Overexpression of PHGPx inhibits hydroperoxide-induced
oxidation, NF
k
B activation and apoptosis and affects
oxLDL-mediated proliferation of rabbit aortic smooth muscle cells
Regina Brigelius-Flohe´
a,b,*, Stefanie Maurer
a,b, Katharina Lo¨tzer
a, Gaby-Fleur Bo¨l
a,
Hanna Kallionpa¨a¨
c, Paulina Lehtolainen
c, Helena Viita
c, Seppo Yla¨-Herttuala
caGerman Institute of Human Nutrition,Uni6ersity of Potsdam,Potsdam-Rehbru¨cke,Arthur-Scheunert-Allee114-116,
14558Bergholz-Rehbru¨cke,Germany
bInstitute for Nutritional Science,Uni6ersity of Potsdam,Potsdam-Rehbru¨cke,Arthur-Scheunert-Allee114-116,
14558Bergholz-Rehbru¨cke,Germany
cA.I.Virtanen Institute and Department of Medicine,Uni6ersity of Kuopio,Kuopio,Finland Received 19 July 1999; received in revised form 1 November 1999; accepted 6 December 1999
Abstract
Rabbit abdominal aortic smooth muscle cells (SMC) were stably transfected with the cDNA of porcine phospholipid hydroperoxide glutathione peroxidase (PHGPx) by means of a retroviral gene transfer technique, to create a model for studying cellular processes relevant to atherogenesis. The transfected cells (SMC/PHGPx) had approximately 4-fold higher PHGPx activity when cultured in the presence of selenite whereas the parental cells did not show any significant increase in PHGPx or total GPx activity upon selenium supplementation. In situ functionality of PHGPx was validated by inhibition of linoleic acid hydroperox-ide-induced toxicity, dihydrorhodamine oxidation, NFkB activation and apoptosis. SMC grown in 1% FCS responded to oxidized LDL (oxLDL) with a marked proliferation, as measured by [3H]thymidine incorporation, irrespective of selenium
supplementa-tion. In SMC/PHGPx grown with or without selenite under control conditions or exposed to native LDL, thymidine incorporation was generally depressed. Also, oxLDL-induced proliferation was lower in SMC/PHGPx compared to untransfected SMC up to 24 h of incubation. After 40 h, however, selenite supplementation restored maximum proliferation response to oxLDL in SMC/PHGPx. The results suggest a proliferative effect of endogenous hydroperoxides in SMC. They further reveal that hydroperoxy lipids of oxLDL contribute to the induction of proliferation, but also suggest involvement of hydroxy lipids in the response to oxLDL. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Glutathione peroxidases; Selenium; Smooth muscle cells; Proliferation; Overexpression; Atherosclerosis; Apoptosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
Apart from the induction of adhesion molecules in endothelial cells, proliferation of smooth muscle cells (SMC) in the subendothelial layer is one of the early events in atherogenesis [1]. Proliferation of SMC finally leads to intima thickening and the development of fibrous plaques. Proliferation is well documented to be stimulated by several compounds, including oxidized LDL (oxLDL), in aortic SMC [2 – 4], in rabbit femoral
SMC [5] and in SMC from aortic segments from hu-man kidneys [6]. SMC are known to take up oxLDL by a particular receptor referred to as the scavenger recep-tor [7]. However, which of the various oxidized
com-pounds in the LDL particle are triggering the
proliferation has not yet been addressed systematically. Oxidized fatty acids belong to the putative stimulators of atherogenesis. They have been found in atheroscle-rotic lesions together with oxLDL [8]. They induce ICAM-1 in HUVEC [9] and obviously enhance TNF-induced CAM expression and PMA-TNF-induced T cell
adhesion in 15-lipoxygenase-transfected endothelial
cells [10]. Their exact involvement in SMC prolifera-tion, however, remains unknown. Hydroxy fatty acids, * Corresponding author. Tel.: +49-33200 88353; fax: +49-33200
88407.
E-mail address:[email protected] (R. Brigelius-Flohe´).
the reduction products of the corresponding hydroper-oxy fatty acids, proved to be even stronger inducers of the endothelial cell adhesion molecule ICAM-1 [9]. They also induce the scavenger receptor CD36
respon-sible for oxLDL uptake in monocytes/macrophages
[11]. In chemically oxidized LDL, oxidized fatty acids were primarily found esterified to cholesterol [12]. They can also be produced by the action of 15-lipoxygenases in cultured endothelial cells [13], macrophages [14], 15-lipoxygenase overexpressing endothelial cells [10] and fibroblasts [15], and after treatment of LDL with purified rabbit reticulocyte 15-lipoxygenase [16]. How-ever, these findings are not undebated [17].
The selenium-containing glutathione peroxidases [18] inhibit the activation of lipoxygenases in vitro [19 – 21] and efficiently reduce products thereof [18]. Their role in the prevention of atherogenesis in vivo is widely discussed, since epidemiological studies revealed a cor-relation of cardiovascular diseases and selenium defi-ciency [22,23]. Therefore, the manipulation of the peroxide metabolism was considered in SMC as a promising approach to analyze the relevance of lipid hydroperoxides present in oxLDL to SMC biology.
While all glutathione peroxidases reduce H2O2 or
soluble alkyl hydroperoxides [24], only the phospho-lipid hydroperoxide glutathione peroxidase (PHGPx) efficiently reduces hydroperoxy groups of complex lipids including those of phospholipids [18,25] and
cholesterolesters [26] even when present in lipoproteins [27]. Overexpression of PHGPx was therefore consid-ered the optimum tool to define the effects of hydroper-oxy and hydrhydroper-oxy lipids of oxLDL on cellular targets. With this in mind PHGPx was overexpressed in SMC, verified its function in situ, and tested the proliferative response to oxLDL in rabbit aortic SMC.
