Summary Container-grown black spruce (Picea mariana (Mill.) B.S.P.) seedlings were planted in trays containing a sand and peat mixture, and placed in a climate-controlled green-house. One group of seedlings was kept well-watered, and another group was subjected to three cycles of drought. Gas exchange analysis showed that mesophyll photosynthetic func-tion was largely unimpaired by drought. In contrast, stomatal conductance was sensitive to drought, although it became less sensitive with each drought cycle. Both stomatal and meso-phyll conductances increased with time in control and drought-stressed seedlings, but mesophyll conductance increased with time more rapidly than did stomatal conductance. Limitation of photosynthetic rate was dominated by the mesophyll. In control seedlings, relative stomatal limitation increased from 6 to 16% by the end of the experiment. In drought-stressed seedlings, relative stomatal limitation of photosynthesis reached 40% during the first drought, but decreased to near control values immediately after rewatering. Because the third, most severe drought had only a minor effect on stomatal conductance, relative stomatal limitation of photosynthesis was similar to that in control seedlings by the end of the experiment. Inhibition of ontogenetic change during drought stress may be responsible for the apparent acclimation of mesophyll photosynthetic processes. We conclude that it would be more effective to select for high photosynthetic capacity than for reduced stomatal sensitivity when breeding for in-creased drought resistance in black spruce seedlings.
Keywords: acclimation, container stock, drought resistance, mesophyll conductance, Picea mariana, stomatal limitation, stomatal sensitivity.
Introduction
Young conifer seedlings are subject to a variety of environ-mental stresses when they are planted on clear-cut sites. Of these stresses, drought is perhaps the most prevalent (Burdett 1990). Even in the boreal forest, a decrease in water content of the surface horizons of the soil can subject shallow-rooted, newly planted seedlings to severe water stress. Seedlings that can acclimate to the first drought may be better able to survive subsequent droughts. Because water stress inhibits the growth
of conifer seedlings, any physiological or morphological ad-justment that ameliorates the growth reduction due to water stress will improve their rate of establishment.
The growth of a plant depends on a series of physical and biochemical processes that transfer CO2 from the surrounding air into organic molecules, and, for this reason, much work on acclimation to water stress in trees has focused on the role of stomata in limiting gas exchange in response to stress (e.g., Unterscheutz et al. 1974, Seiler 1985, van den Driessche 1991). Because mesophyll processes involved in the transfer of CO2 into the chloroplasts and CO2 fixation may also be limit-ing durlimit-ing drought stress, there have been several studies of photosynthetic as well as stomatal acclimation to water stress in trees (e.g., Seiler and Johnson 1985, 1988, Walters and Reich 1989). However, few studies have examined the relative contributions of stomatal and nonstomatal acclimation to net photosynthetic acclimation to drought in trees (Epron and Dreyer 1990, Seiler and Cazell 1990) and nonwoody plants (Hutmacher and Krieg 1983, Matthews and Boyer 1984, Cor-nic et al. 1987).
To identify the traits that contribute most to drought accli-mation in black spruce seedlings, we subjected seedlings to three cycles of drought and measured their photosynthetic responses to CO2 during the drought and recovery periods. The objectives of this study were (a) to test for acclimation to drought stress in net photosynthesis of black spruce seedlings over three drought cycles, (b) to determine the relative sto-matal and nonstosto-matal contributions to the drought response, and (c) to identify the mesophyll processes, if any, involved in acclimation.
Materials and methods
Seedling growing conditions
Black spruce seeds from a lowland Quebec population were planted in nursery containers (Rigipot, IPL, St-Damien, Que-bec) with 50 ml cavities filled with a 3/1 (v/v) peat/vermiculite medium. Seedlings were raised under standard conditions in a commercial greenhouse for one growing season, hardened outside and then placed in black plastic bags in a coldroom at 2 °C for 3 months.
