated with it (National Toxicology Program, 1986). High-molecular-weight (HMW) PAHs are resistant to bacterial metabolism and slow to evaporate, increasing their residence time in aquatic environments (Clark, 1989). Past work has shown that PAHs can have both positive and negative effects on mi-crophytobenthic communities (Bott et al., 1978; Farke et al., 1985; Plante-Cuny et al., 1993). For example, studies have shown that the positive effects of PAHs on microphytobenthic production and abundance are caused by reduced grazing by meiofauna and macrofauna (Farke et al., 1985; Plante-Cuny et al., 1993; Carman et al., 1997). Changes in microphytobenthos abundances are likely to have significant effects on food webs in coastal systems because of their important role as a food resource for many benthic invertebrates (Levinton and Bianchi, 1981; Levinton et al., 1984; Miller et al., 1996). Moreover, many of the benthic invertebrate communities in shallow productive coastal environments, such as salt marshes, provide an important trophic link to commercial fisheries. Thus, any effect of PAHs on the benthic community has the potential to be manifested in higher trophic levels in these coastal systems.
diversity of primary producers (i.e., vascular plants, microphytobenthos, phytoplankton, and macroalgae), resulting in a heterogeneous mixture of organic matter sources in coastal sediments. Plant pigment biomarkers have been widely used to identify different sources of algal organic matter in aquatic systems and to differentiate trophic dynamics in aquatic food webs (Mantoura and Llewellyn, 1983; Bianchi et al., 1993, 1996). Chlorophyll-a is used to determine general algal biomass, while carotenoids are more class specific (Wright et al. 1991). For example, the carotenoid fucoxanthin is a biomarker for the presence of diatoms, while zeaxanthin is a biomarker for cyano-bacteria (Wright et al., 1991; Bianchi et al., 1996). Phaeopigments, such as phaeophytins and phaeophorbides, are the decay products of chlorophylls; phaeophorbides are typically used as markers for metazoan grazing (Schuman and Lorenzen, 1975; Welschmeyer and Lorenzen, 1985; Bianchi et al., 1988, 1995; Bennett et al., 1999). Thus, plant pigments can be used to determine the composition and heterotrophic consumption of benthic algal sources in wetland ecosystems.
Louisiana wetlands represent 41% of the coastal wetlands in the US (Turner and Gosselink, 1975; Turner, 1997); many of these wetlands have been chronically exposed to petroleum-hydrocarbon pollution from oil exploration in the Gulf of Mexico (Fang, 1990). In particular, coastal salt marshes of Pass Fourchon, LA, were subjected to produced water discharge from the petrochemical pumping processes of Chevron until 1994, creating a dramatic PAH gradient over a very short distance (ca. 1 km) (Rabalais et al., 1991; Means and McMillin, 1993; Means, 1995). Carman et al. (1997) suggested that there was an enhancement of microphytobenthic abundance due to reduced meiofaunal grazing in PAH-contaminated sediments from Pass Fourchon. However, to fully understand the complexity of parameters that control the abundance and com-position of microphytobenthos in Pass Fourchon sediments, further work is needed on the interactive effects of contaminants, nutrients, and macrofaunal grazing.
In this study, the effects of PAH contamination and macrofaunal grazing on the abundance and composition of microphytobenthos in sediments were investigated using a laboratory microcosm experiment. This laboratory experiment was conducted in conjunction with a 2-year field study in an effort to understand the effects of PAH contamination on the salt marsh at Pass Fourchon, LA (Bennett et al., 1999). Our overall hypothesis was that PAH concentrations in sediments influence the classes and relative abundances of microphytobenthos, which affect the trophic and population dynamics of the epibenthic gastropod herbivore Littorina irrorata. Thus, the objectives for the microcosm portion of the study were to determine the effects of PAH concentration and grazing by L. irrorata on the abundance and composition of microphytobenthos in sediments from Pass Fourchon, LA, and to determine the effects of PAH concentration on the somatic growth and food resources of L. irrorata.
