Summary Specific chloroplast proteins, gas exchange and dry matter production in oak (Quercus robur L.) seedlings and clonal cherry (Prunus avium L. × pseudocerasus Lind.) plants were measured during 19 months of growth in climate-control-led greenhouses at ambient (350 vpm) or elevated (700 vpm) CO2. In both species, the elevated CO2 treatment increased the
PPFD saturated-rate of photosynthesis and dry matter produc-tion. After two months at elevated CO2, Prunus plants showed
significant increases in leaf (55%) and stem (61%) dry mass but not in root dry mass. However, this initial stimulation was not sustained: treatment differences in net assimilation rate (A) and plant dry mass were less after 10 months of growth than after 2 months of growth, suggesting acclimation of A to elevated CO2 in Prunus. In contrast, after 10 months of growth
at elevated CO2, leaf dry mass of Quercus increased (130%)
along with shoot (356%) and root (219%) dry mass, and A was also twice that of plants grown and measured at ambient CO2.
The amounts of Rubisco and the thylakoid-bound protein cytochrome f were higher in Quercus plants grown for 19 months in elevated CO2 than in control plants, whereas in Prunus there
was less Rubisco in plants grown for 19 months in elevated CO2
than in control plants. Exposure to elevated CO2 for 10 months
resulted in increased mean leaf area in both species and in-creased abaxial stomatal density in Quercus. There was no change in leaf epidermal cell size in either species in response to the elevated CO2 treatment. The lack of acclimation of
photosynthesis in oak grown at elevated CO2 is discussed in
relation to the production and allocation of dry matter. We propose that differences in carbohydrate utilization underlie the differing long-term CO2 responses of the two species.
Keywords: carbon dioxide, cytochrome f, dry matter, gas ex-change, Prunus, Quercus robur, Rubisco.
Introduction
Many reports have detailed the effects of elevated CO2 on
growth and dry matter partitioning of a range of woody peren-nials (see reviews by Cure and Acock 1986, Eamus and Jarvis 1989, Krupa and Kickert 1989, Ceulemans and Mousseau
1994, Gunderson and Wullschleger 1994, Atkinson 1996). Dry mass increases induced by elevated CO2 are often
accompa-nied by increases in net assimilation rate (A), at least in the short term (Long 1991). However, enhancement of A may disappear after longer-term exposure to elevated CO2.
Several explanations have been proposed to account for photosynthetic acclimation to elevated CO2, including reduced
activity of sinks for carbohydrate, and the associated problems of source--sink imbalance (Clough et al. 1981, Thomas and Strain 1991, Long and Drake 1992). Under elevated CO2,
when A greatly exceeds the capacity for photosynthate utiliza-tion, it has been shown that sugars accumulate and modulate the transcription of chloroplast proteins (Van Oosten and Bes-ford 1994, 1995, Wilkins et al. 1994). Because the amount of Rubisco can limit the light-saturated rate of A at low CO2
concentrations (von Caemmerer and Farquhar 1981, Besford et al. 1985), photosynthetic acclimation has also been attrib-uted to a loss of the amount or activity, or both, of Rubisco (Ceulemans and Mousseau 1994), and various photosynthetic enzymes and their thylakoid proteins, including cytochrome f which is an integral part of the cytochrome b6f complex
re-sponsible for electron transfer between photosystem II and photosystem I (Besford et al. 1990, Van Oosten and Besford 1995).
Because Quercus and Prunus are believed to behave differ-ently in their responses to elevated CO2 (Gunderson et al. 1993,
Wilkins et al. 1994), we first set out to determine whether allocation of dry matter to different organs in these species is altered by exposure to elevated CO2 (700 vpm). We then
investigated whether changes in photosynthetic potential in response to elevated CO2 could explain the differences in dry
mass. To investigate possible mechanisms underlying photo-synthetic acclimation to elevated CO2, we compared the
con-tents of Rubisco and cytochrome f in leaves exposed to ambient CO2 with those of leaves exposed to elevated CO2. We
found that, although both Quercus and Prunus had different responses to CO2 enrichment, in both species growth responses
to elevated CO2 reflected changes in dry matter production,
whereas carbon allocation responses to elevated CO2 reflected
changes in net assimilation.