Stomatal and mesophyll limitations of photosynthesis in black spruce
seedlings during multiple cycles of drought
J. D. STEWART,
1,2A. ZINE EL ABIDINE
2and P. Y. BERNIER
11 Forestry Canada, Quebec Region, C.P. 3800, Sainte-Foy, Quebec G1V 4C7, Canada
2 Centre de Recherche en Biologie Forestière, Faculté de Foresterie et de Géomatique, Université Laval, Sainte-Foy, Québec G1K 7P4, Canada
Received June 7, 1993
In January, the seedlings were moved to a greenhouse and transplanted from the nursery containers to 15 × 60 cm trays, each containing 10 seedlings. The trays were filled to a depth of 10 cm with a 3/1 (v/v) fine sand/peat mixture. Trays were watered daily by an automatic overhead watering boom equipped with fine spray nozzles. Seedlings were fertilized using the watering system twice per week, with each tray receiving approximately 47 mg N,P,K (20-20-20) and 1.5 mg MgSO4. Greenhouse day/night temperatures were 19--24/11--20 °C, and day/night relative humidity was 20--90/65--100%; both varied with external weather conditions. The seedlings were maintained in a 16-h photoperiod using high pressure sodium vapor lamps (Lumalux GTE, Sylvania Canada Ltd.) that provided 120 µmol m−2 s−1 PAR at seedling level. Tray positions on the bench were randomized twice a week.
Bud burst occurred in early February, and the seedlings were held under constant conditions until shoot elongation was completed. At the beginning of the treatment period, seedlings averaged 14.0 cm in height and 1.7 cm in diameter.
Cyclic drought and recovery treatment
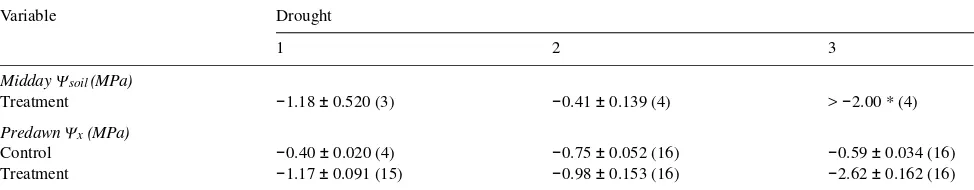
Beginning March 11, the cyclic drought and recovery treat-ment was initiated by withholding water from the seedlings. Control seedlings continued to be watered daily by hand. Drought was allowed to progress until turgor loss (wilting) became visible in the current shoots of some seedlings, where-upon the drought was terminated. Three such drought episodes lasting 10, 10 and 8 days, respectively, were completed. Each drought period was followed by a 7-day recovery period, during which all seedlings were watered daily and fertilized as described above. Each pair of drought and recovery periods comprised a drought treatment cycle, and the three cycles occupied 49 days. Soil water potential (Ψsoil) was measured with calibrated gypsum blocks (Soilmoisture Equipment Cor-poration, Santa Barbara, CA), and predawn xylem water po-tential (Ψx) was measured with a pressure chamber (PMS Instruments, Corvallis, OR) in neighboring nonexperimental trays. The severity of the droughts as measured by soil and predawn xylem water potentials is shown in Table 1 (see also Zine El Abidine et al. 1995).
At intervals during the drought and recovery cycles, one seedling from each of four trays was selected at random for gas exchange measurements. Each seedling was excavated and
placed in a Ray Leach ‘‘Conetainer’’ (Stuewe and Sons, Cor-vallis, OR) in a controlled environment chamber. On each measurement day, all four seedlings were either from the control group or from the treatment group.
The response of net photosynthesis (Anet) to intercellular CO2 concentration (ci), i.e., the A/ci relationship, was measured by the nonsteady state method (Davis et al. 1987, McDermit et al. 1989) in a closed-circuit gas analysis system (LI-6200, Li-Cor Inc., Lincoln, NB). To standardize the measurement process, the seedlings were placed in a controlled environment chamber and kept in the dark in a loosely closed box until needed. The concentration of CO2 in the chamber was elevated as a result of respiration by the observer and was crudely regulated to between 600 and 700 µmol mol−1. Water-cooled high pressure sodium and mercury lamps were suspended above the cuvette and provided 1000 µmol m2 s−1 PAR at the level of the cuvette. For additional heat dissipation, a transpar-ent acrylic tray filled with water was placed between the lamp and the cuvette. A fan circulated ambient air around the cuvette to increase convective cooling. Each seedling was allowed to adjust to the measurement light conditions for 30 min before being placed in the 4-l cuvette. Once the seedling was sealed in the gas exchange cuvette, the relative humidity was raised to 85% by pumping ambient air through a gasing wash bottle, filled with water at room temperature, into the gas exchange system in open mode. The high humidity represented the upper calibration limit of the humidity sensor (personal communica-tion, J.M. Welles, Li-Cor Inc., Lincoln, NB) and was used to avoid stomatal closure in response to high VPD in the water-stressed seedlings (Li-Cor 1991), which would have prevented the gas exchange analysis and is known to provoke heteroge-neous stomatal closure in some species. After closure, the system was equilibrated under the measurement conditions for 20 to 30 min before measurements were taken.
