Phosphorus supply affects the photosynthetic capacity of loblolly
pine grown in elevated carbon dioxide
JAMES D. LEWIS, KEVIN L. GRIFFIN, RICHARD B. THOMAS and BOYD R. STRAIN
Department of Botany, Duke University, Durham, NC 27708-0339, USA
Received November 3, 1993
Summary
Effects of phosphorus supply and mycorrhizal status on the response of photosynthetic capacity to elevated CO2 were investigated in loblolly pine (Pinus taeda L.) seedlings. Seedlings were grown in
greenhouses maintained at either 35.5 or 71.0 Pa CO2 in a full factorial experiment with or without
mycorrhizal inoculum (Pisolithus tinctorius (Pers.) Coker & Couch) and with an adequate or a limiting supply of phosphorus. Assimilation versus internal CO2 partial pressure (Ci) curves were used to estimate
maximum Rubisco activity (Vc,max), electron transport mediated ribulose 1,5-bisphosphate regeneration
capacity (Jmax), phosphate regeneration capacity (PiRC) and daytime respiration rates (Rd).
Nonmycor-rhizal seedlings grown with limiting phosphorus had significantly reduced Vc,max and PiRC compared to
seedlings in other treatments. Elevated CO2 increased photosynthetic capacity in nonmycorrhizal
seed-lings in the low phosphorus treatment by increasing PiRC, whereas it induced phosphorus limitation in mycorrhizal seedlings in the low phosphorus treatment and did not affect the photosynthetic capacity of seedlings in the high phosphorus treatment. Despite the variety of effects on photosynthetic capacity, seedlings in the elevated CO2 treatments had higher net assimilation rates than seedlings in the ambient
CO2 treatments. We conclude that phosphorus supply affects photosynthetic capacity during long-term
exposure to elevated CO2 through effects on Rubisco activity and ribulose 1,5-bisphosphate regeneration
rates.
Keywords: ectomycorrhizae, electron transport capacity, phosphate regeneration capacity, photosyn-thesis, Pinus taeda, Rubisco activity.
Introduction
Photosynthesis is typically limited by the capacity of Rubisco to fix CO2 and the
capacity to regenerate ribulose 1,5-bisphosphate (RuBP) (Farquhar et al. 1980). Under some conditions, phosphate (Pi) may limit photosynthesis if Pi utilization during CO2 assimilation and RuBP regeneration exceed the capacity for Pi release
during starch and sucrose synthesis (Sharkey 1985). Phosphate-regeneration limita-tion of light-saturated photosynthesis has been observed in C3 plants after short-term
(minutes to hours) exposure to elevated partial pressures of CO2 (Sharkey 1985,
Walker and Sivak 1985, Sage and Sharkey 1987, Labate and Leegood 1988) and has been proposed as the mechanism underlying photosynthetic inhibition after long-term (days to weeks) exposure to elevated CO2 (Sage et al. 1989). Two proposed
mechanisms for reduced photosynthetic capacity after long-term exposure to ele-vated CO2 are that either phosphorus limitation (Conroy et al. 1986, Duchein et al.
limits phosphate regeneration capacity (PiRC). The interactive effects of phosphorus supply and source--sink balance on photosynthetic capacity after long-term exposure to elevated CO2 have not been examined.
Long-term exposure to elevated CO2 may induce or compound the effects of
phosphorus deficiency on photosynthesis (Conroy et al. 1988, 1992, Morin et al. 1992, Duchein et al. 1993). Photosynthetic capacity of plants grown at elevated CO2
also recovers from phosphorus stress more slowly, resulting in lower photosynthetic rates than that of plants grown at ambient CO2 (Conroy et al. 1986, 1990). However,
because photosynthesis may be affected by tissue Pi concentrations resulting from phosphorus deficiency (Leegood and Furbank 1986, Brooks et al. 1988, Labate and Leegood 1988, Lauer et al. 1989, Morin et al. 1992), increased PiRC may not always affect Pi limitation of CO2 fixation (Brooks et al. 1988, Bowes 1991). As a result, it
is possible that Pi limitation of photosynthesis after long-term exposure to elevated CO2 may not involve changes in PiRC.
Reduced photosynthetic capacity during long-term exposure to elevated CO2 may
also result from photosynthate source--sink imbalance. Photosynthetic inhibition in plants grown in elevated CO2 can be reversed by removing leaves (Peet 1984) or
reducing root restriction (Thomas and Strain 1991). Parasitic fungal endophytes may also affect photosynthesis by increasing photosynthate sink strength (Marks and Clay 1990). Similarly, ectomycorrhizal fungi increase host tree photosynthetic rates under ambient CO2 conditions by increasing photosynthate sink strength (Ekwebelam and
Reid 1983, Reid et al. 1983, Nylund and Wallander 1989, Dosskey et al. 1990, Rousseau and Reid 1990). Because ectomycorrhizal fungi increase photosynthate sink strength as well as increase phosphorus uptake in phosphorus-limited plants, they may play an important role in offsetting photosynthetic inhibition during long-term exposure to elevated CO2.