2. Materials and methods
2.1. Site description
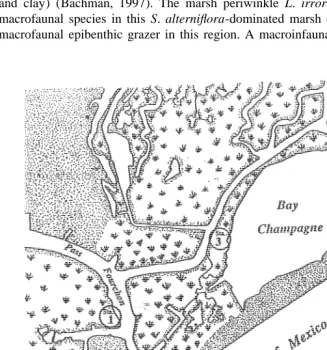
alterniflora salt marsh located between Bayou LaFourche and Bay Champagne, near
Pass Fourchon, LA (Fig. 1). This location is in close proximity to a field station owned and operated by the Louisiana Universities Marine Consortium (LUMCON). The tidal range at Port Fourchon is ca. 0.3 m and the salinity ranges from 4 to 26 ppt. Stations 1 and 3 represented the High- and Low-PAH contamination stations, respectively, that were described in a field study by Bennett et al. (1999). The range of total PAH concentrations in sediments at Stations 1 and 3 between 1996 and 1997 were ca. 2 to 18 ppm and 0.01 to 0.1 ppm, respectively (Bennett et al., 1999). Sediments at the High-PAH site were more coarse (32% silt and clay) than at the Low-PAH site (80% silt and clay) (Bachman, 1997). The marsh periwinkle L. irrorata was used as a model macrofaunal species in this S. alterniflora-dominated marsh due to its dominance as a macrofaunal epibenthic grazer in this region. A macroinfaunal species was not used in
this study because macroinfauna do not represent a significant fraction of the benthic community in northern Gulf coastal systems.
2.2. Microcosm experiment
A microcosm experiment was performed using a 23339 factorial design, with two sediment conditions, three snail conditions, and nine exposure times, resulting in the following treatments: Low- and PAH sediments without snails; Low- and High-PAH sediments with snails collected from High-High-PAH sediments in the field (Station 1); Low- and High-PAH sediments with snails collected from Low-PAH sediments (Station 3). Five replicates of each treatment (30 total microcosms) were maintained for a period of 60 days and nondestructively sampled on Days 0, 4, 12, 20, 28, 36, 44, 52, and 60. Snails and sediments were collected from both the High-PAH site (Stations 1a for sediments and 1b for snails) and the Low-PAH site (Stations 3a for sediments, and 3b for snails). The experiment consisted of Low-Exposure snails (from Station 3b) in both High- and Low-PAH sediments (Stations 1a and 3a, respectively) and High-Exposure snails (from Station 1b) in both sediment treatments. Controls for both sediment treatments were also used to examine microphytobenthic communities in the absence of snails.
Sediments and snails were collected on July 1996 from field Stations 1 and 3 at Pass Fourchon, LA. Sediments were sieved (500mm mesh) to remove macrofauna and then added to Petri dish microcosms of 14 cm diameter to a depth of approximately 1 cm. Meiobenthos, which represented a significant fraction of total benthic community (Chandler and Fleeger, 1983; Carman et al., 1997) in Pass Fourchon sediments, were not removed for the microcosm experiment. To prevent the escape of snails, each Petri dish microcosm was wrapped with plastic screening and secured with a rubber band, with the screening extended from the bottom of the dish to a height of 30.5 cm, and was capped with Petri dish lids. A portion of the screening was above the water line and provided ample space for snails to move in and out of the water.
Thirty Petri dishes were placed randomly in a flow-through water table. Ambient marsh water of 13‰ salinity was filtered (5 mm) to remove particles and continuously circulated through a wet table. Approximately 70% of the water was replaced every 8 days. Water temperature was maintained at approximately 288C, light penetration was 20
22 21
mmol m s , pH was approximately 7.8, and the dissolved oxygen concentration was
21
8 mg l . Lighting from banks of 40-W fluorescent lights was cycled on an approximate 12:12 light / dark cycle. Although light intensity was lower than that found in the field, an algal film was observed on microcosms surface sediments in the early stages of the experiment, suggesting that light was not a limiting factor.