Gas exchange measurements were taken continuously as the seedling depleted the CO2 in the closed system. Observations of CO2 and water fluxes were averaged for every 10 µmol mol−1 change in CO2 concentration. Under severe water stress, some seedlings had completely closed stomata, and the gas exchange was too slow to be measured. These seedlings were not included in the analysis.
Gas exchange parameters calculated were Anet, ci, CO2 com-pensation point (Γ), transpiration rate (E) and stomatal con-ductance to water vapor (gs). The program of the LI-6200 was
Table 1. Midday soil water potentials (Ψsoil) and predawn xylem water potentials (Ψx) measured at the end of each drought period before rewatering. Means ± SE and number of observations are presented. All four soil water potentials measured at the end of the third drought were at or beyond the range of the gypsum resistance blocks used.
altered to account for the leak rate of the system as described in Li-Cor (1991). When the CO2 depletion rate slowed in the lower part of the curve, CO2 was removed by opening the CO2 scrubbing circuit of the LI-6200 for 45 s, followed by a 5-min equilibration time before logging of observations resumed. The average change in CO2 concentration per unit time during the scrub cycle was similar to that during the continuous upper portion of the curve. Ambient CO2 concentration used for leak rate corrections was measured immediately after finishing a curve. The needles were dried for 48 h in a forced air oven at 70 °C, and dry weights of new and old needles were deter-mined on cooled samples.
Curve-fitting and parameter calculations
Various nonlinear functions were fitted to the CO2 response (A/ci) data. Two three-parameter functions, the monomolecular (or Mitscherlich) function and the rectangular hyperbolic func-tion, did not produce satisfactory fits. The four-parameter nonrectangular hyperbola suggested by Jones (1983) gave the best fit to the data:
Anet=
Amax(ci−Γ− AnetRi)
Km+ ci−Γ− AnetRi
. (1)
The fitted parameters (Km, Amax, Γ and Ri) of the hyperbolic function correspond to physiological properties of the photo-synthetic system. The symbol Amax is the CO2 saturated rate of net photosynthesis and represents the maximum rate of RuBP regeneration by the Calvin cycle (Farquhar et al. 1980, von Caemmerer and Farquhar 1981). The symbol Γ is the CO2 compensation point, which is primarily determined by pho-torespiration rates. The symbol Km is a constant for Rubisco affinity for CO2, but it may also be influenced by the light reactions (Jones 1983). The symbol Ri is a measure of the resistance to liquid phase transport of CO2 within the meso-phyll, but may also be determined by the activity of the car-boxylase which may be acting well below its maximum rate in the lower, near-linear portion of the response curve (Jones 1983). The carboxylation efficiency of Rubisco associated with changes in concentration or activity (Vrubisco) was calcu-lated from the first derivative of the function, evaluated at A = 0, and is closely related to 1/Ri.
Stomatal and mesophyll conductances to CO2 for an atmos-pheric concentration of 350 µmol mol−1 were obtained from the response curve data following the procedure described by Jones (1985). Stomatal conductance (gs) defined the slope of the supply curve connecting ca with ci on the demand curve. Mesophyll conductance (gm) was calculated as the slope of the demand (A/ci) curve at its intersection with the supply curve. Stomatal limitation of photosynthesis (Ls) was calculated as in Jones (1985, Equation 17):
Ls=
1/gs
1/gs+ 1/gm
. (2)
Statistical analysis
For the control seedlings, the calculated variables were tested for linear and quadratic responses over the four control meas-urement dates. Analyses of variance and a priori contrasts were performed to assess (a) the differences in response among the three drought cycles, and (b) the effect of the drought and recovery treatment versus control conditions. Before the analysis of variance, data variables were transformed as neces-sary to uphold the assumptions of normality and heteroscedas-ticity for the analysis of variance. No transformation was required for Amax, Vrubisco and gs. All analyses were performed using the General Linear Models procedure of SAS. Effects were considered statistically significant when the probability of a Type I error was 0.05 or less.