2. Methods
2.1. Cell culture
Rabbit abdominal aortic SMC (RAASMC) [10,28]
and 8CRE and 8CRIP cells (both modified from NIH
3T3) [29] were grown in DMEM (Seromed)
supple-mented with 5 or 10% FCS, 100 U/ml penicillin, 100
mg/ml streptomycin and 4 mM L-glutamine. If
men-tioned, cells were supplemented with sodium selenite at the concentrations indicated. A 1000-fold stock solu-tion of selenite (Sigma) in water was prepared, filtered sterile and diluted by addition to the cell culture media. Selenium deficiency was obtained by growing the cells in an FCS batch (Seromed, batch 698E) which con-tained 8.7mg selenium/l, resulting in a selenium concen-tration of 5.5 nM when 5% was used in the growth medium. Despite not knowing the bioavailability and the form in which selenium was present in the FCS, this batch led to the lowest GPx activities obtainable with-out selenium supplementation.
2.2. Construction of the retro6iral 6ector
All cloning work was done according to conventional procedures [30].
2.2.1. Construction of pLPHGPxRNL
The cDNA of the pig PHGPx [31] was first trans-ferred into the retroviral vector pLRNL [32]. The full
PHGPx cDNA was taken from pMM3 [33] asNdeI×
BamHI fragment and ligated into pBluescript II KS+
(Stratagene). To facilitate subsequent cloning proce-dures, an oligonucleotide containing an additional
BamHI restriction-site was inserted between the KpnI
and NdeI site of the vector pBluescript/PHGPx
result-ing in pBluescript/PHGPx/b (Fig. 1A). Bluescript/
PHGPx/b and pLRNL were digested with BamHI,
fragments (PHGPx, 0.8 kbp; pLNRL, 6.4 kbp) isolated
via electroelution, ligated, and transformed into Es
-cherichia coli XL1-Blue (Stratagene). The resulting pLPHGPxRNL (Fig. 1B) was analyzed by restriction for the right orientation of PHGPx gene.
2.2.2. Production of PHGPx6ectors
pLPHGPxRNL was first packaged in an ecotrophic
packaging cell line, 8CRE, and subsequently in an
amphotropic packaging cell line, 8CRIP. Three days
after infection of8CRIP, cells were split and put under
selection (Geneticin, 400 mg/ml). First clones (8CRIP/ PHGPx) were visible after 14 – 16 days of the selection. More than 70 clones were expanded and analyzed for the retroviral transcript by Northern blots and by activity of PHGPx. Five clones with the highest PHGPx activity were chosen for the transfection of target cells.
2.2.3. Infection of target cells
SMC were infected with supernatants from selected
8CRIP/PHGPx clones. On day 4 after infection, cells
were put under selection (400 mg/ml Geneticin) and
clones were isolated 12 – 16 days later.
2.3. Acti6ity of glutathione peroxidases
Glutathione peroxidase activity was measured with the glutathione reductase-coupled test adapted for cul-tured cells as described [34].
2.3.1. Hydroperoxides
H2O2 was purchased from Sigma.
Phosphatidyl-choline hydroperoxide (PCOOH) was prepared by
oxi-dizing phosphatidylcholine (Sigma) with soybean
lipoxygenase type IV (Sigma) in the presence of 3% deoxycholate, freed from detergent and concentrated by solid phase extraction with SEP PACK 18 reverse phase cartridges (Waters-Millipore), and quantified ac-cording to Ref. [35]. Linoleic acid hydroperoxide (LOOH) was prepared according to Ref. [36], by oxi-dizing linoleic acid (Sigma) with soybean lipoxygenase type IV (Sigma).
2.4. Assays for in situ PHGPx function
Cytotoxicity of different hydroperoxides was mea-sured by estimation of MTT reduction [37].
2.4.1. Intracellular oxidation
The amount of intracellular oxidation upon incuba-tion with linoleic acid hydroperoxide (LOOH) was measured fluorographically via oxidation of dihy-drorhodamine (DHR 123) according to Ref. [38]. The 48-well plates with a confluent monolayer of cells grown with or without sodium-selenite in DMEM with
5% FCS for 4 days were loaded with 20mM DHR 123
(Molecular Probes, Netherlands) for 45 min at 37°C in Hank’s balanced salt solution (HBSS), washed and
treated with 30 – 60 mM LOOH in serum-free DMEM
for 60 min at 37°C. After washing with HBSS, cells were scanned in a fluorescence reader (Cytofluor II,
Perceptives Biosystems, Germany) withlex485 nm and
lem 530 nm.
2.4.2. Electrophoretic mobility shift assay
Cells were grown without or with selenium (100 nM sodium-selenite) for 3 days in DMEM with 10% FCS. Then medium was changed to serum-free DMEM for 24 h before stimulation. Linoleic acid hydroperoxide was added at the concentrations indicated for 60 min at 37°C. Nucleic protein extracts were prepared according to Ref. [39].
A DNA fragment representing the consensus binding
motif of the KB site (5%-AATTCA CAAAGA
GGGACT TTCCCC TACATC CATTG-3%) was used
for gel shift analyses. Binding reaction was performed
at room temperature with 5 mg protein of the nuclear
extract, 10 fmol of labelled DNA and 3 mg poly(dIdC)
(Pharmacia Biotech) in 15 mM Hepes, 1 mM EDTA, 1
mM DTT, 10% (w/v) glycerol in 10 ml. After 30 min
samples were immediately subjected to gel
electrophore-sis on a native 4% polyacrylamide gel in 0.25×TBE at
200 V.
2.4.3. Apoptosis
Cells grown in DMEM, 5% FCS, with or without 100 nM sodium-selenite for 3 – 4 days were seeded at 2×104 cells/well in 24-well plates and left to adhere
overnight. Then media were changed to DMEM 5% FCS containing the peroxides indicated and cells incu-bated for 8 h. Thereafter cells were lysed and superna-tants assayed immediately by the Cell Death Detection
ELISAplus (Boehringer Mannheim) according to
manu-factors’ instructions.