We have examined the roles of phosphorus supply and mycorrhizal infection in photosynthetic response to elevated CO2. Two hypotheses were tested: (1) PiRC will
not increase in phosphorus-limited plants after long-term exposure to elevated CO2,
and (2) mycorrhizal infection will result in increased PiRC after long-term exposure to elevated CO2. To test the interactive effects of mycorrhizae and phosphorus supply,
photosynthetic capacity was measured on loblolly pine seedlings (Pinus taeda L.) grown at 35.5 or 71.0 Pa CO2 in a full factorial experiment with either a limiting or
an adequate supply of phosphorus and with or without mycorrhizal inoculum. A biochemical model of photosynthesis (Harley and Sharkey 1991) was used to estimate maximum Rubisco activity, electron transport capacity and PiRC from assimilation versus internal CO2 partial pressure (Ci) curves.
Materials and methods
Growth conditions
main-tained either at 35.0 or 67.5 Pa CO2 (ambient and elevated CO2). Day/night
tempera-tures were 28/17 °C in both chambers. Seed flats were watered as needed with deionized water and were treated weekly with modified one-half strength Hoagland’s nutrient solution.
Approximately 14 days after germination, uniformly sized seedlings were trans-planted to 2.75-l pots filled with a 1/1 (v/v) mixture of gravel and vermiculite. Seedlings in the mycorrhizal treatment were inoculated with vermiculite containing hyphae of Pisolithus tinctorius (Pers.) Coker & Couch by placing inoculum directly on the roots during transplanting. Nonmycorrhizal seedlings were not inoculated. After transplanting, seedlings were placed in one of four greenhouses maintained at either 35.5 or 71.0 Pa CO2. The day/night temperatures were initially 28/17 °C, but
the night temperature was increased to 22 °C after five weeks.
Two phosphorus treatments were initiated three days after transplanting. In the low phosphorus (Low-P) treatment, seedlings were fertilized with a nutrient solution containing 0.083 mM KH2PO4. Seedlings in the high phosphorus (High-P) treatment
were fertilized with 0.5 mM KH2PO4. All seedlings were treated with a nutrient
solution consisting of one-half strength modified Hoagland’s solution lacking phos-phorus. Seedlings were watered with the appropriate nutrient solution every third day and with deionized water on days when the nutrient solution was not applied. The experimental design was a full factorial with three replicates per treatment for a total of 24 seedlings (2 CO2 treatments × 2 mycorrhizal treatments × 2 phosphorus
treatments × 3 replicates).
Gas exchange measurements
Net photosynthesis and conductance were measured on intact needles by infrared gas analysis in an open-type gas exchange system. Gases of the desired CO2 partial
pressures were obtained by mixing CO2-free and CO2-enriched gases from high
pressure cylinders by means of two mass flow controllers (MKS Instruments Inc., Andover, MA, USA). The air stream was humidified to saturation at room tempera-ture by bubbling through deionized water acidified with phosphoric acid to minimize CO2 absorption. The final vapor pressure was set by flowing the air stream through
a glass condensing column in a small refrigerated box (Intertech Corp., Princeton, NJ, USA). Carbon dioxide concentrations were measured with an infrared gas analyzer (LI-6262, Li-Cor Inc., Lincoln, NE, USA). Incoming and outgoing relative humidities, and leaf and air temperatures were measured with a Parkinson-type leaf cuvette (PLCB-002, Analytical Development Corp., Hoddesdon, U.K.). Photosyn-thetic photon flux densities were set to 1200 µmol m−2 s−1 at the cuvette surface. All
gas exchange parameters were calculated according to Field et al. (1989), with the exception that photosynthesis and conductance were calculated on a total needle surface area basis rather than on a projected surface area basis.
surfaces of each needle were photosynthetic. It was also assumed that the fascicle shape approximates that of a cylinder, with each needle occupying one third of the cylinder volume. All fascicles stretched completely across the cuvette. By measuring the cylinder diameter and the inside cuvette length, the total surface area of the needles was then calculated.
Gas exchange measurements were initiated after five months of growth. Measure-ments were made on three fully expanded needles from one fascicle of each seedling. Seedlings to be measured were randomly selected and one or two seedlings were measured each day between 1000 and 1600 h EDT. Each seedling was equilibrated for 45 min at the growth CO2 and then was measured at six CO2 partial pressures
from 8 to 100 Pa CO2, alternating between 2 and 21 kPa O2 at each external CO2
partial pressure (Ca). Seedlings were allowed to equilibrate at each new Ca (typically requiring 20 min or less) and after each change in O2 partial pressure (typically
requiring 10--15 min).
Model parameterization
Photosynthesis was modeled according to Farquhar et al. (1980) and von Caemmerer and Farquhar (1981), as modified by Harley and Sharkey (1991) to include condi-tions where assimilation may be limited by PiRC (Sharkey 1985). This model incorporates seven parameters: the Michaelis-Menten constant of Rubisco for CO2
(Kc), the Michaelis-Menten constant of Rubisco for O2 (Ko), the specificity factor of
Rubisco for CO2 versus O2 (τ), the maximum rate of CO2 fixation by Rubisco
(Vc,max), the maximum capacity for electron transport (Jmax), the phosphate regenera-tion capacity (PiRC), and the daytime respiraregenera-tion rate (Rd). In the model of Harley
and Sharkey (1991), PiRC is referred to as TPU (triose phosphate utilization capac-ity). Model parameters, parameter units and temperature parameters used to describe the temperature dependency of each model parameter are listed in Table 1.