Results
Conductance to CO2 and photosynthetic limitation
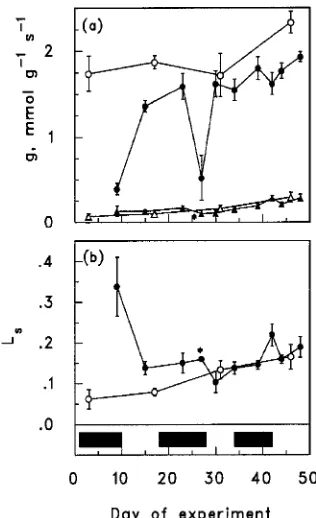
Stomatal conductance of control seedlings increased with seedling age, though only during Cycle 3 (Figure 1). Seedlings recovering from drought showed a similar trend, but stomatal conductance was generally lower in recovering seedlings than in control seedlings, especially in Cycles 1 and 3 (Cycle 2
produced only a mild stress; Table 1). Drought caused a large decrease in stomatal conductance, but recovery following re-watering was rapid. The drought-induced reduction in stomatal conductance was least in Cycle 3, even though this was the most severe drought.
Mesophyll conductance increased linearly with age in con-trol (Table 2) and drought-stressed seedlings (Figure 1), and there was no significant difference between control and drought-stressed seedlings (Table 3).
In control seedlings, the relative stomatal limitation of pho-tosynthesis increased linearly with age (Table 2), rising from 6% at the beginning of the experiment to about 16% at the end (Figure 1). Stomatal limitation increased to about 40% during the period of maximum drought stress in Cycle 1, but the effect was much less during the drought periods in Cycles 2 and 3 (Figure 1). Relative stomatal limitation quickly declined to near control values on rewatering, and there were no signifi-cant differences between recovering and control seedlings in Cycles 2 and 3 (Table 3). Overall, the rate of photosynthesis was less affected by stomatal conductance than by mesophyll conductance.
Gas exchange parameters
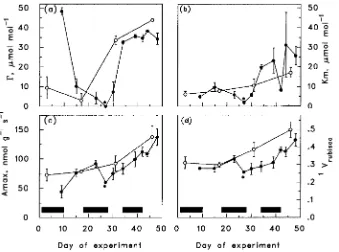
Most of the gas exchange parameters measured in control seedlings increased with time (Table 2 and Figure 2), whereas the effect of drought varied among the gas exchange parame-ters and drought cycles.
Gas exchange parameters of both control and recovering seedlings showed increases mostly in Cycle 3 (Table 3); how-ever, Γ also increased from Cycle 1 to Cycle 2. There was a significant difference in Γ between control and recovering seedlings late in Cycles 1 and 2 (Table 2 and Figure 2). By Cycle 3, Γ was lower in recovering seedlings than in control seedlings. Both control and recovering seedlings exhibited similar Amax. Drought only affected Amax during Cycles 1 and 2 (Figure 2 and Table 3). The value of Km decreased with drought, but tended to recover to values greater than those of control seedlings on rewatering, but this effect was only statis-tically significant in Cycle 2 (Table 2 and Figure 2). There was a significant difference in Vrubisco between control and recov-ering seedlings late in Cycle 2 (Table 2 and Figure 2). Drought only affected Vrubisco during Cycle 2.
Discussion
Ontogenetic changes
As seedlings go through a series of drought cycles, they re-spond not only to water stress, but also to increasing age and ontogenetic state. The A/ci curves of control seedlings showed changes in photosynthetic properties over time. The values of Amax, Vrubisco and Km increased with age (Figure 2), resulting in an increase in mesophyll conductance with age (Figure 1). All of these processes changed in concert, suggesting that this ontogenetic response is a reflection of the increasing photosyn-thetic competence of the needles as they mature (Ludlow and Wilson 1971, Field 1987).
The apparent trend in Γ may not be real, because values Table 2. Regression analysis of the linear response of the measured
variables in control seedlings with respect to time. The parameter estimates for intercept and slope, and the t-test probability that slope is not equal to zero are presented.