2.4.4. Proliferation assay
Cells were grown in DMEM medium, 10% FCS, in the presence or absence of 100 nM sodium selenite. They were seeded at 3×103cells/ml into 96-well plates
and allowed to adhere for 24 h. Then serum was deprived for 30 h to obtain quiescence. Thereafter, cells were incubated with 1% FCS (control), 1% FCS plus 5
or 20mg nLDL/ml, 1% FCS plus 5 or 20mg oxLDL/ml
for 24 or 40 h. At the end of a stimulation period 10 ml
[3
H]thymidine (0.5mCi/well) was added and incubations
were continued for additional 2 h. The radioactive medium was aspirated, cells were washed twice in
ice-cold PBS buffer (200ml) followed by 5% TCA exposure
for 2 h at room temperature. The TCA solution was
removed by aspiration and 75 ml of 1 N NaOH was
added to solubilize the precipitated material. After
fur-ther 2 h 75 ml of 1 N HCl was added to each well for
neutralization. Incorporated radioactivity was
quantified by liquid scintillation counting.
2.4.5. Oxidation of LDL
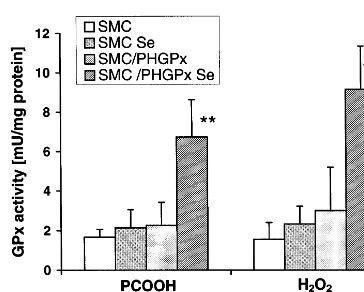
SDS-Fig. 2. Selenium-dependent glutathione peroxidase activities of con-trol (smooth muscle cells, SMC) and phospholipid hydroperoxide glutathione peroxidase (PHGPx)-transfected (SMC/PHGPx) smooth muscle cells. Cells were grown for 4 days in DMEM, 10% FCS, with and without selenium (100 nM sodium selenite) supplementation. Then cells were harvested and PHGPx activity measured with phos-phatidylcholine hydroperoxide (PCOOH) and total glutathione per-oxidase activity measured with H2O2 as substrates. Activity is expressed in mU/mg protein. Values are means from seven individual cultures9S.D. *PB0.01, **PB0.001 vs selenium-supplemented SMC.
In contrast, the parental cell line, SMC, did not show any significant increase in GPx activities upon selenium supplementation neither when measured with PCOOH
nor with H2O2. This data implies that the parental
SMC are practically devoid of both cGPx and PHGPx, and the increase of PHGPx acitivity seen upon selenium
supplementation in SMC/PHGPx is almost exclusively
due to the overexpression of the transfected PHGPx gene. The stability of increased PHGPx activity in the
SMC/PHGPx clone was verified over a period of 2
years.
In absolute terms, the overexpression achieved is modest, but comparable with overexpression obtained by others [45 – 50]. When high expression rates of, e.g. cGPx were obtained [51,52], they proved to be unstable over time. The modest but stable overexpression of PHGPx was therefore considered satisfactory to study the impact of improved lipid hydroperoxide metabolism in SMC.
3.2. Validation of in situ functionality of o6erexpressed PHGPx
3.2.1. Peroxide-cytotoxicity
As expected from previous studies [46,47,49] overex-pressed PHGPx reduced the cytotoxicity of different hydroperoxides like PCOOH and linoleic acid
hy-droperoxide in SMC/PHGPx compared to SMC (not
shown). Cytoprotection correlated with the PHGPx
activity and was highest in SMC/PHGPx grown in
selenium supplemented medium.
3.2.2. Intracellular DHR oxidation
To further prove the functionality of transfected PHGPx, intracellular oxidation of dihydrorhodamine 123 (DHR 123) after challenging cells with LOOH was measured. DHR 123 belongs to the oxidant-sensing fluorescent probes detecting a broad range of oxidizing reactions that may be increased during intracellular oxidative stress, e.g. induced by LOOH [53]. As shown in Fig. 3, intracellular DHR 123 oxidation upon LOOH exposure increased in SMC in a concentration depen-dent manner and was independepen-dent of selenium
supple-mentation. In contrast, in SMC/PHGPx, intracellular
oxidation was by far lower and could not be further reduced upon selenium supplementation. Thus, the low
increase in PHGPx activity in SMC/PHGPx grown
without selenium supplementation is already sufficient
to withdraw LOOH from reacting with
dihydrorhodamine.
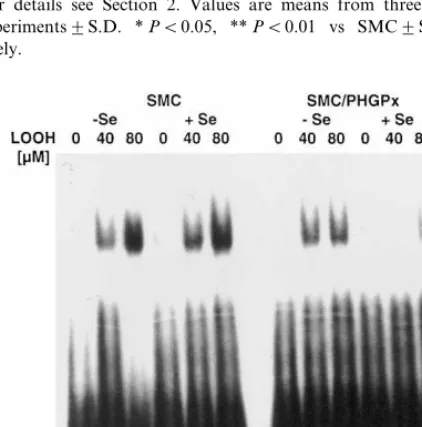
3.2.3. Hydroperoxide-induced acti6ation of NFkB The SMC line used in this study belongs to the few
examples in which NFkB can be activated by
hydroper-oxides (Fig. 4). In unsupplemented SMC, 1 h
incuba-tion with 40 and 80 mM LOOH, respectively, led to a
PAGE of apolipoproteins according to Ref. [41]. For
oxidation, native LDL (1 mg/ml) was incubated with
0.2 mM FeCl3at 37°C. After 3 h, iron was removed by
gel filtration. Oxidative modification of LDL was
esti-mated according to [42]. A total of 7.790.7 nmol
MDA/mg protein was considered adequate (native
LDL: 1.290.2 nmol/mg protein).