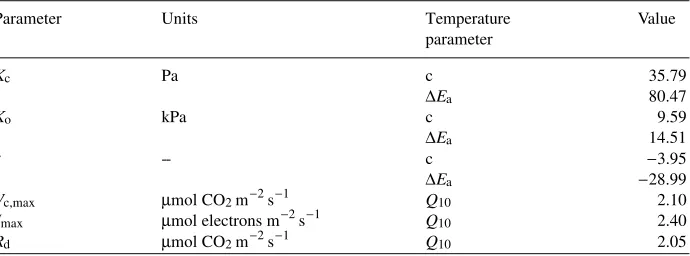
Table 1. List of model parameters and the parameters used to describe their temperature dependencies. Temperature parameters for Kc, Ko and τ are from Harley et al. (1992). Those for Vc,max and Jmax are from
Collatz et al. (1991); Rd is from Higginbotham (1974).
Parameter Units Temperature Value
parameter
Kc Pa c 35.79
∆Ea 80.47
Ko kPa c 9.59
∆Ea 14.51
τ -- c −3.95
∆Ea −28.99
Vc,max µmol CO2 m−2 s−1 Q10 2.10
Jmax µmol electrons m−2 s−1 Q10 2.40
The parameters Kc, Ko and τ were calculated from the general equation (Harley et al. 1992):
Parameter = exp(c−∆Ea/RTl), (1)
where c is a scaling constant, ∆Ea is the activation energy, R is the universal gas
constant, and Tl is the leaf temperature. The substrate specificity factor (τ) is relatively constant across several species (Jordan and Ogren 1984, Woodrow and Berry 1988), whereas Kc varies among C3 species (Evans and Seemann 1984, Keys
1986). However, because no values for Kc, Ko and τ of Rubisco in loblolly pine have
been published, the values and temperature dependencies of these parameters were calculated using the equations of Harley et al. (1992), which are based on values determined for Rubisco in spinach (Spinacia oleracea L.) (Jordan and Ogren 1984). Values for the remaining four parameters, Vc,max, Jmax, PiRC and Rd, were obtained as in Harley et al. (1992), following the procedures outlined below. At saturating light and Ci less than 20 Pa, RuBP concentrations are assumed to be nonlimiting and the net assimilation rate (A) can be represented by the following equation (Harley et al. 1992):
Best fit values were calculated for Vc,max and Rd in Equation 3 by nonlinear least
squares regression techniques on data collected at Ci less than 20 Pa.
As Ci increases, assimilation becomes limited by either the rate of electron transport or the rate of inorganic phosphate turnover during triose phosphate utiliza-tion. Using the values of Vc,max and Rd calculated for each curve, it was possible to obtain best fit estimates for Jmax and PiRC by nonlinear least squares regression techniques to fit entire A versus Ci curves to the following equation:
Wp = 3PiRC +
VcO Ciτ
. (6)
Wc is the rate of carboxylation limited by the activity of Rubisco and obeys Michaelis-Menten kinetics, reflecting the amount, activation state and kinetic prop-erties of Rubisco, and the competitive interaction between CO2 and O2. Wj is the rate
of carboxylation limited by the rate of RuBP regeneration in the Calvin cycle and is mediated by the rate of electron transport (J). Wp is the rate of assimilation limited
by the supply of inorganic phosphate. Wp is mediated by the rate of phosphate release during triose phosphate utilization (PiRC) in the formation of starch and sucrose, and the rate of Rubisco oxygenase activity expressed as a function of the rate of CO2
fixation by Rubisco (Vc). To calculate Wp, it was assumed that 50% of glycolate
produced during photorespiration was recovered. For Vc,max, Jmax and Rd, estimated values were corrected for temperature using the following equation:
Parameter = Parameter Q10((Tl− 25.0)/10), (7)
where Q10 is the increase in activity for every 10 °C increase in temperature, and Tl
is the leaf temperature. No temperature correction for PiRC was used, because of limited data on the temperature dependency of this parameter (Harley and Sharkey 1991). To simplify comparisons between modeled and measured curves, all parame-ter values and net assimilation rates were corrected to 25 °C.
Estimating oxygen sensitivity and relative stomatal limitation
Oxygen sensitivity (i.e., the change in net assimilation rate due to a change in oxygen concentration) was estimated by comparing net assimilation rates measured at 2 and 21 kPa O2 when Ca = 71.0 Pa with the following equation (Sharkey 1985):
Gas diffusion limitation (also referred to as relative stomatal limitation (RSL), i.e., the reduction in net assimilation rate resulting from the reduction in Ci relative to Ca
due to the cumulative resistances to gas diffusion between the atmosphere and the mesophyll) was calculated at Ca = 35.5 and 71.0 Pa with the following equation (Farquhar and Sharkey 1982):
Statistical analyses
Data collected at 21 kPa O2 were used to estimate all parameters except oxygen
sensitivity. Least squares estimates of model parameters were calculated from the assimilation versus Ci curves by the nonlinear regression model fitting function in SYSTAT (SYSTAT 1992). Least squares estimates of treatment means were com-pared by ANOVA, and Scheffe’s test was used for pairwise comparisons.