Variable Intercept Slope Probability
gs 1.64 0.0114 0.070
gm 0.035 0.0049 0.001
Ls 0.048 0.0025 0.002
Amax 59.5 1.47 0.002
Γ −0.089 0.934 0.001
Km 4.45 0.248 0.002
Vrubisco 0.261 0.0046 0.001
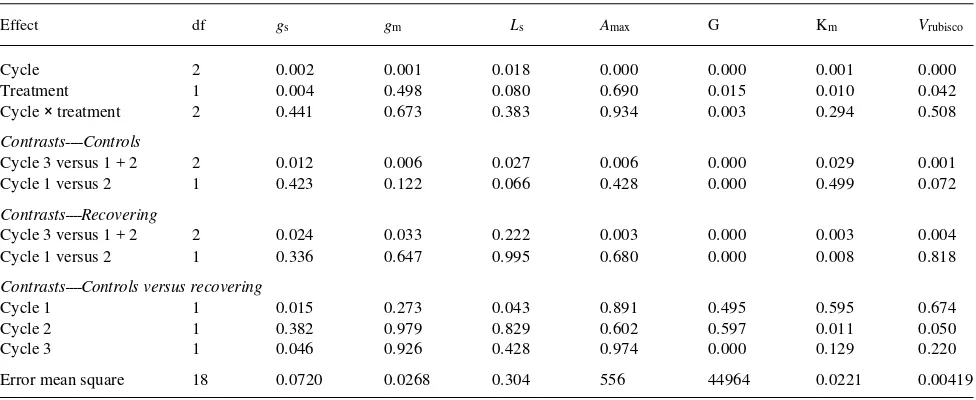
Table 3. Statistical summary for gas exchange and photosynthetic parameters for control seedlings and treatment seedlings at the end of the recovery periods following each drought. Probability values from a two-way ANOVA with contrasts are presented.
Effect df gs gm Ls Amax G Km Vrubisco
Cycle 2 0.002 0.001 0.018 0.000 0.000 0.001 0.000
Treatment 1 0.004 0.498 0.080 0.690 0.015 0.010 0.042
Cycle × treatment 2 0.441 0.673 0.383 0.934 0.003 0.294 0.508
Contrasts----Controls
Cycle 3 versus 1 + 2 2 0.012 0.006 0.027 0.006 0.000 0.029 0.001
Cycle 1 versus 2 1 0.423 0.122 0.066 0.428 0.000 0.499 0.072
Contrasts----Recovering
Cycle 3 versus 1 + 2 2 0.024 0.033 0.222 0.003 0.000 0.003 0.004
Cycle 1 versus 2 1 0.336 0.647 0.995 0.680 0.000 0.008 0.818
Contrasts----Controls versus recovering
Cycle 1 1 0.015 0.273 0.043 0.891 0.495 0.595 0.674
Cycle 2 1 0.382 0.979 0.829 0.602 0.597 0.011 0.050
Cycle 3 1 0.046 0.926 0.428 0.974 0.000 0.129 0.220
below 10 µmol mol−1 are not likely to occur in a C3 plant (normal range is 30 to 70 µmol mol−1). The data points at the bottom of the curve, which have the greatest influence on the value of Γ, have a lower precision than those at the top of the curve, due to the very low rates of gas exchange at low ca. Unlike the other gas exchange parameters, the calculated val-ues of Γ were sensitive to the form of equation used to fit the data. The monomolecular function gave more realistic and consistent values of Γ than the nonrectangular hyberbolic function, but the overall fit was not as good, especially for the upper portion of the curve.
Stomatal conductance increased with age. Relative stomatal limitation of photosynthesis also increased with age, because mesophyll conductance increased faster than stomatal conduc-tance (Table 2 and Figure 2). Although both gs and gm increase with age in most plants, the relative rates of increase vary among species (e.g., Ludlow and Wilson 1971, Field 1987, Mebrahtu and Hanover 1991). Consequently, the change in relative stomatal limitation of photosynthesis can be positive, negative or constant, depending on the species.
Acclimation
Acclimation in drought-stressed seedlings is manifested as either a change in the degree of response to drought, or a persistent difference between recovering seedlings and the controls. Although the values of photosynthetic variables in treated and control seedlings followed similar trends with time, acclimation responses to the drought and recovery cycles were seen. The finding that gs, Amax and Γ reacted to drought and rewatering in the first two cycles, but not in the third and most severe drought cycle, indicates that these variables exhib-ited decreasing sensitivity to drought (Figures 1 and 2). It is possible that stomatal sensitivity to drought decreased with
both seedling age and drought history. Although a reduction in stomatal sensitivity with age has been reported for orange trees (Syvertsen et al. 1981), stomatal sensitivity to drought usually increases with age, especially in agricultural species (Field 1987).
A persistent difference between the control and recovering seedlings 7 days after drought relief was observed for gs, Γ, Km and Vrubisco (Figures 1 and 2, and Table 3). However, only gs showed a consistent difference over the three cycles. The responses of the other mesophyll photosynthesis variables were either significant only in the second cycle (Km and Vrubisco), or inconsistent in trend (Γ), possibly due to measure-ment error. In general, mesophyll photosynthetic function, as reflected by mesophyll conductance, was largely independent of water stress. In a broad range of species, stomatal closure occurs before mesophyll photosynthetic function is greatly affected by water stress (Gollan et al. 1985, Teskey et al. 1986, Kirschbaum 1987, Grieu et al. 1988, Grossnickle and Russell 1991). In several studies, mesophyll photosynthetic capacity under saturating CO2 showed little response to dehydration at relative water contents as low as 50--70%, which is beyond the usual turgor loss point (Kaiser 1987, Cornic et al. 1989, Epron and Dreyer 1990).