In separate samples prepared the same way, hy-droperoxide and hydroxide levels. were analyzed as cholesteryl hydro(pero)xy linoleate by HPLC [12,43] or by absorption at 234 nm of extracted lipids [44]. Both methods proved a hydro(pero)xide content of 1 – 2 mmol/mg protein.
3. Results
3.1. Success of transfection
Twelve Geneticin-resistant clones of PHGPx-trans-fected cells were expanded for further analysis. They all exhibited the transfected porcine PHGPx mRNA in Northern blot data (not shown). Three of them, show-ing the highest mRNA content, were tested for PHGPx activity and the selenium dependency thereof. The
finally selected clone, SMC/PHGPx, still expressed low
PHGPx (measured with PCOOH) and total GPx (mea-sured with H2O2) activity under selenium limiting
concentration-dependent activation of NFkB. Corre-sponding to the unchanged GPx activity, selenium sup-plementation did not show any effect. This is in contrast to other studies in which selenium
supplemen-Fig. 5. Selenium-dependent initiation of apoptosis by linoleic acid hydroperoxide (LOOH) in control (smooth muscle cells, SMC) and phospholipid hydroperoxide glutathione peroxidase (PHGPx)-trans-fected (SMC/PHGPx) smooth muscle cells. (A) DNA fragmentation (expressed as absorbance at 450 nm) in untreated (control) and cells exposed to 40 mM LOOH for 8 h. Values are means from three
individual experiments9S.D. *PB0.01; **PB0.005. (B) DNA fragmentation in SMC/PHGPx9Se treated with different concentra-tions of LOOH for 18 h. Values are means from three individual experiments9S.D. **PB0.0001. For details see Section 2. Fig. 3. Selenium-dependent linoleic acid hydroperoxide
(LOOH)-in-duced intracellular oxidation in control (smooth muscle cells, SMC) and phospholipid hydroperoxide glutathione peroxidase (PHGPx)-transfected (SMC/PHGPx) smooth muscle cells. Cells were grown for 4 days in DMEM, 10% FCS, with and without selenium (100 nM sodium selenite) supplementation. Then cells were loaded with dihy-drorhodamine 123 (DHR 123) and treated with LOOH for 1 h at the concentrations indicated. Oxidation was measured by fluorescence. For details see Section 2. Values are means from three individual experiments9S.D. *PB0.05, **PB0.01 vs SMC9Se, respec-tively.
Fig. 4. Selenium-dependent activation of NFkB by linoleic acid
hydroperoxide (LOOH) in control (smooth muscle cells, SMC) and phospholipid hydroperoxide glutathione peroxidase (PHGPx)-trans-fected (SMC/PHGPx) smooth muscle cells. Cells were grown for 4 days in DMEM, 10% FCS, with and without selenium (100 nM sodium selenite) supplementation. 24 h prior to stimulation serum was withdrawn, then cells were stimulated with LOOH for 60 min at the concentrations indicated. NFkB activation was analyzed as
de-scribed in Section 2.
tation led to an inhibition of NFKB activation due to an increased activity of endogenous glutathione
peroxi-dases [54 – 57]. In SMC/PHGPx, the degree of NFkB
activation was slightly decreased in selenium deficiency and almost completely abrogated in selenium supple-mented cells. Supershift experiments with the respective
antibodies against the different NFkB subunits
iden-tified the activated band as composed of p65 and p50 but not c-rel (data not shown).
3.2.4. Hydroperoxide-induced apoptosis
LOOH (40 mM, 8 h) led to apoptotic cell death in
SMC as measured by a quantitative sandwich-enzyme-immunoassay allowing the determination of mono- and oligonucleosomes in the cytosol of apoptotic cells. The degree of apoptosis was independent of the selenium
status in control cells (Fig. 5A). In SMC/PHGPx,
LOOH-induced apoptosis was clearly inhibited even under selenium limiting conditions. This is in accor-dance with the decreased intracellular oxidation upon
LOOH treatment of SMC/PHGPx (see Fig. 3),
short period of time can be counteracted by the small increment of transfected PHGPx. Selenium-supplemen-tation, thus, could lead only to a small additional inhibition of apoptosis.
In contrast, after 18 h of LOOH treatment apoptosis
dramatically increased also in selenium-deficient SMC/
PHGPx (Fig. 5B). Under these conditions the low additional PHGPx activity obviously is not sufficient to prevent apoptotic cell death. In selenium supplemented
SMC/PHGPx apoptosis was still completely abolished
in agreement with the high PHGPx activity.
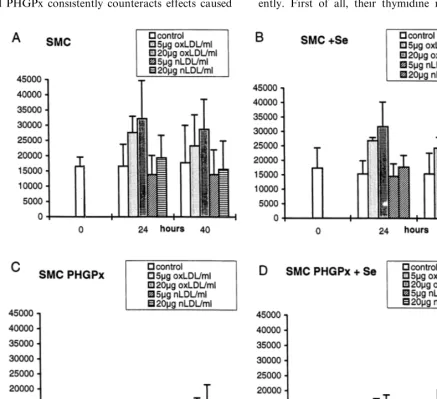
3.2.5. oxLDL-induced SMC proliferation
The experiments described so far prove that overex-pressed PHGPx consistently counteracts effects caused
by added hydroperoxides. The test system can thus be used to identify effects of hydroperoxides possibly present in more complex systems such as the
prolifera-tive response of SMC and SMC/PHGPx to oxLDL. In
untransfected SMC, the rate of thymidine incorpora-tion remained unchanged over a period of 40 h, irre-spective of selenium supplementation (Fig. 6A,B). Native LDL did not significantly affect the prolifera-tion of SMC. oxLDL induced a significant and dose-de-pendent increase of the proliferation. This proliferative response was not affected by selenium supplementation, which is consistent with the lack of endogenous se-lenoperoxidases in SMC.