Effects of elevated CO
2
on chloroplast components, gas exchange and
growth of oak and cherry
C. J. ATKINSON,
1J. M. TAYLOR,
1D. WILKINS
2and R. T. BESFORD
21
Horticulture Research International, East Malling, West Malling, Kent, ME19 6BJ, U.K. 2
Horticulture Research International, Littlehampton, West Sussex, BN17 6LP, U.K.
Received March 7, 1996
Materials and methods
Plant culture
Quercus robur L. seedlings were grown individually in 275 cm3 containers from acorns collected in the autumn of 1992, near Nancy, Champenoux (France). Before germination, the acorns were planted and placed in cold storage at 2 °C for 1 month and then transferred to two temperature-controlled greenhouses (see Wilkins et al. 1994). In one greenhouse CO2
was held constantly at 350 vpm (ambient), whereas in the other it was held at 700 vpm (elevated). In mid-summer, the plants were repotted in 5 dm3 pots at which time they were inoculated with the ectomycorrhizal fungus Thelephora terrestris Echr. Fr. (supplied by INRA-Nancy).
Cherry plants of the clone ‘‘Colt,’’ a cross between two wild Prunus species (P. avium L. ×P. pseudocerasus Lind.), were obtained by micropropagation. After one month of hardening-off, the micropropagated plants were transferred in February to the greenhouses in which the Quercus seedlings were grow-ing. In mid-summer, the plants were repotted in 15 dm3 pots.
For both species, the pots were free-draining to minimize accumulation of ABA and other substances from the roots that might inhibit photosynthesis. Fewer plants of Prunus were available at the start of the experiments, but on three sub-sequent occasions (in April, August and October of the first year), approximately 40 additional micropropagated plants were transferred to the greenhouses, after hardening-off. Some of the Quercus and Prunus plants were used for destructive analysis, and some plants were grown for a further two years at their respective CO2 concentrations.
Both greenhouses were ventilated at 25 °C. Air temperature was continuously monitored with thermographs, and statistical analyses showed no significant difference in air temperature between the greenhouses. To minimize the risk of confounding effects that possible differences in mineral nutrition might have on A (see Eamus and Jarvis 1989, Sinclair 1992, Ceule-mans and Mousseau 1994, Wilkins et al. 1994), from March onward, all plants were fertilized weekly with nutrient solution (stock solution contained dm--3: KNO3, 43 g; MgSO4.7H2O,
22 g; NH4NO3, 40 g; NH4H2PO4, 7 g; MnSO4.H2O, 0.2 g; plus
micronutrients and chelated iron) diluted 200-fold with cal-cium-rich tap water. Osmocote slow-release fertilizer (15,11,13, N,P,K) was applied in June.
The analysis of variance was based on the variation within a single greenhouse for each treatment, differences between the greenhouses were then compared. Where appropriate, the sig-nificance of the differences is shown along with the standard error of the difference of means.
Leaf gas exchange
Leaf gas exchange rates (net assimilation (A), transpiration (E) and leaf conductance to water vapor (g)) were measured on several occasions during the growing season. Two portable gas exchange systems were used (LCA2, Analytical Development Corp. Ltd., Hoddesdon, U.K., and Ciras-1, PP Systems,
LCA2 was used to measure gas exchange at the CO2
concen-tration in which the plants were grown. A minimum of ten plants, with three leaves of similar physiological age per plant, was used for each measurement. Although A varied with plant age and time of year, the general pattern of treatment differ-ences was similar (data not shown). To investigate the causes of the treatment differences in A, it was measured at a PPFD of 860 µmol m--2 s--1 in plants from both treatments at both CO
2
concentration. The leaves were not preconditioned to the measurement CO2 concentration.
Extraction of Rubisco and cytochrome f and immunodetection by Western blotting
In September 1994, after 19 months in the greeenhouses, young, fully expanded leaves of Prunus and Quercus were analyzed for Rubisco large subunit (LSU) and thylakoid bound cytochrome f as described by Mehta et al. (1992), Besford et al. (1993) and Van Oosten and Besford (1995). To avoid con-founding of the results by leaf-level differences in light accli-mation, we assayed leaves from full sun and from shade (< 50% full sun). Western blotting was carried out as described by Besford (1990) and Van Oosten and Besford (1995). Treat-ment comparisons were based on separate leaf extracts, loaded on an equal leaf area basis, from at least three plants per treatment; each sample was blotted twice and typical blots are presented (Figure 1).