Snails were depurated for 1 day prior to their addition to microcosms in order to remove any residual gut contents remaining from the field, then added in groups of three to each of 20 microcosms; the remaining ten microcosms represented treatments with no
22
snails (grazing controls). Snail density in the microcosms (|190 snails m ) was
22
pigment analysis, after which sediments were sampled on Day 4 and approximately every 8 days following for a period of 60 days. Snail wet-weights were measured at every sampling period in order to monitor snail biomass changes in the four different L.
irrorata treatments. Sediment PAHs, snail gut pigments, and total sedimentary carbon
and nitrogen were measured at the beginning (Day 0), midpoint (Day 28), and endpoint (Day 60) of the experiment. Snails removed at Day 28 were replaced by L. irrorata from a nonstudy site in order to maintain consistent population density within each treatment.
2.3. PAHs
Surface sediments (0–1 cm) and whole snail tissues were extracted for PAHs according to the methods of Means (1998). All PAH analyses were performed on a GC / MS Hewlett-Packard 5890 gas chromatograph coupled to a Hewlett-Packard 5970B Mass Selective Detector (Carman et al., 1996; Means, 1998).
2.4. Total carbon and nitrogen
Lyophilized sediments were prepared for organic carbon and nitrogen (POC and PON) analyses by placing them in a glass desiccator along with a small beaker containing 25 ml of 12 N HCl for 24 h in order to remove inorganic carbon. Following acid treatment, analyses for POC and PON were performed using a Carlo Erba NA1500 NCS system. Acetanilide (71.09% C and 10.36% N) was used as a standard. Reproducibility between replicate samples always differed ,5% for both C and N.
2.5. Plant pigments
Surface sediments and dissected digestive tracts of L. irrorata were lyophilized, weighed, and extracted for pigments with 100% acetone. Samples were sonicated at 40 W within a beaker of ice for 40 s and allowed to sit overnight at 248C (Bianchi et al., 1995). Extracts were filtered through a Gelman cartridge filter (25 mm diam., 0.2mm pore size) prior to analysis by HPLC.
Reversed-phase HPLC analysis was conducted according to the method of Wright et al. (1991) as modified by Bianchi et al. (1996). The Wright et al. (1991) method has been shown to be the most effective means for separating over 50 carotenoids, chlorophylls and their derivatives, including elution of the isomers lutein and zeaxanthin. High purity HPLC standards for chlorophylls-a and -b were obtained from Sigma, whereas standards for fucoxanthin and lutein were obtained from Hoffman LaRoche. Total phaeophytins and phaeophorbides (a and b) were made in the laboratory according to procedures described by Welschmeyer (1994). Purity of all standards was spectro-photometrically verified. Pigment identification was based on retention times of standards and confirmed with a Waters 996 spectral diode-array. All peaks were quantified with calculated response factors using a Waters Millennium software package.
21
2.6. Statistical analyses
Analysis of variance (ANOVA) analyses were conducted using the software STAT-GRAPHICS Plus 2.0. Values of P,0.05 were considered to be significant. When ANOVA differences were significant, the Fisher’s Least Significant Difference, LSD, a posteriori multiple range test was used to detect differences among effects. A 23339 repeated measures ANOVA design was used to detect significant differences among sediment type, snail type, PAH concentration, and time effects in the microcosm experiment. Regressions and correlations were determined using both STATGRAPHICS
Plus 2.0 and SigmaPlot 1.02 software.
3. Results
3.1. Microcosm sediments and L. irrorata
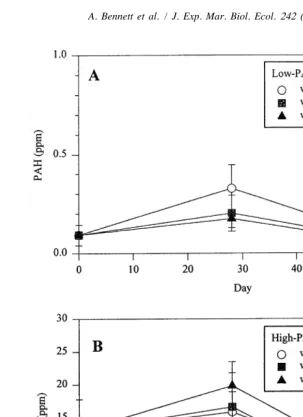
PAH concentrations in the Low-PAH sediment treatments ranged from 0.1 to 0.2 ppm (Fig. 2), whereas PAH concentrations in the High-PAH sediments were significantly higher (P,0.05) and ranged from 5 to 18 ppm (Fig. 2b). A loss of PAHs in both High-and Low-PAH sediments was observed between Days 28 High-and 60 High-and was consistent across all three of the High-PAH treatments.