The PHGPx-transfected cells behave quite differ-ently. First of all, their thymidine incorporation was
Fig. 6. Proliferation of control (smooth muscle cells, SMC) and phospholipid hydroperoxide glutathione peroxidase (PHGPx)-transfected (SMC/PHGPx) smooth muscle cells treated with native and oxidized LDL (oxLDL). SMC (9Se) (A and B) and SMC/PHGPx (9Se) (C, D) were seeded at 3×103cells/ml each into 96 well plates, allowed to adhere and made quiescent by serum deprivation for 24 h. Then they were stimulated with either native LDL (nLDL, 5 and 20mg/ml) or oxLDL (5 and 20mg/ml) all in the presence of 1% FCS9Se. For controls, only
lower than in SMC from the beginning (Fig. 6C,D). It further decreases with time, irrespective of selenium supplementation and exposure to native LDL. In terms of absolute proliferation rates, the response to oxLDL
blunted in selenium-deprived SMC/PHGPx, when
com-pared to control SMC. The relative proliferation of
oxLDL-exposed versus unstimulated SMC/PHGPx,
however, tended to be increased. This trend was more
pronounced in selenium supplemented SMC/PHGPx,
i.e. under optimized PHGPx activity (compare Fig. 6C and D). After 24 h of oxLDL exposure of
selenium-supplemented SMC/PHGPx the proliferation was still
low compared to identically treated SMC. After 40 h,
however, the proliferation of the SMC/PHGPx is
prac-tically the same as that of pertinent controls (compare Fig. 6D with B).
Clearly, thus, expression of functional PHGPx affects the oxLDL response of SMC in a complex manner in lowering basic proliferation rates, but increasing the oxLDL proliferative response, after long term exposure, to that of control cells.
4. Discussion
The design of a cellular model to study the effect of hydroperoxides on SMC proliferation proved not to be trivial. One of the very few enzymes known to effi-ciently reduce hydroperoxides including complex
hy-droperoxy lipids is PHGPx which, being a
selenoprotein, is not easily overexpressed. Transfection of mammalian cells with selenoprotein genes does not routinely result in a substantial overexpression, because many factors of the complex system of selenoprotein biosynthesis may determine the expression level (see [58] for review) apart from gene dose and promoter efficiency. In particular, transfection with glutathione peroxidase genes usually yielded moderate overexpres-sion rates comparable to those obtained here with SMC and sometimes required cotransfection with cDNA of selenophosphate synthetase (SelD) to support selenium incorporation [49,56]. Expression tended to decrease with passages [51,52], especially when it was high in the beginning [51]. With respect to this background forma-tion of a SMC line, stably overexpressing PHGPx for a period of 2 years by a factor of 4 versus parental control cells is an important tool to study physiological responses to hydroperoxides.
The particular SMC line proved to be particularly suited for the studies since the basic level of any GPx was negligible, as evident from extremely low activities and insignificant increase of GPx activities upon
supple-mentation with selenium. The PHGPx activity in SMC/
PHGPx can thus be correlated to physiological effects without any concern about interference of other se-lenoperoxidases. The PHGPx activity therein can be
modulated by physiological selenium supplementation. The still moderate PHGPx expression at optimum sele-nium supplementation, i.e. 100 nM sodium selenite, is not anticipated to affect any expression of other se-lenoproteins in a way different from control SMC grown under identical conditions. Neither is it associ-ated with selenium toxicity.
It was nevertheless attempted to validate the system by demonstrating that the PHGPx overexpressed in SMC really does in situ what it is supposed to do according to present knowledge.
As an efficient antioxidant device, PHGPx is
sup-posed to prevent oxidant-induced toxicity [25], and it did so in SMC, as overexpressed PHGPx did in ECV 304 [49], in FL5-12 cells [45], in RBL-2H3 cells [46] and in the guinea pig cell line 104C1 [47].
Direct evidence of intracellular reduction of
exoge-nous alkyl hydroxides is presented by competing out dihydrorhodamine oxidation by LOOH.
Selenoperoxidases have been implicated in the
pre-vention of hydroperoxide induced apoptosis [59,60] as did PHGPx when overexpressed in SMC.
Both, cGPx [54] and PHGPx [56] have been shown
to inhibit NFkB activation. Selective expression of
PHGPx, which is low in terms of absolute activity even at optimum selenium supplementation,
com-pletely abrogated LOOH-induced NFkB activation
in SMC.
While the results described above are consistent with the generally accepted biological role of PHGPx, the SMC proliferation study surprised in several respects. First of all, PHGPx transfection generally suppressed proliferation rates in unstimulated SMC and, in con-trast to control cells, the transfected ones incorporated less thymidine with prolonged cultivation in 1% FCS. This observation is not related to impaired cell viability, as judged by unchanged MTT reduction (data not shown). Neither is it a peculiarity of the particular cell clone, but it has been observed repeatedly with other clones and other PHGPx-transfected cell lines in the laboratory and with cGPx-transfected cells (P. Arrigo, personal communication). The reasons of this be-haviour are unclear but would comply with a prolifera-tive role of endogenous hydroperoxides as, e.g. suggested by hydroperoxides associated with growth factor receptor stimulation [61].
The blunted response to oxLDL stimulation seen in
unsupplemented and selenium supplemented SMC/
PHGPx versus SMC is consistent with an enhanced reductive capacity abrogating the oxidant potential of engulfed oxLDL. This would imply that indeed hy-droperoxy lipids present in oxLDL are responsible for the proliferative response, since only these components can be considered substrates of PHGPx.
this also happens in vivo. Strong evidence for a role of lipoxygenases and their reaction products in atherogen-esis has been presented recently by Cyrus et al. [62] in
mice made deficient in ApoE and 12/15 lipoxygenase.