Leaf cell number and stomatal density
Stomatal density and leaf epidermal cell number per unit area were quantified from epidermal impressions of the abaxial surface using a silicone-based dental product (Provil M, Bayer, Lever Kusen, Germany). During the growing season, leaf im-pressions were taken of the central region around the midrib of the youngest fully expanded leaf from each of ten plants. Positive impressions were made with nail varnish and viewed with a projection light microscope at low magnification (45×). For each leaf impression, three fields of view were selected for analysis and the mean calculated.
Distribution and production of dry matter, and leaf mineral analysis
The allocation of dry mass was used to describe the relation-ship between leaf canopy, stem development and wood pro-duction (radial growth). Main stem extension growth (branches not included) was determined in relation to radial wood production. For Quercus in particular, the phenological development of the main stem was quantified with respect to the number of growth flushes produced. Total plant leaf area and mean individual leaf area were measured with a leaf area meter (Model LI-3050A, Li-Cor, Inc., Lincoln, NE) on ten leaves from each of ten Quercus plants per treatment, and on 20 leaves from each of five Prunus plants per treatment.
were separated from coarse roots (> 2 mm diameter), and cut to a length of 2--5 cm. The length of coarser roots was meas-ured by hand with a ruler. Fine root length was determined with a root length scanner (Comair, Commonwealth Aircraft Corp. Ltd., Melbourne, Australia). A calibration curve was used to convert counts to root length, i.e., actual root length = --0.2246 + 0.9655 (L) + 0.00123 (L2) where L is the estimated root
length from the scan.
Leaf mineral status was determined on oven-dried material from 20 plants each of Quercus and Prunus, by a standard Kjeldhal digestion procedure. Nitrogen and phosphorus were analyzed colorimetrically with an auto-analysis system (Tech-nicon Instruments Corp., Tarrytown, NY), and calcium, mag-nesium and potassium were analyzed by atomic absorption and emission spectroscopy.
Results
Leaf development, gas exchange, and chloroplast proteins
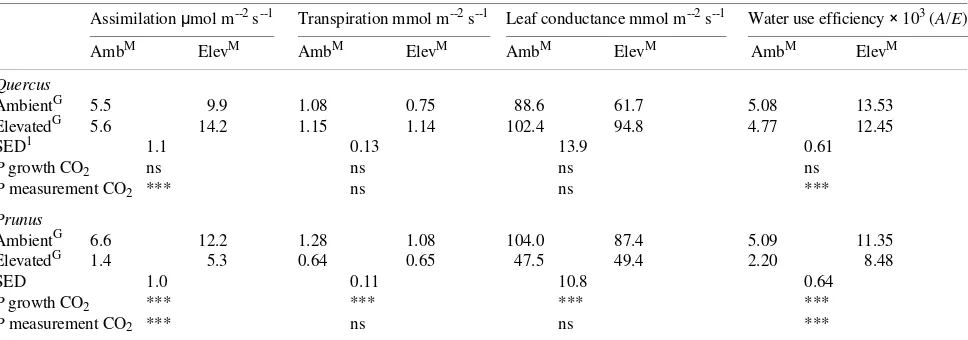
Light-saturated A of Quercus plants grown for 10 months at elevated CO2 and measured at the growth CO2 concentration,
was significantly greater (158%) than in plants grown and measured in ambient CO2 (Table 1). Both g and E increased
slightly in Quercus leaves grown in elevated CO2 and
meas-ured at ambient CO2, whereas partial stomatal closure occurred
in Quercus leaves grown in ambient CO2 and measured at the
elevated CO2 concentration. Net assimilation rate increased
twofold for Prunus plants grown at ambient CO2 and measured
at the elevated CO2 concentration, compared to Prunus plants
grown and measured at ambient CO2 concentration. However,
A of Prunus grown and measured at elevated CO2 was not
Table 1. Gas exchange characteristics (assimilation, transpiration, leaf conductance and WUE) measured at 20 °C under light-saturating conditions for young, fully expanded leaves of Quercus robur and Prunus avium×pseudocerasus exposed for 10 months to either ambient or elevated CO2.
Superscript M denotes measurement CO2 concentration, superscript G denotes growth CO2 concentration.