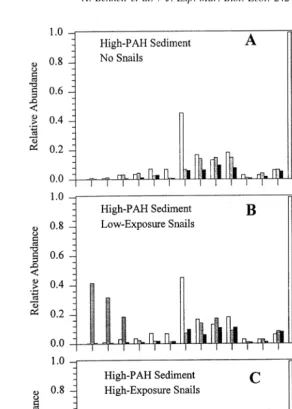
Relative abundances of PAHs in the High-PAH sediments (Fig. 3) reflect PAH concentrations normalized to the most abundant PAH isomer (Steinhauer and Boehm, 1992). When examining the relative abundances of certain PAH groups with a variety of molecular weights the proportion of HMW PAHs increased with time in High-PAH sediments. In High-PAH sediments, Low-Exposure and High-Exposure snail treatments had similar trends in low molecular weight (LMW) and HMW PAH relative abundances within each sediment treatment throughout the duration of the experiment (Fig. 3). The relative abundances of PAHs in Low-PAH sediments had a broader and more evenly distributed range of molecular weights than High-PAH sediments (data not shown).
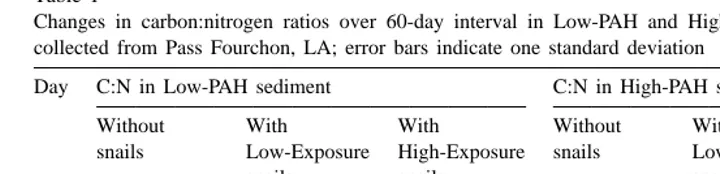
Initial (Day 0) C:N ratios were significantly higher (P,0.0001) in Low-PAH sediments (60.966.03) than in High-PAH sediments (19.861.71), (Table 1). The C:N ratios of sediments in the three Low-PAH sediment treatments all declined significantly (P,0.02) over the course of the experiment, with some sporadic changes in treatments involving snails (Table 1). The C:N ratios of the Low-PAH sediments on Day 60 were similar to the initial C:N ratios of the High-PAH sediments. There were no significant changes in the C:N ratios of High-PAH sediments over the 60 days (Table 1).
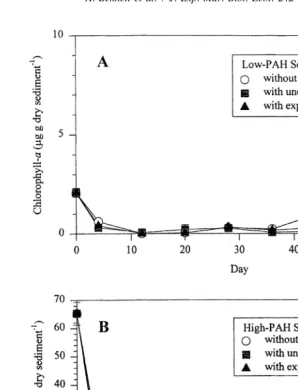
High-PAH sediments had a significantly higher initial (Day 0) concentration of
21
chlorophyll-a (65.3064.01 mg g dry sed. ) than did the Low-PAH sediments
21
(2.0960.22 mg g dry sed. , P,0.0001) (Fig. 4). Chlorophyll-a concentrations
21
declined to below 10mg g dry sed. in all of the High-PAH sediment treatments by Day 12 of the experiment and remained below this level for the remainder of the experiment. Chlorophyll-a concentrations in Low-PAH sediments also declined from Day 0 (2.09mg
21 21
Fig. 2. Changes in total PAH concentrations over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation.
21
the experiment (up to a range of 0.30 to 2.05mg g dry sed. by Day 60), particularly in the treatment without L. irrorata.
Fig. 3. Relative abundances of specific PAHs and PAH groups in High-PAH sediment treatments with No Snails (A), Low-Exposure snails (B), and High-Exposure snails (C) of the microcosm experiment. Napth5 napthalene, Phenan5phenanthrene, Dibenzo5dibenzothiophene, Fluoran5fluoranthene, Benzan5 benzanthracene, Benzo(b)f5benzo(b)fluoranthene, Benzo(k)f5benzo(k)fluoranthene, Benzo(a)p5 benzo(a)pyrene.