It is possible that we observed only an apparent acclimation. Drought stress treatments can suspend or slow down the nor-mal process of photosynthetic development (Ludlow and Ng 1974). During the recovery periods between droughts, the ontogenetic trends in gs, Γ, Amax and Vrubisco continued at roughly the same rates in both control and drought-stressed seedlings (cf. slopes in Figures 1 and 2). The apparent accli-mation response could, therefore, be due to an offset in the development curves of control and drought-stressed seedlings caused by the slowing or cessation of development during the drought periods.
Photosynthetic limitation and drought resistance
The finding that stomatal limitation was less than mesophyll limitation in black spruce has also been observed in other conifers (Teskey et al. 1986, Grieu et al. 1988, Seiler and Cazell 1990, Grossnickle and Russell 1991) and in C3 plants in general (Bunce 1977, Briggs et al. 1986, Ni and Pallardy 1992). The relative stomatal limitation at the beginning of the experiment was lower than most reported values; however, stomatal limitation increased with seedling age and was simi-lar to values found in other black spruce trees (calculated from data in Macdonald and Lieffers 1990, Greenway et al. 1992).
The functional consequences of the drought-insensitivity of mesophyll photosynthetic processes combined with the small relative stomatal limitation of photosynthesis are illustrated by plotting Anet against ci, as determined at the operating point (ca = 350 µmol mol−1, Figure 3). For all seedlings pooled, there was a poor correlation between Anet and ci (R2 = 0.019). How-ever, when the data were separated into well-watered (includ-ing recover(includ-ing seedl(includ-ings) and drought-stressed subsets, the regressions explained 45 and 31% of the variation, respec-tively. In drought-stressed seedlings, photosynthesis decreased with decreasing ci as the stomata closed, whereas in well-wa-tered seedlings the relationship was negative. The photosyn-thetic rate largely determined ci and stomatal conductance had little influence. Thus, contrary to observations on several spe-cies by Wong et al. (1979), photosynthetic rate in our seedlings was largely uncoupled from stomatal behavior, except during periods of maximum drought when stomatal conductance was very low.
In cases of heterogeneous stomatal closure, the calculated
values of ci are higher than the real values, which can give the appearance of mesophyll photosynthesis decreasing along with stomatal conductance. The lack of such a coordinated response to drought stress in our experiment supports our assumption that heterogeneous stomatal closure did not occur. Although there have been no reports of heterogeneous sto-matal closure in conifers, we minimized the probability of patchy stomatal closure by taking measurements soon after full light exposure in high humidity on homobaric leaves that had been exposed to a slowly increasing drought stress.
Gollan et al. (1985) postulated that the ability to maintain constant mesophyll photosynthetic processes during water stress, where photosynthesis is reduced only by a reduction in stomatal conductance, is characteristic of a tolerance to soil drought. In black spruce, mesophyll response was negligible under drought conditions that produced soil water potentials of
−1.5 MPa. Limited mesophyll response to drought has been noted in several species including Abies bornmuelleriana Matt. (−1.0 MPa, Guehl et al. 1991), Eucalyptus pauciflora Sieb. ex Spreng. (Kirschbaum 1987), Pseudotsuga macro-carpa (Torr.) Mayr (−1.5 MPa) and Pseudotsuga menziesii (Mirb.) Franco (−1.9 MPa, Grieu et al. 1988). In Cedrus atlantica Manetti, the entire drought response was due to changes in mesophyll processes (Grieu et al. 1988, Guehl et al. 1991).
The drought resistance strategy of black spruce seedlings appears to be one of maximizing, rather than optimizing, photosynthetic rate (Tan et al. 1992). Under drought condi-tions, when stomatal closure limits CO2 uptake as well as water loss, photosynthetic efficiency remains relatively unaffected. Maximizing growth, particularly of the roots, is important in establishment of young seedlings (van den Driessche 1987, Burdett 1990). Although high mesophyll photosynthetic ca-pacity may be of little value during severe droughts when stomata close completely, during moderate droughts the main-tenance of photosynthetic capability may improve the overall net carbon gain and growth of black spruce seedlings.