Results
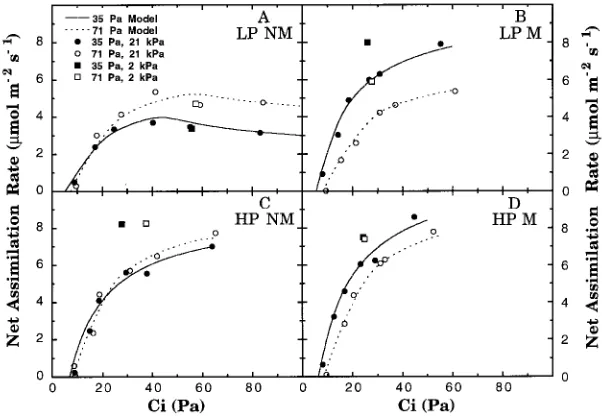
Representative modeled and measured assimilation versus Ci curves for all
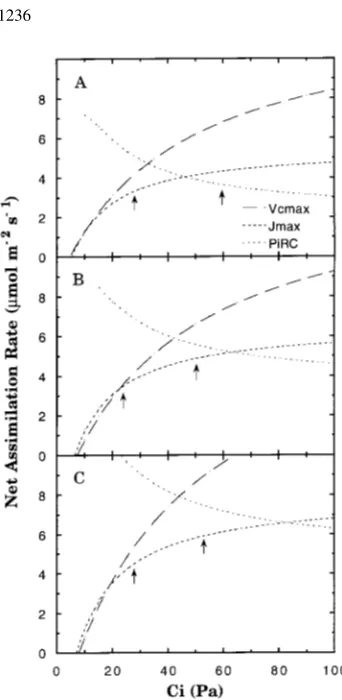
treat-ments are shown in Figure 1. In nonmycorrhizal, Low-P seedlings grown at 35.5 Pa, electron transport capacity limited net assimilation rate when Ca was 35.5 Pa, but
PiRC limited net assimilation rate when Ca was 71.0 Pa (Figure 2A). However, in nonmycorrhizal, Low-P seedlings grown at 71.0 Pa, electron transport capacity limited net assimilation rate at 71.0 Pa Ca (Figure 2B). For comparison, a
repre-sentative curve of regulation of net assimilation rate by Rubisco activity and RuBP regeneration capacity in a mycorrhizal, High-P seedling grown at 71.0 Pa is included. Under nonlimiting phosphorus conditions, carboxylation was limited by electron transport capacity at both Ca, regardless of CO2 treatment (Figure 2C).
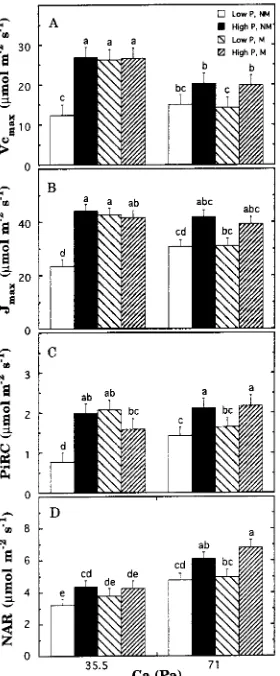
There was a significant three-way interaction among CO2 supply, phosphorus
supply and mycorrhizal status on the estimated value of Vc,max (P = 0.017). The
Figure 1. Representative measured and modeled assimilation versus Ci curves corrected to 25 °C for
seedlings grown at 35.5 and 71.0 Pa CO2. Seedlings in a given treatment were grown with either an
elevated CO2 treatment resulted in significantly decreased Vc,max in all phosphorus
treatments except nonmycorrhizal, Low-P seedlings, where estimated values were unchanged (Figure 3A). Additionally, in contrast to the Vc,max of Low-P seedlings in the ambient CO2 treatment, the Vc,max of Low-P seedlings in the elevated CO2
treatment was not significantly affected by mycorrhizal treatment. As a result, the
Vc,max of mycorrhizal, Low-P seedlings in the elevated CO2 treatment was
signifi-cantly lower than those of both mycorrhizal and nonmycorrhizal seedlings grown with adequate phosphorus.
There was also a significant three-way interaction among CO2 supply, phosphorus
supply and mycorrhizal status on PiRC (P = 0.004). The elevated CO2 treatment
resulted in significantly increased PiRC in nonmycorrhizal, Low-P seedlings and mycorrhizal, High-P seedlings, but did not significantly affect PiRC in the other two treatments (Figure 3C). The effect of CO2 treatment on PiRC response to mycorrhizal Figure 2. Modeled assimilation versus Ci curves obtained assuming Rubisco activity (Vc,max), electron
transport mediated RuBP regeneration capacity (Jmax) or PiRC solely limited net assimilation rates of
Low-P, nonmycorrhizal seedlings grown at 35.5 Pa (A) and 71.0 Pa CO2 (B) and High-P, mycorrhizal
inoculation was similar to the response observed with Vc,max. Mycorrhizal inoculation of Low-P seedlings grown at ambient CO2 resulted in significantly increased PiRC
compared to nonmycorrhizal seedlings, whereas mycorrhizal treatment did not significantly affect the PiRC of Low-P seedlings grown at elevated CO2. As a result,
the PiRC of Low-P seedlings grown at elevated CO2 was significantly lower than
those of High-P seedlings, regardless of mycorrhizal treatment. The three-way interaction among CO2 supply, phosphorus supply and mycorrhizal status on Jmax
was not significant (P = 0.106); means for all treatments are shown to indicate the functional relationships with Vc,max, PiRC and net assimilation rate (Figure 3B).