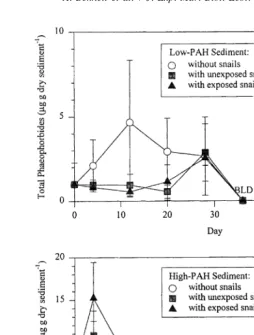
irrorata peaked at Day 12 and on Day 28 in Low-PAH treatments with L. irrorata. Phaeophorbides were not detectable in Low-PAH treatments from Day 36 throughout the remainder of the experiment.
Table 1
Changes in carbon:nitrogen ratios over 60-day interval in Low-PAH and High-PAH microcosm sediments collected from Pass Fourchon, LA; error bars indicate one standard deviation
Day C:N in Low-PAH sediment C:N in High-PAH sediment
Without With With Without With With
snails Low-Exposure High-Exposure snails Low-Exposure High-Exposure
snails snails snails snails
0 60.8666.03 60.8666.03 60.8666.03 19.7961.71 19.7961.71 19.7961.71 28 18.6365.30 73.95656.72 17.4862.68 54.89632.62 49.73648.83 20.0062.98 60 19.2960.21 17.4160.58 15.9462.49 77.98663.20 19.0567.55 30.1361.66
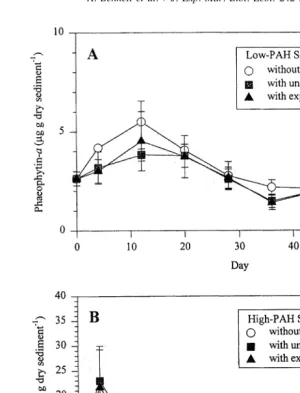
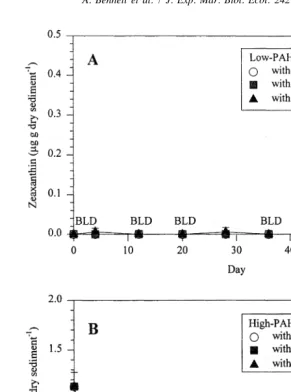
experiment in the treatments with Low-PAH sediments without L. irrorata (Fig. 7). Fucoxanthin, zeaxanthin, and chlorophyll-a showed the same rapid decay in High-PAH sediments within the first ten days of the experiment (Figs. 4, 7 and 8). Fucoxanthin
2
concentrations were significantly correlated with chlorophyll-a (r 50.82) in all treatments throughout the duration of the microcosm experiment (data not shown). Zeaxanthin was rarely detected in Low-PAH sediments (Fig. 8). Zeaxanthin con-centrations in High-PAH sediments did not change significantly from Day 28 to Day 60, and did not show any significant differences due to the presence of L. irrorata (Fig. 8). In all four treatments containing snails, L. irrorata experienced weight loss over the course of the 60 days of the microcosm experiment (Fig. 9). High-Exposure snails (preexposed to High-PAH concentrations) experienced more total wet weight loss over the 60 day period than did Low-Exposure snails (collected from Low-PAH sediments). In all treatments L. irrorata experienced some weight gain during the early stages of the experiment (Fig. 9). This weight gain occurred consistently earlier in the High-Exposure snails than in Low-Exposure snails. We were not able to detect significant differences in PAH concentrations in whole animal tissues collected from Low-Exposure and High-Exposure snails at the beginning and end of the experiment due to the high variability (high standard deviations) in replicate concentrations.
4. Discussion
21
Fig. 4. Changes in chlorophyll-a concentrations (mg g dry sediment ) over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation.
HMW compounds are resistant to both weathering and metabolism, and accumulate over time (Carman et al., 1996).
21
Fig. 5. Changes in phaeophytin-a concentrations (mg g dry sediment ) over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation.
21
Fig. 6. Changes in total phaeophorbide concentrations (mg g dry sediment ) over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation. BLD5below limits of detection.
vascular signature by Day 60 of the experiment (ca. 20); over time, carbon resources increased in quality for microbial uptake, as shown by the shift in sedimentary C:N ratios (Table 1). Increases in chlorophyll-a and fucoxanthin concentrations (after Day 40) indicated that increased diatom abundance was largely responsible for decreased C:N ratios in Low-PAH sediments. Zeaxanthin concentrations never recovered after the initial decline, indicating that cyanobacteria did not contribute to observed changes in C:N ratios.