Assimilation µmol m--2 s--1 Transpiration mmol m--2 s--1 Leaf conductance mmol m--2 s--1 Water use efficiency × 103 (A/E)
AmbM ElevM AmbM ElevM AmbM ElevM AmbM ElevM
Quercus
AmbientG 5.5 9.9 1.08 0.75 88.6 61.7 5.08 13.53
ElevatedG 5.6 14.2 1.15 1.14 102.4 94.8 4.77 12.45
SED1 1.1 0.13 13.9 0.61
P growth CO2 ns ns ns ns
P measurement CO2 *** ns ns ***
Prunus
AmbientG 6.6 12.2 1.28 1.08 104.0 87.4 5.09 11.35
ElevatedG 1.4 5.3 0.64 0.65 47.5 49.4 2.20 8.48
SED 1.0 0.11 10.8 0.64
P growth CO2 *** *** *** ***
P measurement CO2 *** ns ns ***
1
SED = Standard error of difference of means; significant differences (P) are shown by the symbols: ns = not significant, * = 0.05, ** = 0.01 and *** = 0.001.
Figure 1. Immunodetection after SDS-PAGE and electroblotting of the large subunit of Rubisco (LSU) in the soluble phase and cytochrome f in the thylakoid preparations from Prunus and Quercus grown in ambient or ele-vated CO2. Molecular weights of
marker proteins are indicated at the banding positions (kDa) in lanes 1 and 12, unshaded young fully expanded leaves were used except in lanes 7 and 9 where shaded leaves were analyzed. A, ambient CO2; E, elevated CO2
significantly different from that of Prunus plants grown and measured at ambient CO2. Stomatal conductance did not
in-crease when Prunus leaves grown in elevated CO2 were
meas-ured at ambient CO2, and there was only a small reduction in
g when ambient-grown Prunus leaves were measured at ele-vated CO2 (Table 1).
The amount of the large subunit of Rubisco (LSU) was less in Prunus plants grown in elevated CO2 than in Prunus plants
grown in ambient CO2, whereas no appreciable treatment
differences were observed in Quercus seedlings (Figure 1). In Prunus, there was no effect of elevated CO2 on the amount of
cytochrome f (although the amount appeared to be enhanced by shading), whereas cytochrome f content was increased in Quercus plants grown in elevated CO2 compared with Quercus
plants grown in ambient CO2 (Figure 1).
Mean individual leaf area in Quercus and Prunus increased (P < 0.001) in response to elevated CO2, from 20.8 to 26.8 cm2
and from 13.6 to 25.4 cm2, respectively. The elevated CO2
treatment increased the number of stomata per unit of abaxial surface between 2 and 3 times in Quercus (data not shown), whereas the treatment had no effect on stomatal density in Prunus. Leaf epidermal cell density was higher for Quercus than for Prunus, indicating a smaller epidermal cell size for oak leaves, but no CO2 response was evident for either species.
Dry matter production and distribution and leaf mineral analysis
After 10 months, there were significant increases in the accu-mulated dry mass of leaves (131%), wood (main stem and all branches by 356%) and roots (219%) of Quercus seedlings growth in elevated CO2 compared to seedlings grown at
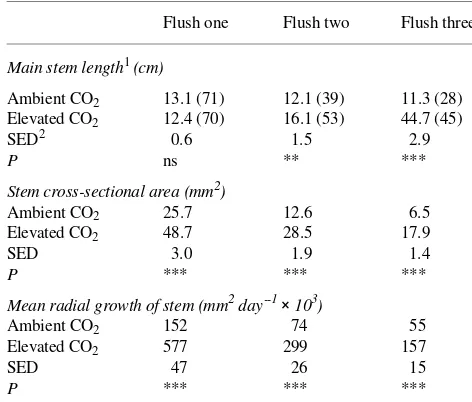
ambi-ent CO2 (Table 2). Main stem extension growth was examined
for each growth flush, and during the first year most of the trees in both treatments produced around three flushes (Table 3). Although the length of the first flush did not differ between treatments, significant treatment differences in stem radial growth (wood production) were apparent in all three flushes
(Table 3). Shoot length of the third flush increased 296% by the elevated CO2 treatment.