The double knock out mice do not develop atheroscle-rosis during the first 15 weeks of observation whereas
heterozygotes (ApoE − / −; 12/15LO − / +) and
con-trols (ApoE − / −, 12/15LO + / +) did. In contrast,
Witting et al. [63] showed that oxidized lipids may not necessarily be connected to atherosclerotic lesions. A probucol metabolite, bisphenol, completely prevented lipid hydro(pero)xide accumulation in arterial walls of Watanabe rabbits, but did not affect the extent of atherosclerotic lesions. These observations may indicate that atherosclerosis can also develop in the absence of measurable amounts of oxidized lipids. They do, how-ever, not rule out a role of oxLDL in early initiating events such as proliferation of SMC, as amply docu-mented [2 – 6] and also confirmed in the present study. Interestingly, the response to oxLDL by PHGPx transfection is only delayed but not abolished. In fact, it is undistinguishable from that of control cells after a 40-h exposure to oxLDL. It may even be rated as
increased when compared to unstimulated SMC/
PHGPx. This clearly rules out that hydroperoxy lipids are the only components in oxLDL directly inducing proliferation. Instead, the delayed proliferative response of the PHGPx overexpressing cells suggests that prod-ucts of PHGPx accumulating with time take over the proliferation-inducing role initially played by the hy-droperoxy lipids. Like peroxy lipids, hydroxy lipids can
induce formation of H2O2 [64] which at low level is
known to trigger proliferation [65]. Moreover, the emerging role of hydroxy lipids as specific stimuli mer-its attention according to the known specificity of PHGPx. 13-Hydroxy linoleic acid, for instance, has
been reported to mediate TNFa release from
macrophages [66], to induce the scavenger receptor in monocytic cells [11] and to induce ICAM-1 in endothe-lial cells more efficiently than LOOH [9].
With regard to the theory that oxLDL plays a critical role in atherogenesis [67] the model experiments suggest that it might be more effective to prevent LDL oxida-tion than to inhibit its proliferative acoxida-tion after it has been taken up by SMC. Even if it were feasible to significantly increase intracellular PHGPx levels in hu-man aortic tissue, e.g. by selenium supplementation, the resulting delay of the proliferative response to oxLDL would probably be clinically irrelevant. This tentative conclusion is, however, not meant to rule out any role of the selenoperoxidases in atherogenesis. A reduction of the extracellular peroxide tone by plasma GPx or the inhibition of lipoxygenases by cellular cGPx or PHGPx [19 – 21,68] might well be relevant in preventing initia-tion and propagainitia-tion of lipid peroxidainitia-tion within the circulation, whereby loading of LDL with both hy-droperoxy and hydroxy lipids would be reduced.
In conclusion, a PHGPx overexpressing cell line has been constructed in which the effects of PHGPx can be investigated individually. The altered response of the cells to hydroperoxides and to oxLDL shows that these cells may be a suitable medium to study the role of this
particular selenoprotein in processes relevant to
atherogenesis.
Acknowledgements
We thank E. Wendt for excellent support in the tremendous cell culture work, G. Aust for indefatigably measuring GPx activites, and J. Neuzil for help with the HPLC analyses. This study was supported by the ‘Deutsche Akademie der Naturforscher’, Leopoldina, the Boehringer Ingelheim Fond, the Finnish Academy, and the European community Biomed II program (BMH4-CT98-3202). S.M. was a recipient of a
scholar-ship of the ‘Graduiertenfo¨rderung des Landes
Brandenburg’.
References
[1] Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J 1993;69:S30 – 7. [2] Chatterjee S. Role of oxidized low density lipoproteins in
atherosclerosis: effect of smooth muscle cell proliferation. Mol Cell Biochem 1992;111:143 – 7.
[3] Auge´ N, Pieraggi M-T, Thiers J-C, Ne`gre-Salvayre A, Salvayre R. Proliferative and cytotoxic effects of mildly oxidized low-den-sity lipoproteins on vascular smooth-muscle cells. Biochem J 1995;309:1015 – 20.
[4] Balagopalakrishna C, Bhunia AK, Rifkind JM, Chatterjee S. Minimally modified low density lipoproteins induce aortic smooth muscle cell proliferation via the activation of mitogen activated protein kinase. Mol Cell Biochem 1997;170:8589. [5] Natarajan V, Scribner W, Hart CM, Parthasarathy S. Oxidized
low density lipoprotein-mediated activation of phospholipase D in smooth muscle cells: a possible role in cell proliferation and atherogenesis. J Lipid Res 1995;36:2005 – 16.
[6] Bjo¨kerud B, Bjo¨kerud S. Contrary effects of lightly and strongly oxidized LDL with potent promotion of growth versus apoptosis on arterial smooth muscle cells, macrophages, and fibroblasts. Arterioscler Thromb Vasc Biol 1996;16:416 – 24.
[7] Pitas RE. Expression of the acetyl low density lipoproptein receptor by rabbit fibroblasts and smooth muscle cells. J Biol Chem 1990;265:12722 – 7.
[8] Yla¨-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086 – 95.
[9] Friedrichs B, Toborek M, Hennig B, Heinevetter L, Mu¨ller C, Brigelius-Flohe´ R. 13-HPODE and 13-HODE modulate cy-tokine-induced expression of endothelial cell adhesion molecules differently. BioFactors 1999;9:61 – 72.
[10] Viita H, Sen CK, Roy S, Siljama¨ki T, Nikkari T, Yla¨-Herttuala S. High expression of human 15-lipoxygenase induces NF-k
[11] Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARg. Cell 1998;93:229 – 40.