As a group, High-P seedlings had significantly higher net assimilation rates than Low-P seedlings at the growth CO2 (Figure 3D; P < 0.001). Effects of nutrient supply Figure 3. Interactive effects of CO2 partial pressure, nutrient supply and mycorrhizal status on Rubisco
were similar across measurement CO2 partial pressures, so only net assimilation rates
collected at the growth CO2 are shown. Seedlings grown at 71.0 Pa had significantly
higher net assimilation rates than seedlings grown at 35.5 Pa when measured at the growth CO2 (P < 0.001). However, there was no effect of growth CO2 when net
assimilation rates were measured at either 35.5 Pa or 71.0 Pa.
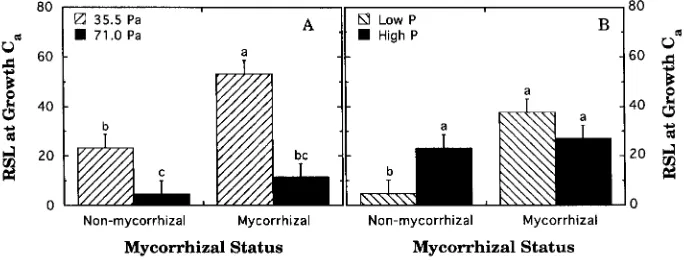
Analysis of variance indicated significant two-way interactions between CO2
supply and mycorrhizal status (P = 0.050), and nutrient supply and mycorrhizal status (P = 0.014) on RSL (Figure 4). The three-way interaction among CO2 supply,
nutrient supply and mycorrhizal status was not significant (P > 0.57). Interaction effects were similar across measurement Ca, so only the data collected at the growth CO2 are shown. Growth and measurement of seedlings at elevated CO2 yielded
significantly reduced RSL values compared to seedlings grown and measured at ambient CO2, regardless of mycorrhizal treatment. Interestingly, RSL was −3.30 ±
1.73 (mean ± SE) for nonmycorrhizal, Low-P seedlings when measured at 71.0 Pa. All other treatments had positive RSL values (data not shown). Relative stomatal limitation and oxygen sensitivity were significantly correlated with PiRC (P < 0.001 and P = 0.031, respectively). Both RSL and oxygen sensitivity increased as PiRC increased (Figure 5).
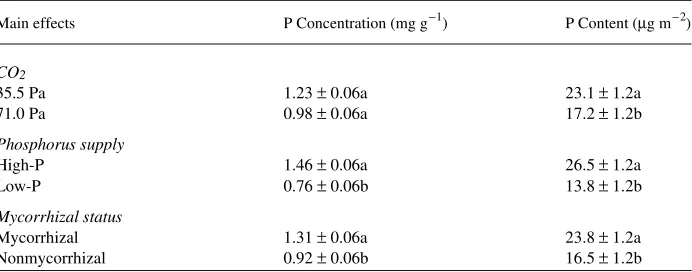
Leaf phosphorus concentration and phosphorus content per unit leaf area for each treatment are shown in Table 2. Mycorrhizal infection and increased phosphorus supply significantly increased leaf phosphorus concentration and phosphorus content per unit leaf area (P ≤ 0.03 in all cases), whereas elevated CO2 resulted in a
nonsignificant decrease in leaf phosphorus concentration (P = 0.069) and a signifi-cant decrease in phosphorus content per unit leaf area (P = 0.016). There were no significant interactions among main effects. Leaf phosphorus concentration is con-sidered limiting to growth when below 0.75 mg g−1 (Allen et al. 1990). Mycorrhizal, Low-P seedlings grown at 71.0 Pa and nonmycorrhizal, Low-P seedlings in both CO2
Figure 4. Interactive effects of CO2 supply and mycorrhizal status (A), and phosphorus supply and
mycorrhizal status (B) on RSL at the growth CO2 partial pressure. Each bar represents the mean of five
treatments had leaf phosphorus concentrations below 0.64 ± 0.18 mg g−1 (mean ± SE). All other treatments had leaf phosphorus concentrations of 0.97 ± 0.15 mg g−1 or higher.
There were no significant main effects on leaf nitrogen concentration, although elevated CO2 resulted in a significant decrease in nitrogen content per unit leaf area
(P = 0.040). Mean specific leaf nitrogen content was 0.336 ± 0.019 mg m−2 (mean ± SE) in seedlings grown at ambient CO2 and was 0.277 ± 0.018 mg m−2 in seedlings
grown at elevated CO2. There was a significant interaction between phosphorus
supply and mycorrhizal status on leaf nitrogen concentration (P = 0.050), but not on nitrogen content per unit leaf area (P = 0.067). For both parameters, no significant differences were determined between treatment means (P > 0.079 in all cases).
Figure 5. Relationships between PiRC and O2 sensitivity at 71 Pa CO2 (A), and PiRC and RSL at 71 Pa
CO2 (B) for all seedlings.
Table 2. Least square means ± SE for effects of CO2, phosphorus supply and mycorrhizal inoculation on
leaf phosphorus levels. Different letters within main effects within columns indicate means that are significantly different at P≤ 0.05 (n = 11).