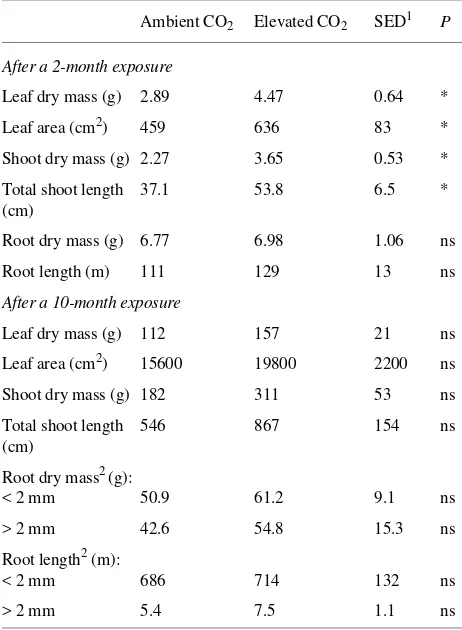
Because Prunus species grow faster than Quercus species, the amount of dry mass produced by Prunus plants was deter-mined after 2 and 10 months of growth. Analysis of 2-month-old Prunus plants showed significant increases in the dry mass of shoots (61%) and leaves (55%) in response to elevated CO2,
but not in roots (Table 4). Although small increases in dry mass in response to elevated CO2 were still evident in 10-month-old
Prunus plants, none of the treatment effects were significant (Table 4). In both species, there were linear relationships between leaf area and stem diameter that were independent of CO2 concentration (data not shown).
Leaf mineral content (N, P, K, Ca and Mg) in Quercus and Prunus was measured in early August and in October, because differences between dates were slight in both species only the August data are presented in Table 5. The only statistically significant effect of elevated CO2 was to lower the foliar
concentrations of potassium in Quercus and calcium in Prunus (Table 5).
Discussion
Acclimation of assimilation as an explanation of differences in dry matter production
Prunus plants were less responsive to elevated CO2 than
Quer-cus seedlings. Compared to the ambient CO2 treatment, the
Table 2. Total dry mass of shoots and roots, leaf area production and total root and shoot length (including main stem plus all branches) of Quercus robur seedlings after 10 months of exposure to either ambient or elevated CO2.
SED = Standard error of difference of means; significant differences (P) are shown by the symbols: ns = not significant, * = 0.05, ** =
Table 3. Main stem length (cm), stem cross-sectional area (mm2) and radial growth (mm2 day--1× 103) of Quercus robur seedlings measured or calculated within three stem flushes over a 4-month period of growth between 6 months and 10 months of exposure to either ambient or elevated CO2.
Flush one Flush two Flush three
Main stem length1 (cm)
Ambient CO2 13.1 (71) 12.1 (39) 11.3 (28)
Elevated CO2 12.4 (70) 16.1 (53) 44.7 (45)
SED2 0.6 1.5 2.9
P ns ** ***
Stem cross-sectional area (mm2)
Ambient CO2 25.7 12.6 6.5
Elevated CO2 48.7 28.5 17.9
SED 3.0 1.9 1.4
P *** *** ***
Mean radial growth of stem (mm2 day--1× 103)
Ambient CO2 152 74 55
Elevated CO2 577 299 157
SED 47 26 15
P *** *** ***
1 Main stem length not including branches. The figures in brackets
refer to the number of seedlings with one, two or three stem flushes.
2 SED = Standard error of difference of means; significant differences
elevated CO2 treatment caused a 27% enhancement in dry
mass production in Prunus after two months and 51% after 10 months compared to a dry mass gain of more than 200% after 10 months in Quercus seedlings. The limited response of Prunus to elevated CO2 may be associated with photosynthetic
acclimation (Besford et al. 1990, Gunderson and Wullschleger 1994, Wilkins et al. 1994). After 10 months of growth in elevated CO2, A, measured at elevated CO2 for Prunus, was not
significantly different from that of plants grown and measured at ambient CO2; whereas, A of Prunus plants grown at elevated
CO2 and measured at ambient CO2 was significantly lower by
about 80%, than A of plants grown and measured at ambient CO2. Because the area of individual Prunus leaves in the
elevated CO2 treatment was about 87% greater than the area of
leaves in the ambient CO2, we also expressed A on a per leaf
basis. Net assimilation rate of leaves grown in elevated CO2
and measured at ambient CO2 was only about 40% that of
leaves grown in ambient CO2 on a per leaf basis, which
sug-gests that photosynthetic acclimation had taken place. Net assimilation rate of Quercus grown and measured at elevated CO2 was over twice that of seedlings grown and
measured at ambient CO2, whereas A of seedlings grown at
elevated CO2 and measured at ambient CO2 was similar to A of
seedlings grown and measured at ambient CO2.Thus, in
con-trast to Prunus, Quercus seedlings exhibited no loss of photo-synthetic activity even after 10 months in elevated CO2. A
comparison of A measured at elevated CO2 of Quercus
seed-lings grown in ambient and elevated CO2, indicated that there
was some gain in photosynthetic capacity in response to the elevated CO2 treatment (Table 1).