[12] Stocker R, Bowry VW, Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxida-tion than does a-tocopherol. Proc Natl Acad Sci USA
1991;88:1646 – 50.
[13] Parthasarathy S, Wieland E, Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modificaton of low density lipoprotein. Proc Natl Acad Sci USA 1989;86:1046 – 50. [14] Rankin SM, Parthasarathy S, Steinberg D. Evidence for a
dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res 1991;32:449 – 56. [15] Sigari F, Lee C, Witztum JL, Reaven PD. Fibroblasts that
overexpress 15-lipoxygenase generate bioactive and minimally modified LDL. Arterioscler Throm Vasc Biol 1997;17:3639 – 45. [16] Belkner J, Stender H, Ku¨hn H. The rabbit 15-lipoxygenase preferentially oxygenates LDL cholesterol esters, and this reac-tion does not require vitamin E. J Biol Chem 1998;273:23225 – 32.
[17] Sparrow CP, Olszewski J. Cellular oxidative modification of low density lipoprotein does not require lipoxygenases. Proc Natl Acad Sci USA 1992;89:128 – 31.
[18] Ursini F, Maiorino M, Brigelius-Flohe´ R, Aumann KD, Roveri A, Schomburg D, Flohe´ L. Diversity of glutathione peroxidases. Methods Enzymol 1995;252:38 – 53.
[19] Smith WL, Lands WEM. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry 1972;11:3276 – 85.
[20] Haurand M, Flohe´ L. Kinetic studies on arachidonate 5-lipoxy-genase from rat basophilic leukemia cells. Biol Chem Hoppe-Seyler 1988;369:133 – 42.
[21] Schnurr K, Hellwing M, Seidemann B, Jungblut P, Ku¨hn H, Rapoport SM, Schewe T. Oxygenation of biomembranes by mammalian lipoxygenases: the role of ubiquinone. Free Radic Biol Med 1996;20:11 – 21.
[22] Salonen LT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial in-farction and serum selenium in a matched-pair longitudinal study. Lancet 1982;2:175 – 9.
[23] Salonen JT, Salonen R, Lappetela¨inen R, Ma¨nenpa¨a¨ PH, Alfthan G, Puska P. Risk of cancer in relation to serum concen-trations of selenium and vitamins A and E: matched case-control analysis of prospective data. Br Med J 1985;290:417 – 20. [24] Flohe´ L. The selenoprotein glutathione peroxidase. In: Dolphin
D, Poulson R, Avramovic O, editors. Glutathione: Chemical, Biochemical and Medical Aspects — Part A. New York: Wiley, 1989:643 – 731.
[25] Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purifica-tion from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glu-tathione peroxidase activity on phosphatidylcholine hydroperox-ides. Biochim Biophys Acta 1982;710:197 – 211.
[26] Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damage in lipid peroxidation. J Biol Chem 1990;265:454 – 61.
[27] Sattler W, Maiorino M, Stocker R. Reduction of HDL- and LDL-associated cholesterylester and phospholipid hydroperox-ides by phospholipid hydroperoxide glutathione peroxidase and ebselen (PZ51). Arch Biochem Biophys 1994;309:214 – 21. [28] Pietila¨ K, Yla¨-Herttuala S, Jaakkola Nikkari T. Metabolism of
glycosaminoglycans and lipids in smooth muscle cells from atherosclerotic rabbit aortas in culture. Atherosclerosis 1980;37:449 – 56.
[29] Danos O, Mulligan C. Safe and efficient generation of recombi-nant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci USA 1988;85:6460 – 4.
[30] Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates and Wiley-Inter-science, Wiley, 1992.
[31] Brigelius-Flohe´ R, Aumann KD, Blo¨cker H, Gross G, Kiess M, Klo¨ppel K-D, Maiorino M, Roveri A, Schuckelt R, Ursini F, Wingender E, Flohe´ L. Phospholipid-hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J Biol Chem 1994;269:7342 – 8.
[32] Miyanohara A, Sharkey MF, Witztum JL, Steinberg D, Fried-man T. Efficient expression of retroviral vector-transducted hu-man low density lipoprotein (LDL) receptor in LDL receptor-deficient rabbit fibroblasts in vitro. Proc Natl Acad Sci USA 1988;85:6538 – 42.
[33] Maiorino M, Aumann KD, Brigelius-Flohe´ R, Doria D, van den Heuvel J, McCarthy J, Roveri A, Ursini F, Flohe´ L. Probing the presumed catalytic triad of selenium-containing peroxidases by mutational analysis of phospholipid hydroperoxide glutathione peroxidase (PHGPx). Biol Chem Hoppe-Seyler 1995;376:651 – 60. [34] Brigelius-Flohe´ R, Lo¨tzer K, Maurer S, Schultz M, Leist M. Utilization of selenium from different chemical entities for se-lenoprotein biosynthesis by mammalian cell lines. BioFactors 1995/1996;5:125 – 31.
[35] Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospho-lipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 1985;839:62 – 70.
[36] Funk MO, Isaac R, Porter NA. Preparation an purification of lipid hydroperoxides from arachidonic and g-linolenic acids.
Lipids 1976;11:113 – 7.
[37] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods 1983;65:55 – 63.
[38] Rothe G, Emmendo¨rffer A, Oser A, Roesler J, Valet G. Flow cytometric measurement of the respiratory burst activity of phagocytes using dihydrorhodamine 123. J Immunol Methods 1991;138:133 – 5.
[39] Schreiber E, Matthias P, Mu¨ller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’ pre-pared from a small number of cells. Nucleic Acids Res 1989;17:6419.
[40] Chung BH, Wilkinson T, Geer JC, Segrest JP. Preparative and quantitative isolation of plasma lipoproteins: rapid, single dis-continuous density gradient ultracentrifugation in a vertical ro-tor. J Lipid Res 1980;21:284 – 91.