We were unable to demonstrate unequivocally that the drought treatments induced true active acclimation rather than delaying the ontogenetic processes in black spruce seedlings. However, our results show that photosynthetic capacity re-mained relatively unaffected by drought treatments causing predawn water potentials of −1.5 MPa, although stomatal con-ductance was greatly reduced under such conditions. Stomatal conductance accounted for less than 20% of photosynthetic limitation under most conditions. Based on the variation in photosynthetic capacity (Amax) and efficiency (Vrubisco) found among black spruce seedlings, and the limited degree of con-trol of photosynthetic rate by the stomata, we conclude that it would be more effective to select for high photosynthetic capacity than for reduced stomatal sensitivity when breeding for increased drought resistance in black spruce (cf. Lauer and Boyer 1992).
Acknowledgments
We thank Pierre Davignon for technical assistance, and Gilles Robi-taille for the loan of equipment. Support for this research was provided Figure 3. The relationship between net photosynthetic rate (Anet) and
by Forestry Canada, Quebec Region, Research Project CFL-44. A Natural Sciences and Engineering Research Council of Canada For-estry Postdoctoral Fellowship awarded to JDS is gratefully acknow-ledged. Dr. André Plamondon contributed greatly to the realization of this postdoctoral position and the research carried out during its tenure.
References
Briggs, G.M., T.W. Jurik and D.M. Gates. 1986. Non-stomatal limita-tion of CO2 assimilation in three tree species during natural drought conditions. Physiol. Plant. 66:521--526.
Bunce, J.A. 1977. Nonstomatal inhibition of photosynthesis at low water potentials in intact leaves of species from a variety of habitats. Plant Physiol. 59:348--350.
Burdett, A.N. 1990. Physiological processes in plantation estab-lishment and the development of specifications for forest planting stock. Can. J. For. Res. 20:415--427.
Cornic, G., I. Papgeorgiou and G. Louason. 1987. Effect of a rapid and a slow drought cycle followed by rehydration on stomatal and non-stomatal components of leaf photosynthesis in Phaseolus vul-garis L. J. Plant Physiol. 126:309--318.
Cornic, G., J.-L. LeGouallec, J.M. Briantais and M. Hodges. 1989. Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). Planta 177:84--90.
Davis, J.E., T.J. Arkebauer and J.M. Norman. 1987. Rapid field meas-urement of the assimilation rate versus internal CO2 concentration relationship in green ash (Fraxinus pennsylvanica Marsh.): the influence of light intensity. Tree Physiol. 3:387--392.
Epron, D. and E. Dreyer. 1990. Stomatal and non-stomatal limitation of photosynthesis by leaf water deficits in three oak species: a comparison of gas exchange and chlorophyll a fluorescence data. Ann. Sci. For. 47:435--450.
Farquhar, G.D., S. von Caemmerer and J.A. Berry. 1980. A biochemi-cal model of photosynthetic CO2 assimilation in leaves of C3 spe-cies. Planta 149:78--90.
Field, C.B. 1987. Leaf-age effects on stomatal conductance. In Sto-matal Function. Eds. E. Zeiger, G.D. Farquhar and I.R. Cowan. Stanford Univ. Press, Stanford, CA, USA, pp 367--384.
Gollan, T., N.C. Turner and E.-D. Schulze. 1985. The responses of stomata and leaf gas exchange to vapor pressure deficits and soil water content. III. In the sclerophyllous woody species Nerium oleander. Oecologia 65:356--362.
Greenway, K.J., S.E. Macdonald and V.J. Lieffers. 1992. Is long-lived foliage in Picea mariana an adaptation to nutrient-poor conditions? Oecologia 91:184--191.
Grieu, P., J.M. Guehl and G. Aussenac. 1988. The effects of soil and atmospheric drought on photosynthesis and stomatal control of gas exchange in three coniferous species. Physiol. Plant. 73:97--104. Grossnickle, S.C. and J.H. Russell. 1991. Gas exchange processes of
yellow-cedar (Chamaecyparis nootkatensis) in response to environ-mental variables. Can. J. Bot. 69:2684--2691.
Guehl, J.M., G. Aussenac, J. Bouachrine, R. Zimmermann, J.M. Pen-nes, A. Ferhi and P. Grieu. 1991. Sensitivity of leaf gas exchange to atmospheric drought, soil drought, and water-use efficiency in some Mediterranean Abies species. Can. J. For. Res. 21:1507-1515. Hutmacher, R.B. and D.R. Krieg. 1983. Photosynthetic rate control in
cotton. Stomatal and nonstomatal factors. Plant Physiol. 73:658--661.