Main effects P Concentration (mg g−1) P Content (µg m−2)
CO2
35.5 Pa 1.23 ± 0.06a 23.1 ± 1.2a
71.0 Pa 0.98 ± 0.06a 17.2 ± 1.2b
Phosphorus supply
High-P 1.46 ± 0.06a 26.5 ± 1.2a
Low-P 0.76 ± 0.06b 13.8 ± 1.2b
Mycorrhizal status
Discussion
Long-term exposure to elevated CO2 may result in reduced photosynthetic capacity
in some C3 plants. Such an effect has been referred to as down regulation of
photosynthetic processes. Phosphorus limitation (Conroy et al. 1986) and reduced sink strength (Stitt 1991, Thomas and Strain 1991) have both been proposed as mechanisms leading to down regulation through reduction of RuBP regeneration capacity. In this study, long-term exposure to elevated CO2 resulted in reduced
photosynthetic capacity in mycorrhizal seedlings grown with limiting phosphorus, apparently due to induced phosphorus deficiency. Long-term exposure to elevated CO2, however, resulted in increased photosynthetic capacity in nonmycorrhizal
seedlings grown with limiting phosphorus. In all other treatments, photosynthetic capacity was unaffected by CO2 supply. This suggests that, under certain conditions,
long-term exposure to elevated CO2 may enhance photosynthetic capacity of some
limited seedlings. However, the photosynthetic capacity of phosphorus-limited seedlings grown at elevated CO2 was still lower than in nonlimited seedlings,
indicating that elevated CO2 did not alleviate phosphorus limitation.
The observed increase in photosynthetic capacity in nonmycorrhizal, Low-P seed-lings in response to growth at elevated CO2 was due primarily to increased PiRC
(Figure 2), which nearly doubled in this treatment. Because short-term exposure to elevated CO2 led to limiting Pi regeneration in nonmycorrhizal, Low-P seedlings
grown at ambient CO2, it might be expected that long-term exposure to elevated CO2
would lead to reallocation of nitrogen from Rubisco to PiRC (Sage et al. 1989, Stitt 1991). However, the increased PiRC observed in the nonmycorrhizal, Low-P treat-ment occurred without a concomitant change in Rubisco activity, indicating that resources were not reallocated from Rubisco activity to PiRC.
The increased PiRC observed in the nonmycorrhizal, Low-P seedlings may instead reflect changes in relative sink activity. Under ambient CO2 conditions, phosphorus
stress generally affects plant growth more than photosynthesis (Marschner 1986, Chapin and Wardlaw 1988). Therefore, the reduced PiRC observed during short-term exposure to elevated CO2 in the nonmycorrhizal, Low-P treatment grown at ambient
CO2 may reflect phosphorus limitation of plant growth leading to sink limitation of
photosynthesis. Elevated CO2 can lead to increased root/shoot ratio in
phosphorus-limited P. taeda seedlings (Lewis et al. 1994), increasing relative sink activity. Increasing relative sink activity would only affect the photosynthetic capacity of seedlings that were sink limited at ambient CO2. In this study, only seedlings in the
nonmycorrhizal, Low-P treatment would reflect the change in relative sink activity. In addition to possible changes in the relative sink activity, the observed lack of changes in biochemical activity and leaf nitrogen concentration also suggest that the CO2 effect on photosynthesis of phosphorus-limited seedlings was due to effects at
the whole-plant level rather than at the leaf level.
Long-term exposure to elevated CO2 may increase the photosynthetic capacity of
1993). Although mycorrhizal, Low-P seedlings were not phosphorus limited at ambient CO2, photosynthetic capacity of mycorrhizal, Low-P seedlings was reduced
approximately 20% by exposure to elevated CO2. This was associated with a
significant decrease in leaf phosphorus concentration to values comparable with those of nonmycorrhizal, Low-P seedlings. Interestingly, the down regulation of photosynthetic capacity was due to decreases in both Rubisco activity and electron transport mediated RuBP regeneration. As a result, mycorrhizal, Low-P seedlings and nonmycorrhizal, Low-P seedlings grown at 71.0 Pa had similar photosynthetic capacities, suggesting that leaf-level responses to phosphorus stress were integrated with whole-plant responses.
Because plant photosynthate source--sink balance may affect the response of photosynthetic capacity to long-term exposure to elevated CO2, mycorrhizal status
may be important in mediating effects of elevated CO2 independent of effects on
plant nutrient uptake. Studies performed under ambient CO2 conditions have shown
a mycorrhizal effect on photosynthesis that can be attributed to increased photosyn-thate sink strength (Reid et al. 1983, Nylund and Wallander 1989, Dosskey et al. 1990, Rousseau and Reid 1990). In the present study, mycorrhizal status did not significantly affect PiRC and, hence, photosynthate sink strength, after long-term exposure to elevated CO2. The lack of response to mycorrhizal status was surprising
given that mycorrhizae significantly decreased root carbohydrate concentrations in three-month-old P. taeda seedlings in a parallel study (Lewis et al. 1994), suggesting that the fungus was an active photosynthate sink (Borges and Chaney 1989). How-ever, the seedlings in this study were two months older than those in the parallel study, and mycorrrhizal growth is characterized by cycles of growth and senescence (Pankow et al. 1989, Kozlowski 1992), so it is possible that the lack of response was due to mycorrhizal senescence.