It has been suggested that light-saturated A at low CO2
concentrations is limited by RuBP-saturated Rubisco activity (e.g., von Caemmerer and Farquhar 1981, Besford et al. 1985), whereas at high CO2 concentrations A may become
increas-ingly dependent on RuBP regeneration through, for example, light harvesting and photosynthetic electron transport capacity Table 4. Total dry mass of shoots and roots, leaf area production and
total root and shoot length(including main stem and branches) of Prunus avium×pseudocerasus exposed for either 2 or 10 months to either ambient or elevated CO2.
Ambient CO2 Elevated CO2 SED1 P
After a 2-month exposure
Leaf dry mass (g) 2.89 4.47 0.64 *
Leaf area (cm2) 459 636 83 *
Shoot dry mass (g) 2.27 3.65 0.53 *
Total shoot length 37.1 53.8 6.5 * (cm)
Root dry mass (g) 6.77 6.98 1.06 ns
Root length (m) 111 129 13 ns
After a 10-month exposure
Leaf dry mass (g) 112 157 21 ns
Leaf area (cm2) 15600 19800 2200 ns
Shoot dry mass (g) 182 311 53 ns
Total shoot length 546 867 154 ns (cm)
Root dry mass2 (g):
< 2 mm 50.9 61.2 9.1 ns
> 2 mm 42.6 54.8 15.3 ns
Root length2 (m):
< 2 mm 686 714 132 ns
> 2 mm 5.4 7.5 1.1 ns
1
SED = Standard error of difference of means; significant differences (P) are shown by the symbols: ns = not significant, * = 0.05, ** = 0.01 and *** = 0.001.
2
Root dry mass and root length were partitioned with respect to root diameter.
Table 5. Mineral nutrient concentration (% dry mass basis) in leaves of Quercus robur after 6 months exposure to ambient or elevated CO2 and
Prunus avium × pseudocerasus after two months of growth at either ambient or elevated CO2.
Nitrogen Phosphorus Potassium Calcium Magnesium
Quercus
Ambient CO2 2.93 0.23 1.31 1.45 0.37
Elevated CO2 2.71 0.21 0.97 1.30 0.38
SED1 0.11 0.04 0.29 0.31 0.05
P ns ns ** ns ns
Prunus
Ambient CO2 3.10 0.39 2.96 3.13 0.47
Elevated CO2 2.92 0.34 2.90 2.88 0.47
SED 0.15 0.03 0.14 0.09 0.01
P ns ns ns * ns
1 SED = Standard error of difference of means; significant differences (P) are shown by the symbols: ns = not significant, * = 0.05, ** = 0.01 and
(von Caemmerer and Farquhar 1981). Leaves of Quercus plants grown at elevated CO2 contained increased amounts of
thylakoid-bound cytochrome f (a potential rate limiting step in photoelectron transport) with no apparent change in the amount of Rubisco LSU. In previous work with Prunus avium grown in elevated CO2 for two years, both Rubisco LSU and
cytochrome f amounts were lower in plants grown in elevated CO2 than in plants grown in ambient CO2 (Wilkins et al. 1994)
and the amounts of Rubisco decreased before changes in composition of thylakoid membranes were observed (Van Oosten and Besford 1995). In our study, Prunus grown in elevated CO2 showed a reduction in the amount of LSU, but
not of cytochrome f.
It has not yet been demonstrated unequivocally that the apparent difference in response between Quercus and Prunus is not an experimental artifact. There is some evidence that photosynthetic acclimation is less likely to occur in field-grown trees than in container-field-grown trees in a greenhouse (Gunderson et al. 1993). In our study, the physical effects of root restriction were minimized by increasing pot size twice in the first year and by using large, deep pots. Furthermore, recent work with Prunusavium has shown that root restriction does not necessarily have a negative affect on the CO2-induced
stimulation of dry matter production (Kerstiens and Hawes 1994).