[41] Mindham MA, Mayes PA. A simple and rapid method for the preparation of apolipoproteins for electrophoresis. J Lipid Res 1992;32:1084 – 8.
[42] Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302 – 10.
[43] Kritharides L, Jessup W, Gifford J, Dean RT. A method for defining the stages of low-density lipoprotein oxidation by the separation of cholesterol- and cholesteryl ester-oxidation prod-ucts using HPLC. Anal Biochem 1993;213:7989.
[44] Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911 – 7.
[45] Lei GX, Evenson J, Patrick D, Sunde RA. Overexpression of phospholipid hydroperoxide glutathione peroxidase in FL5.12 cells partially protects against azo-initiated peroxidation. FASEB J 1995;9:A158.
[46] Imai H, Sumi D, Sakamoto H, Hanamoto A, Arai M, Chiba N, Nakagawa Y. Overexpression of phospholipid hydroperoxide glutathione peroxidase suppressed cell death due to oxidative damage in rat basophile leukemia cells (RBL-2H3). Biochem Biophys Res Commun 1996;222:432 – 8.
lipid hydroperoxide-mediated injury. Biochem Biophys Res Commun 1996;219:486 – 91.
[48] Sun Q, Kojima H, Komura S, Ohishi N, Yagi K. Effect of selenium on human phospholipid hydroperoxide glutathione per-oxidase expression and host cell susceptibility to lipid hydroper-oxide-mediated injury. Biochem Mol Biol Int 1997;42:957 – 63. [49] Brigelius-Flohe´ R, Friedrichs B, Maurer S, Streicher R.
Determi-nants of PHGPx expression in a cultured endothelial cell line. Biomed Environ Sci 1997;10:163176.
[50] Diamond AM, Kataoka Y, Murray J, Chengying D, Folks TM, Sandstrom PA. A T-cell model for the biological role of sele-nium-dependent glutathione peroxidase. Biomed Environ Sci 1997;10:246 – 52.
[51] Mirault ME, Tremblay A, Beaudoin N, Tremblay M. Overex-pression of selenoglutathione peroxidase by gene transfer en-hances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem 1991;266:20752 – 60.
[52] Kelner MJ, Bagnell RD, Uglik SF, Montoya MA, Mullenbach GT. Heterologous expression of selenium-dependent glutathione peroxidase affords cellular resistance to paraquat. Arch Biochem Biophys 1995;323:40 – 6.
[53] Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intra-cellular oxidants: comparison with 2%,7% -dichlorodihydrofluores-cein diacetate, 5 (and 6)-carboxy 2%,7%-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 1999;27:146 – 59.
[54] Kretz-Remy C, Mehlen P, Mirault ME, Arrigo AP. Inhibition of I kappa B-alpha phosphorylation and degradation and subse-quent NF-kappa B activation by glutathione peroxidase overex-pression. J Cell Biol 1996;133:1083 – 93.
[55] Makropoulos V, Bruning T, Schulze-Osthoff K. Selenium-medi-ated inhibition of transcription factor NF-kappa B and HIV-1 LTR promoter activity. Arch Toxicol 1996;70:277 – 83. [56] Brigelius-Flohe´ R, Friedrichs B, Maurer S, Schultz M, Streicher
R. IL-1-induced NFKB activation is inhibited by overexpression of PHGPx in a human endothelial cell line. Biochem J 1997b;328:199 – 203.
[57] Kim IY, Stadtman TC. Inhibition of NF-kappaB DNA binding and nitric oxide induction in human T cells and lung adenocar-cinoma cells by selenite treatment. Proc Natl Acad Sci USA 1997;94:12904 – 7.
[58] Low SC, Berry MJ. Knowing when not to stop: selenocysteine incorporation in eukaryotes. TIBS 1996;21:203 – 8.
[59] Sandstrom PA, Tebbey PW, van Cleave S, Buttke TM. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-as-sociated glutathione peroxidase deficiency. J Biol Chem 1994;269:798 – 801.
[60] Kayanoki Y, Fujii J, Islam KN, Suzuki K, Kawata S, Mat-suzawa Y, Taniguchi N. The protective role of glutathione peroxidase in apoptosis induced by reactive oxygen species. J Biochem 1996;119:817 – 22.
[61] Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, Chan C, Chavez C. EGF-Receptor phosphorylation and sig-naling are targeted by H2O2redox stress. Am J Respir Cell Mol Biol 1998;19:786 – 98.
[62] Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene di-minishes atherosclerosis in apo E deficient mice. J Clin Invest 1999;103:1597 – 604.
[63] Witting P, Pettersson K, O8stlund-Lindqvist A-M, Westerlund C, Wa¨gberg M, Stocker R. Dissociation of atherogenesis from aortic accumulation of lipid hydro(pero)xides in Watanabe heri-table hyperlipidemic rabbits. J Clin Invest 1999;104:213 – 20. [64] Santanam N, Auge´ N, Zhou M, Keshava C, Parthasarathy S.
Overexpression of human catalase gene decreases oxidized lipid-induced cytotoxicity in vascular muscle cells. Arterioscler Thromb Vasc Biol 1999;19:1912 – 7.
[65] Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans 1996;24:1028 – 32.
[66] Schade FU, Engel R, Ha¨rtling S, Holler J, Jakobs D. The role of unsaturated fatty acids in endotoxin-induced macrophage activa-tion. Immunobiology 1993;187:283 – 302.
[67] Steinberg D. Low density lipoprotein oxidation and its pathobio-logical significance. J Biol Chem 1997;272:20963 – 6.
[68] Weitzel F, Wendel A. Selenoenzymes regulate the activity of leukocyte 5 lipoxygenase via the peroxide tone. J Biol Chem 1993;268:6288 – 92.