In addition to inducing source--sink imbalances, elevated CO2 may cause stomatal
limitation of photosynthesis by reducing stomatal conductance. However, estimates of RSL of photosynthesis at 71.0 Pa Ca indicated that stomatal conductance generally did not significantly limit photosynthesis of seedlings grown at elevated CO2 (e.g.,
DeLucia et al. 1985, Ehret and Jolliffe 1985, Thomas and Strain 1991). A seemingly anomalous observation in this study was the negative RSL estimates at 71.0 Pa for nonmycorrhizal, Low-P seedlings. This indicates reversed CO2 sensitivity
(decreas-ing photosynthesis with increas(decreas-ing CO2); actual stomatal limitation in these seedlings
was zero. Reversed CO2 sensitivity is indicative of Pi regeneration limitation (Harley
and Sharkey 1991) and provides additional evidence that nonmycorrhizal, Low-P seedlings were limited by PiRC. Both RSL and O2 sensitivity, which has also been
used to infer when PiRC limits photosynthesis (Harley and Sharkey 1991), were significantly correlated with PiRC.
In summary, we demonstrated that phosphorus supply, but not mycorrhizal status, affects the photosynthetic response to long-term exposure to elevated CO2 in loblolly
increasing PiRC (Figures 1 and 3). However, long-term exposure to elevated CO2 can
lead to a reduction of photosynthetic capacity in seedlings supplied with phosphorus in amounts at which photosynthetic capacity is not phosphorus limited at ambient CO2, e.g., mycorrhizal, Low-P plants (Figures 1 and 3). As a result, the phosphorus
status of plants must be considered when interpreting the response of photosynthetic capacity to elevated CO2. Additionally, plants not currently limited by phosphorus
supply in the field may become phosphorus limited as atmospheric CO2 partial
pressures increase.
Acknowledgments
We thank the Duke Phytotron Staff, Larry Giles, Beth Guy and Elizabeth Thomas for technical assistance. David Tissue and two anonymous reviewers improved earlier drafts of this manuscript. Seeds were generously provided by Dr. Paul Belonger of Georgia-Pacific. This research was supported by the Department of Energy, CO2 Research Division, contract DE-FG05-87ER60575 and the Electric Power
Research Institute. The Duke Phytotron is supported by the National Science Foundation, grant No. BSR 87-06429.
References
Allen, H.L., P.M. Dougherty and R.G. Campbell. 1990. Manipulation of water and nutrients----practice and opportunity in Southern US pine forests. For. Ecol. Manage. 30:437--453.
Borges, R.G. and W.R. Chaney. 1989. Root temperature affects mycorrhizal efficacy in Fraxinus pennsylvanica Marsh. New Phytol. 112:411--417.
Bowes, G. 1991. Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant
Cell Environ. 14:795--806.
Brooks, A., K.C. Woo and S.C. Wong. 1988. Effects of phosphorus nutrition on the response of photosynthesis to CO2 and O2, activation of ribulose bisphosphate carboxylase and amounts of
ribulose bisphosphate and 3-phosphoglycerate in spinach leaves. Photosynth. Res. 15:133--141. Chapin, F.S., III and I.F. Wardlaw. 1988. Effect of phosphorus deficiency on source--sink interactions
between flag leaf and developing grain in barley. J. Exp. Bot. 39:165--177.
Collatz, G.J., T.J. Ball, G. Grivet and J.A. Berry. 1991. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer. Agric. For. Meteorol. 54:107--136.
Conroy, J.P., P.J. Milham and E.W.R. Barlow. 1992. Effect of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2. Plant Cell Environ. 15:843--847.
Conroy, J.P., P.J. Milham, M.L. Reed and E.W. Barlow. 1990. Increases in phosphorus requirements for CO2-enriched pine species. Plant Physiol. 92:977--982.
Conroy, J.P., M. Küppers, B. Küppers, J. Virgona and E.W.R. Barlow. 1988. The influence of CO2
enrichment, phosphorus deficiency and water stress on the growth, conductance and water use of Pinus radiata D. Don. Plant Cell Environ. 11:91--98.
Conroy, J.P., R.M. Smillie, M. Küppers, D.I. Bevege and E.W. Barlow. 1986. Chlorophyll a fluorescence and photosynthetic and growth responses of Pinus radiata to phosphorus deficiency, drought stress, and high CO2. Plant Physiol. 81:423--429.
DeLucia, E.H., T.W. Sasek and B.R. Strain. 1985. Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynth. Res. 7:175--184.
Dosskey, M.G., R.G. Linderman and L. Boersma. 1990. Carbon-sink stimulation of photosynthesis in Douglas-fir seedlings by some ectomycorrhizas. New Phytol. 115:269--274.
Duchein, M.-C., A. Bonicel and T. Betsche. 1993. Photosynthetic net CO2 uptake and leaf phosphate
concentrations in CO2 enriched clover (Trifolium subterraneum L.) at three levels of phosphate
nutrition. J. Exp. Bot. 44:17--22.
Ekwebelam, S.A. and C.P.P. Reid. 1983. Effect of light, nitrogen fertilization, and mycorrhizal fungi on growth and photosynthesis of lodgepole pine seedlings. Can. J. For. Res. 13:1099--1106.
Evans, J.R. and J.R. Seemann. 1984. Differences between wheat genotypes in specific activity of ribulose 1,5-bisphosphate carboxylase and the relationship to photosynthesis. Plant Physiol. 74:759--765. Farquhar, G.D. and T.D. Sharkey. 1982. Stomatal conductance and photosynthesis. Annu. Rev. Plant
Physiol. 33:317 - - 345.