An absence of acclimation of A has been observed in several Quercus species after long-term exposure to elevated CO2
(Gunderson et al. 1993, Ceulemans and Mousseau 1994), although there are exceptions. Of the temperate deciduous tree species examined by Bunce (1992), only Quercus robur showed photosynthetic acclimation. No conclusion can be reached for Prunus, because there have been few studies of the effects of elevated CO2 on this species (see Bazzaz et al. 1990,
Wilkins et al. 1994). However, we found no evidence that environmentally-based factors could explain the differences between Quercus and Prunus. In the elevated CO2 treatment,
Quercus roots represented 45% of total dry mass, whereas in Prunus only 20% of total dry mass was partitioned to roots. Acclimation of A may be an effective mechanism for restrict-ing the rate of aerial growth and thereby restorrestrict-ing a more even distribution of dry mass between root and shoot. The mainte-nance of this ratio is an important component of the ability of the root to sustain the shoot, particularly with respect to the supply of water and nutrients. Low nutrient status can impose a limitation on A especially in plants grown in elevated CO2
(Norby et al. 1992). In our experiments, the possibility of soil nutrient depletion, at elevated CO2, influencing leaf nitrogen
and A was minimized by weekly applications of nutrient solu-tion. Mineral analyses indicated that the loss of photosynthetic capacity in Prunus grown at elevated CO2 was not the result of
foliar nutrient deficiency.
Dry matter allocation and sink development influences on assimilation
The elevated CO2 treatment had a pronounced effect on the
1986). Shoot length of the first flush did not differ with respect to CO2 treatment, but later extension growth, particularly of the
third flush, was significantly different for trees grown at ele-vated CO2. Radial growth (wood production) was significantly
greater in all three flushes in plants grown at elevated CO2.
These findings highlight the effects of elevated CO2 on carbon
allocation and meristematic function with respect to apical growth and cambial production, which in turn will have impli-cations for the development of the tree canopy to maturity. The switch to a more indeterminate pattern of Quercus shoot growth in response to elevated CO2 is important, because the
stimulation of new apical meristems creates new sinks for carbohydrate utilization, which may explain why photosyn-thetic capacity was maintained in the elevated CO2 treatment.
In conclusion, we found important differences in the behav-ior of two woody species to elevated CO2. Quercus robur did
not show photosynthetic acclimation, whereas Prunus did. This difference appeared to be associated with the ability of Quercus to maintain its photosynthetic potential as a result of a stable content of Rubisco. A stable Rubisco content in leaves exposed to elevated CO2 may reflect the ability of Quercus to
develop new sinks for carbohydrate utilization.
Acknowledgments
This work was sponsored by the European Commission (Climate Change Impacts, Project EV5V-CT92-0093) and the Ministry of Ag-riculture, Fisheries and Food, U.K. We are grateful to Drs. J.D. Quin-lan, B.H. Howard and Prof. H.G. Jones for their comments, to Martin Ridout for statistical advice, the greenhouse staff at HRI-Littlehamp-ton for their assistance with growing the plants and for technical help from Ann Lucas and Lorraine Taylor (HRI-East Malling).
References
Atkinson, C.J. 1996. Global changes in atmospheric carbon dioxide: The influence on terrestrial vegetation. In Plant Response to Air Pollution. Eds. M. Iqbal and M. Yunus. John Wiley and Sons Ltd., Sussex, U.K., pp 99--133.
Bazzaz, F.A., J.S. Coleman and S.R. Morse. 1990. Growth responses of major co-occurring tree species of the Northeastern United States to elevated CO2. Can. J. For. Res. 20:1479--1484.
Besford, R.T. 1990. The greenhouse effect: Acclimation of tomato plants growing in high CO2, relative changes in Calvin Cycle
enzymes. J. Plant Physiol. 136:458--463.
Besford, R.T., L.J. Ludwig and A.C. Withers. 1990. The greenhouse effect: Acclimation of tomato plants growing in high CO2
photosyn-thesis and ribulose-1,5-bisphosphate carboxylase protein. J. Exp. Bot. 41:925--931.