21
Fig. 7. Changes in fucoxanthin concentrations (mg g dry sediment ) over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation.
meiobenthos (harpacticoid copepods) in experimental sediments collected from Cocod-rie, LA (Carman et al., 1997). Our results support previous findings that meiobenthos can significantly ‘‘graze-down’’ microphytobenthos in Louisiana salt marsh sediments (Carman et al., 1997).
21
Fig. 8. Changes in zeaxanthin concentrations (mg g dry sediment ) over a 60-day interval in Low-PAH (A) and High-PAH (B) microcosm sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation. BLD5below limits of detection.
Fig. 9. Average wet-weight changes (g) of L. irrorata per sampling period over the 60-day duration of the microcosm experiment in Low-PAH (A) and High-PAH (B) sediments collected from Pass Fourchon, LA. Error bars indicate one standard deviation.
sediments were exposed at low tide, the range of light levels observed in the field when
2 21
the sites were covered with water at slack and high tides (250–500 mmol m s , Bennett et al., 1999) was similar to the light levels used in this experiment (ca. 270
2 21
mmol m s ). Diatoms were observed growing in thick mats on the surface of the microcosm water table.
The overall weight loss of L. irrorata in all microcosm treatments may have been due to a limitation of microalgal resources because of meiobenthic grazing (Fig. 9). Average dry-weights of L. irrorata in populations collected from Grand Isle, LA, were shown to have the least amount of weight change (175 to 201 mg dry weight) during the months (May to Sept.) with the highest temperatures (23 to 278C) (Shirley et al., 1978). This period of minimal weight change was considered to be a thermal-neutral zone where respiration and thermal stresses were in ‘‘balance’’. Weight losses of snails collected from the High-PAH station at Pass Fourchon were greater than in snails collected from the Low-PAH station. Despite the greater weight loss by the High-Exposure snails, these snails were clearly more active and contributed more to microphytobenthic loss in the early stages of the experiment than did the Low-Exposure snails. Developed resistance has been demonstrated in benthic invertebrates exposed to environmental contaminants over several generations (Klerks and Levinton, 1989).
5. Conclusions
A microcosm study examining the interactive effects between PAHs, microphyto-benthos, and L. irrorata in sediments from Pass Fourchon, LA, indicated that the effect of contaminants on benthic food webs can be quite complex. Concentrations of total PAHs declined over the duration of the experiment in all microcosms. There was a pattern of increasing proportional relative abundance of HMW PAHs in High-PAH sediments. Overall, L. irrorata had no effect on the proportional abundance of LMW and HMW PAHs in High- and Low-PAH sediments. Initially, High-PAH sediments collected from the field had a higher abundance of cyanobacteria and diatoms than Low-PAH sediments. These results support earlier work which suggests that PAHs can actually enhance microphytobenthic abundance due to either loss of benthic grazers or direct uptake of PAHs as a resource.
to High-PAH conditions, and became resource-limited after the initial decline in microphytobenthos.
Acknowledgements
This work was supported by grants from the Department of Energy and Office of Naval Research (ONR). We thank Corey Lambert and Sid Mitra for reviewing earlier drafts of this manuscript; we also thank Sid Mitra for his help with the PAH data. Brenda Bachman assisted with field collections. Ed Chesney provided invaluable assistance with setting up laboratory space at the LUMCON facility.
References
Admiraal, W., 1984. The ecology of estuarine sediment-inhabiting diatoms. Prog. Phycol. Res. 3, 269–322. Bachman, B.M., 1997. The effects of produced water on the trophic relationship of microalgae and meiofauna.
M.S. Thesis, Louisiana State University.
Bauer, J.E., Capone, D.G., 1985a. Effects of four aromatic organic pollutants on microbial glucose metabolism and thymidine incorporation in marine sediments. Appl. Environ. Microbiol. 49, 828–835.