Farquhar, G.D., S. von Caemmerer and J.A. Berry. 1980. A biochemical model of photosynthetic CO2
assimilation in leaves of C3 species. Planta 149:78--90.
Field, C.B., J.T. Ball and J.A. Berry. 1989. Photosynthesis: principles and field techniques. In Plant Physiological Ecology: Field Methods and Instrumentation. Eds. R.W. Pearcy, J. Ehlringer, H.A. Mooney and P.W. Rundel. Chapman and Hall, New York, pp 209--253.
Harley, P.C. and T.D. Sharkey. 1991. An improved model of C3 photosynthesis at high CO2: reversed O2
sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth. Res. 27:169--178. Harley, P.C., R.B. Thomas, J.F. Reynolds and B.R. Strain. 1992. Modelling photosynthesis of cotton
grown in elevated CO2. Plant Cell Environ. 15:271--282.
Higginbotham, K.O. 1974. The influence of canopy position and the age of leaf tissue on growth and photosynthesis in loblolly pine. Ph.D. Dissertation, Duke University, Durham, 248 p.
Jordan, D.B. and W.L. Ogren. 1984. The CO2/O2 specificity of ribulose 1,5-bisphosphate
carboxy-lase/oxygenase: dependence on ribulose bisphosphate concentration, pH and temperature. Planta 161:308--313.
Keys, A.J. 1986. Rubisco: its role in photorespiration. Phil. Trans. R. Soc. Lond. B 313:325--336. Kozlowski, T.T. 1992. Carbohydrate sources and sinks in woody plants. Bot. Rev. 58:107--222. Labate, C.A. and R.C. Leegood. 1988. Limitation of photosynthesis by changes in temperature. Factors
affecting the response of carbon dioxide assimilation to temperature in barley leaves. Planta 173:519--527.
Lauer, M.J., S.G. Pallardy, D.G. Blevins and D.D. Randall. 1989. Whole leaf carbon exchange charac-teristics of phosphate deficient soybeans (Glycine max L.). Plant Physiol. 91:848--854.
Leegood, R.C. and R.T. Furbank. 1986. Stimulation of photosynthesis by 2% oxygen at low temperatures is restored by phosphate. Planta 168:84--93.
Lewis, J.D., R.B. Thomas and B.R. Strain. 1994. Effect of elevated CO2 on mycorrhizal colonization of
loblolly pine (Pinus taeda L.) seedlings. Plant Soil. In press.
Marks, S. and K. Clay. 1990. Effects of CO2 enrichment, nutrient addition, and fungal
endophyte-infec-tion on the growth of two grasses. Oecologia 84:207--214.
Marschner, H. 1986. Mineral nutrition of higher plants. Academic Press, London, 674 p.
Morin, F., M. André and T. Betsche. 1992. Growth kinetics, carbohydrate, and leaf phosphate content of clover (Trifolium subterraneum L.) after transfer to a high CO2 atmosphere or to high light and
ambient air. Plant Physiol. 99:89--95.
Nylund, J.-E. and H. Wallander. 1989. Effects of ectomycorrhiza on host growth and carbon balance in a semi-hydroponic cultivation system. New Phytol. 112:389--398.
Pankow, W., W. Niederer, U. Weiser, B. Schmid, T. Boller and A. Wiemken. 1989. Biochemical symptoms of stress in the mycorrhizal roots of Norway spruce (Picea abies). Trees 3:65--72. Peet, M.M. 1984. CO2 enrichment of soybeans. Effects of leaf/pod ratio. Physiol. Plant. 60:38--42.
Reid, C.P.P., F.A. Kidd and S.A. Ekwebelam. 1983. Nitrogen nutrition, photosynthesis and carbon allocation in ectomycorrhizal pine. Plant Soil 71:414--432.
Rousseau, J.V.D. and C.P.P. Reid. 1990. Effects of phosphorus and ectomycorrhizas on the carbon balance of loblolly pine seedlings. For. Sci. 36:101--112.
Sage, R.F. and T.D. Sharkey. 1987. The effect of temperature on the occurrence of O2 and CO2 insensitive
photosynthesis in field grown plants. Plant Physiol. 84:658--664.
Sage, R.F., T.D. Sharkey and J.R. Seemann. 1989. Acclimation of photosynthesis to elevated CO2 in five
C3 species. Plant Physiol. 89:590--596.
Sharkey, T.D. 1985. Photosynthesis of cotton plants exposed to elevated levels of carbon dioxide in the field. Photosynth. Res. 12:191--203.
Stitt, M. 1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells.
SYSTAT. 1992. SYSTAT: Statistics. Edn. 5.2. Systat Inc., Evanston, IL, 724 p.
Thomas, R.B. and B.R. Strain. 1991. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol. 96:627--634.
von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosyn-thesis and the gas exchange of leaves. Planta 153:376--387.
Walker, D.A. and M.N. Sivak. 1985. Can phosphate limit photosynthetic carbon assimilation in vivo? Physiol. Veg. 23:829--841.
Woodrow, I.E. and J.A. Berry. 1988. Enzymatic regulation of photosynthetic CO2 fixation in C3 plants.