Besford, R.T., C.M. Richardson, J.L. Campos and A.F. Tiburcio. 1993. Effect of polyamines on stabilization of molecular complexes in thylakoid membranes of osmotically stressed oat leaves. Planta 189:201--206.
Besford, R.T., A.C. Withers and L.J. Ludwig. 1985. Ribulose bisphos-phate carboxylase activity and photosynthesis during leaf develop-ment in the tomato. J. Exp. Bot. 36:1530--1541.
Ceulemans, R. and M. Mousseau. 1994. Effects of elevated atmos-pheric CO2 on woody plants. New Phytol. 127:425--446.
Clough, J.M., M.M. Peet and P.J. Kramer. 1981. Effects of high atmospheric CO2 and sink size on rates of photosynthesis of a
soybean cultivar. Plant Physiol. 67:1007--1010.
Cure, J.D. and B. Acock. 1986. Crop response to carbon dioxide doubling: A literature survey. Agric. For. Meteorol. 38:127--145. Eamus, D. and P.G. Jarvis. 1989. The direct effects of an increase in
the global atmospheric CO2 concentration on natural and
commer-cial temperate trees and forests. Adv. Ecol. Res. 19:1--55. Gunderson, C.A., R.J. Norby and S.D. Wullschleger. 1993. Foliar gas
exchange responses of two deciduous hardwoods during 3 years of growth in elevated CO2: No loss of photosynthetic enhancement.
Plant Cell Environ. 16:797--807.
Gunderson, C.A. and S.D. Wullschleger. 1994. Photosynthetic accli-mation in trees to rising atmospheric CO2: A broader perspective.
Photosynth. Res. 39:369--388.
Hanson, P.J., R.E. Dickson, J.G. Isebrands, T.R. Crow and R.K. Dixon. 1986. A morphological index of Quercus seedling ontogeny for use in studies of physiology and growth. Tree Physiol. 2:273--281. Kerstiens, G. and C.V. Hawes. 1994. Response of growth and carbon
allocation to elevated CO2 in young cherry (Prunus avium L.)
saplings in relation to root environment. New Phytol. 128:607--614.
Krupa, S.V. and R.N. Kickert. 1989. The greenhouse effect: Impacts of ultraviolet-B (UV-B) radiation, carbon dioxide (CO2), and ozone
(O3) on vegetation. Environ. Pollut. 61:263--393.
Long, S.P. 1991. Modification of the response of photosynthetic pro-ductivity to rising temperature by atmospheric CO2 concentrations:
Has its importance been underestimated? Plant Cell Environ. 14:729--739.
Long, S.P. and B.G. Drake. 1992. Photosynthetic CO2 assimilation and
rising atmospheric CO2 concentration. In Crop Photosynthesis:
Spatial and Temporal Determinants. Eds. N.R. Baker and H. Thomas. The Hague: Elsevier Science Publishers B.V., pp 69--103. Mehta, R.A., T.W. Fawcett, D. Porath and A.K. Mattoo. 1992.
Oxida-tive stress causes rapid membrane translocation and in vivo degra-dation of ribulose-1,5-bisphosphate carboxylase oxygenase. J. Biol. Chem. 267:2810--2816.
Norby, R.J., C.A. Gunderson, S.D. Wullschleger, E.G. O’Neill and M.K. McCracken. 1992. Productivity and compensatory responses of yellow-poplar trees in elevated CO2. Nature 357:322--324.
Sinclair, T.R. 1992. Mineral nutrition and plant growth response to climate change. J. Exp. Bot. 43:1141--1146.
Thomas, R.B. and B.R. Strain. 1991. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol. 96:627--634.
von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376--387.
Van Oosten, J.-J. and R.T. Besford. 1994. Sugar feeding mimics effect of acclimation to high CO2-rapid down regulation of RuBisCo
small subunit transcripts but not of the large subunit transcripts. J. Plant Physiol. 143:306--312.
Van Oosten, J.-J. and R.T. Besford. 1995. Some relationships between gas-exchange, biochemistry and molecular biology of photosynthe-sis during leaf development of tomato plants after transfer to differ-ent carbon dioxide concdiffer-entrations. Plant Cell Environ. 18:1253--1266.
Wilkins, D., J.-J. Van Oosten and R.T. Besford. 1994. Effects of elevated CO2 on growth and chloroplast proteins in Prunus avium.