Bauer, J.E., Capone, D.G., 1985b. Degradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and napthalene in intertidal marine sediments. Appl. Environ. Microbiol. 50, 81–90. Bauer, J.E., Kerr, R.P., Bautista, M.F., Decker, C.J., Capone, D.G., 1988. Stimulation of microbial activities
and polycyclic aromatic hydrocarbon degradation in marine sediments inhabited by Capitella capitata. Mar. Environ. Res. 25, 63–84.
Bennett, A., Bianchi, T.S., Means, J.C., 1999. The effects of PAH contamination and grazing on the abundance and composition of microphytobenthos in salt marsh sediments (Pass Fourchon, LA): II. A field study. Estuar. Coast. Shelf Sci. (submitted).
Bianchi, T.S., Dawson, R., Sawangwong, P., 1988. The effects of macrobenthic deposit-feeding on the degradation of chloropigments in sandy sediments. J. Exp. Mar. Biol. Ecol. 122, 243–255.
Bianchi, T.S., Demetropoulos, A., Hadjichristophorou, M., Argyrou, M., Baskaran, M., Lambert, C., 1996. Plant pigments as biomarkers of organic matter sources in sediments and coastal waters of Cyprus (eastern Mediterranean). Estuar. Coast. Shelf Sci. 42, 103–115.
Bianchi, T.S., Findlay, F., Dawson, R., 1993. Organic matter sources in the water column and sediments of the Hudson River Estuary: the use of plant pigments as tracers. Estuar. Coast. Shelf Sci. 36, 359–376. Bianchi, T.S., Lambert, C., Biggs, D.C., 1995. Distribution of chlorophyll a and phaeopigments in the
northwestern Gulf of Mexico: a comparison between fluorometric and high-performance liquid chromatog-raphy measurements. Bull. Mar. Sci. 56 (1), 25–32.
Bott, T.L., Rogenmuser, K., Thorne, P., 1978. Effects of No. 2 fuel oil, Nigerian crude oil, and used crankcase oil on benthic algal communities. J. Environ. Sci. Health A13, 751–779.
Bunch, J.N., 1987. Effects of petroleum releases on bacterial numbers and microheterotrophic activity in the water and sediment of an Arctic marine ecosystem. Arctic 40, 172–183.
Carman, K.R., Fleeger, J.W., Pomarico, S.M., 1997. Response of a benthic food web to hydrocarbon contamination. Limnol. Oceanogr. 42 (3), 561–571.
Carman, K.R., Means, J.C., Pomarico, S.C., 1996. Response of sedimentary bacteria in a Louisiana salt marsh to contamination by diesel fuel. Aquat. Microbiol. Ecol. 10, 231–241.
Chandler, G.T., Fleeger, J.W., 1983. Meiofaunal colonization of azoic estuarine sediment in Louisiana: Mechanisms of dispersal. J. Exp. Mar. Biol. Ecol. 69, 175–188.
Clark, R.B. (Ed.), 1989. Marine Pollution, third ed., Clarendon Press, Oxford, p. 172.
Fang, C.S., 1990. Petroleum drilling and production operations in the Gulf of Mexico. Estuaries 13, 89–97. Farke, H., Wonneberger, K., Gunkel, W., Dahlmann, G., 1985. Effects of oil and a dispersant on intertidal organisms in field experiments with a mesocosm, the Bremerhaven Caisson. Mar. Environ. Res. 15, 97–114.
Griffiths, R.P., Caldwell, B.A., Broich, W.A., Morita, R.Y., 1981. Long-term effects of crude oil on uptake and respiration of glucose and glutamate in Arctic and subarctic marine sediments. App. Environ. Microbiol. 42, 792–801.
Kennish, M.J. (Ed.), 1998. Pollution Impacts On Marine Biotic Communities, CRC Press, Boca Raton, p. 310. Klerks, P.L., Levinton, J.S., 1989. Rapid evolution of metal resistance in a benthic oligochaete inhabiting a
metal-polluted site. Biol. Bull. 176, 135–141.
Levinton, J.S., Bianchi, T.S., 1981. Nutrition and food limitation of deposit-feeders: I. The role of microbes in the growth of mud snails (Hydrobiidae). J. Mar. Res. 39 (3), 531–545.