Jones, H.G. 1983. Plants and microclimate. A quantitative approach to environmental plant physiology. Cambridge University Press, Cambridge, U.K., 323 p.
Jones, H.G. 1985. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 8:95--104.
Kaiser, W.M. 1987. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 71:142--149.
Kirschbaum, M.U.F. 1987. Water stress in Eucalyptus pauciflora. Comparison of effects on stomatal conductance with effects on the mesophyll capacity for photosynthesis and investigation of a possi-ble involvement of photoinhibition. Planta 171:466--473. Lauer, M.J. and J.S. Boyer. 1992. Internal CO2 measured directly in
leaves. Abscisic acid and low leaf water potential cause opposing effects. Plant Physiol. 98:1310-1316.
Li-Cor, Inc. 1991. A protocol for measuring assimilation rate versus internal CO2 concentration using the LI-6200. Application Note #103. LI-COR Inc., Lincoln, NB.
Ludlow, M.M. and T.T. Ng. 1974. Water stress suspends leaf aging. Plant Sci. Lett. 3:235--240.
Ludlow, M.M. and G.L. Wilson. 1971. Photosynthesis of tropical pasture plants. III. Leaf age. Aust. J. Biol. Sci. 24:1077--1087. Macdonald, S.E. and V.J. Lieffers. 1990. Photosynthesis, water
rela-tions, and foliar nitrogen of Picea mariana and Larix laricina from drained and undrained peatlands. Can. J. For. Res. 20:995--1000. Matthews, M.A. and J.S. Boyer. 1984. Acclimation of photosynthesis
to low leaf water potentials. Plant Physiol. 74:161--166.
McDermit, D.K., J.M. Norman, J.E. Davis, T.J. Arkebauer, J.T. Ball, J.M. Welles and S.R. Roemer. 1989. CO2 Response curves can be measured with a field portable closed-loop photosynthesis system. Ann. Sci. For. 46(suppl.):416--420.
Mebrahtu, T. and J.W. Hanover. 1991. Leaf age effects on photosyn-thesis and stomatal conductance of black locust seedlings. Photo-synthetica 25:537--544.
Ni, B.-R. and S.G. Pallardy. 1992. Stomatal and nonstomatal limita-tions to net photosynthesis in seedlings of woody angiosperms. Plant Physiol. 99:1502--1508.
Seiler, J.R. 1985. Morphological and physiological changes in black alder induced by water stress. Plant Cell Environ. 8:219--222. Seiler, J.R. and B.H. Cazell. 1990. Influence of water stress on the
physiology and growth of red spruce seedlings. Tree Physiol. 6:69--77.
Seiler, J.R. and J.D. Johnson. 1985. Photosynthesis and transpiration of loblolly pine seedlings as influenced by moisture-stress condi-tioning. For. Sci. 31:742--749.
Seiler, J.R. and J.D. Johnson. 1988. Physiological and morphological responses of three half-sib families of loblolly pine to water-stress conditioning. For. Sci. 34:487--495.
Syvertsen, J.P., M.L. Smith Jr. and J.C. Allen. 1981. Growth rate and water relations of citrus leaf flushes. Ann. Bot. 47:97--105. Tan, W.X., T.J. Blake and T.J.B. Boyle. 1992. Drought tolerance in
faster-growing and slower-growing black spruce (Picea mariana) progenies. I. Stomatal and gas exchange responses to osmotic stress. Physiol. Plant. 85:639--644.
Teskey, R.O., J.A. Fites, L.J. Samuelson and B.C. Bongarten. 1986. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol. 2:131--142.
Unterscheutz, P., W.F. Reutz, R.R. Geppert and W.K. Ferrell. 1974. The effect of age, preconditioning and water stress on the transpira-tion rates of Douglas-fir (Pseudotsuga menziesii) seedlings of sev-eral ecotypes. Physiol. Plant. 32:214--221.
van den Driessche, R. 1991. Influence of container nursery regimes on drought resistance of seedlings following planting. II. Stomatal conductance, specific leaf area, and root growth capacity. Can. J. For. Res. 21:566--572.
von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376--387.
Walters, M.B. and P.B. Reich. 1989. Response of Ulmus americana seedlings to varying nitrogen and water status. 1. Photosynthesis and growth. Tree Physiol. 5:159--172.