Levinton, J.S., Bianchi, T.S., Stewart, S., 1984. What is the role of particulate organic matter in benthic invertebrate nutrition? Bull. Mar. Sci. 35 (3), 270–282.
Mantoura, R.F.C., Llewellyn, C.A., 1983. The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal. Chem. Acta 151, 297–314.
Means, J.C., 1995. Influence of salinity upon sediment–water partitioning of aromatic hydrocarbons. Mar. Chem. 51, 3–16.
Means, J.C., 1998. Compound-specific GC / MS analysis of alkylated and parent polycyclic aromatic hydrocarbons in waters, sediments, and aquatic organisms. J. Assoc. Off. Anal. Chem. 81, 657–672. Means, J.C., McMillin, D.J., 1993. Fate and transport of particle-reactive normal, alkylated and heterocyclic
aromatic hydrocarbons in a sediment–water–colloid system. USMMS OCS Study MMS-93-0018, Final Report, pp. 153.
Miller, D.C., Geider, R.J., MacIntyre, H.L., 1996. Microphytobenthos: The ecological role of the ‘‘secret garden’’ of unvegetated, shallow-water marine habitats. II. Role in sediment stability and shallow-water food webs. Estuaries 19, 202–212.
Mitra, S., 1997. Polycyclic aromatic hydrocarbon distribution coefficients with urban estuarine sediments. Ph.D. Dissertation, Virginia Institute of Marine Science, College of William and Mary.
National Toxicology Program, 1986. Toxicology and carcinogenesis studies of marine diesel fuel and JP-5 Navy fuel in B6C3F . NTP Technical Report. NTP TR 310.1
Plante-Cuny, M.R., Salen-Picard, C., Grenz, C., Plante, R., Alliot, E., Barranguet, C., 1993. Experimental field study of the effects of crude oil, drill cuttings and natural biodeposits on microphyto- and macrozoobenthic communities in a Mediterranean area. Mar. Biol. 117, 355–366.
Rabalais, N.N., McKee, B.A., Reed, D.J., Means, J.C., 1991. Fate and effects of nearshore discharges of OCS produced waters. Vol. II. Technical Report. OCS Study / MMS 91-0005. US Department of the Interior, Mineral Management Service, Gulf of Mexico OCS Region, New Orleans, LA.
Schuman, F.R., Lorenzen, C.J., 1975. Quantitative degradation of chlorophyll by a marine herbivore. Limnol. Oceanogr. 15, 59–69.
Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. (Eds.), 1993. Environmental Organic Chemistry, Wiley, New York, p. 681.
Shirley, T.C., Denoux, G.W., Stickle, W.B., 1978. Seasonal respiration in the marsh periwinkle, Littorina
irrorata. Biol. Bull. 154, 322–334.
Steinhauer, M.S., Boehm, P.D., 1992. The composition and distribution of saturated and aromatic hydrocarbons in nearshore sediments, river sediments, and coastal peat of the Alaskan Beaufort Sea: implications for detecting anthropogenic hydrocarbon inputs. Mar. Environ. Res. 33, 223–253.
Turner, R.E., 1997. Wetland loss in the Gulf of Mexico: multiple working hypotheses. Estuaries 20 (1), 1–13. Turner, R.E., Gosselink, J.G., 1975. A note on standing crop of Spartina alterniflora in Texas and Florida.
Contrib. Mar. Sci. 19, 13–18.
Welschmeyer, N.A., 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and phaeopigments. Limnol. Oceanogr. 39 (8), 1985–1992.
Welschmeyer, N.A., Lorenzen, C.J., 1985. Chlorophyll budgets: zooplankton grazing and phytoplankton growth in a temperate fjord and the Central Pacific Gyres. Limnol. Oceanogr. 30, 1–21.
Wright, S.W., Jeffrey, S.W., Mantoura, R.F.C., Llewellyn, C.A., Bjornland, D., Repeta, D., Welschmeyer, N., 1991. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 77, 183–196.