SPECIAL SECTION
Commentary from the Italian Association of Sleep Medicine on the AASM manual
for the scoring of sleep and associated events: For debate and discussion
q
Liborio Parrino
a,*,1, Raffaele Ferri
b,1, Marco Zucconi
c,1, Francesco Fanfulla
d,1 aSleep Disorders Center, Department of Neurosciences, University of Parma, ItalybSleep Research Center, Department of Neurology I.C., Oasi Institute for Research on Mental Retardation and Brain Aging (IRCCS), Troina, Italy cSleep Disorders Center, Department of Neurology, Scientific Institute and University Ospedale San Raffaele, Vita-Salute University, Milan, Italy dSleep Center, Pulmonary Division, Istituto Scientifico di Pavia, Fondazione S. Maugeri, IRCCS, Italy
a r t i c l e
i n f o
Article history:
Received 24 October 2008
Received in revised form 16 May 2009 Accepted 22 May 2009
Available online 28 June 2009
Keywords:
Sleep Scoring rules Arousals CAP
Sleep-related movements Respiratory events
a b s t r a c t
In 2007, the American Academy of Sleep Medicine (AASM) completed a new manual for the scoring of sleep and associated events. The AASM manual is divided into separate sections relative to the parame-ters reported for polysomnography. The present commentary, accomplished by a Task Force of the Italian Association of Sleep Medicine, focuses on sleep scoring data, arousal rules, movement and respiratory events. Comparisons with the previous Rechtschaffen and Kales system are detailed and a number of methodological weaknesses are pointed out. Major comments address the 30-s scoring epochs, the restrictive approach to arousals and EEG activating patterns, the incomplete quantification of motor events and the thresholds for the definition of hypopnea. Since the new AASM manual is an iterative pro-cess, proposals for discussion and re-examination of the agreed criteria with other national and interna-tional organizations are encouraged.
Ó2009 Elsevier B.V. All rights reserved.
1. Prologue
In June 2007, the American Academy of Sleep Medicine (AASM) completed a new manual for the scoring of sleep and associated events[1]. The manual, presented during the 21st Annual Meeting of the Associated Professional Sleep Societies, was preceded by the March 2007 issue of the Journal of Clinical Sleep Medicine compris-ing seven evidence-based review articles[2]. Each article was orga-nized to provide a historical perspective, a review and evidence grading the validity or reliability of measures and a discussion of fu-ture areas for research. Over 1500 total articles were reviewed reflecting the PubMed literature on human studies published in Eng-lish between 1968 and 2004. Under the overall supervision of a steering committee, the task force members developed a consensus
process which broadly represents current knowledge and expertise in the field. Besides the rules for sleep staging and arousals, agree-ment was acheived for cardiac, moveagree-ment and respiratory events, and for the first time, criteria for sleep in children were detailed.
The rules and specifications in the visual scoring, however, re-tain much of the framework of Rechtschaffen and Kales (R&K) rules
[3], and this reverberates negatively upon the entire arrangement of the manual. Software and hardware companies have been in-volved in polysomnography data acquisition and scoring, but sleep is still interpreted as a discontinuous process artificially divided in discrete standardized epochs. This approach is different from pub-lished material and clinical evidence, which in the past decades has emphasized the necessity to use alternative scoring methods. Re-cently, Schulz[4]published a contribution provocatively entitled ‘‘Rethinking sleep analysis.” In this perspective, the Italian school has always supported the R&K stage definition approach, which, however, needs to be implemented by analysis of the continuity and consolidation of sleep to recover most of the information ne-glected by the conventional scoring measures. A more dynamic ap-proach is not limited to arousals and cyclic EEG patterns during sleep, but also includes motor and respiratory events, which have been demonstrated to undergo ordered sequences in a number of pathological conditions such as periodic limb movements and sleep apnea syndrome.
Since the new AASM scoring manual is an iterative process, future editions of the manual will undoubtedly require a
1389-9457/$ - see front matterÓ2009 Elsevier B.V. All rights reserved. doi:10.1016/j.sleep.2009.05.009
q
In order to generate a healthy discussion and debate to promote scientific progress we are inviting comments from the readership as Letters to the Editor which will be published in subsequent issues of the journal after appropriately editing the contents.
* Corresponding author. Address: Clinica Neurologica – Centro di Medicina del Sonno, Università deglI Studi, Via Gramsci, 14, 43100 Parma, Italia. Tel./fax: +11 39 521 287913.
E-mail address:liborio.parrino@unipr.it(L. Parrino).
1 Task Force on the AASM Manual for the Scoring of Sleep and Associated Events on
behalf of the Governing Board of the Italian Association of Sleep Medicine: Franco Ferrillo (President), Gian Luigi Gigli (Vice-President), Liborio Parrino (Secretary), Alberto Braghiroli (Treasurer).
Contents lists available atScienceDirect
Sleep Medicine
re-examination of evidence to include additional elements and ad-dress the rapidly evolving science of the metrics of examining sleep. The present commentary, carried out by an ad hoc task force of the Italian Association of Sleep Medicine, was written in a per-spective of acknowledgement and integration.
2. Sleep stages: the unsolved problem
The standardized R&K criteria for sleep staging set up in 1968
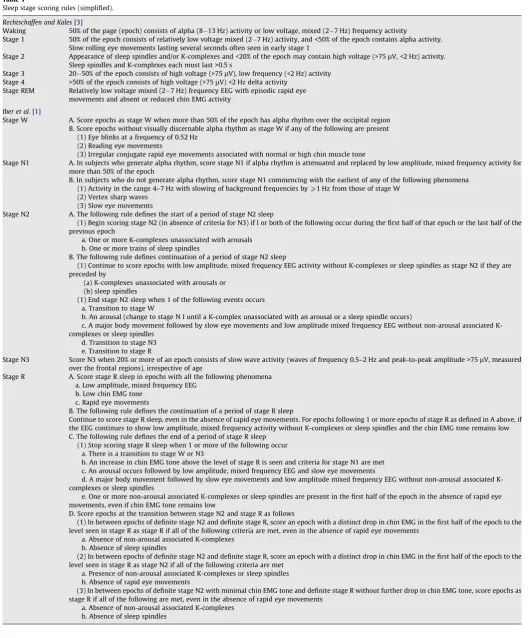
[3]have constituted the only internationally accepted system for sleep scoring for almost 40 years, and an enormous body of data has been produced and published in the scientific literature with this system. The new AASM manual [1]has the scope to revise R&K sleep staging and to address digital methodology as well as the scoring of arousals, respiratory events, sleep related movement disorders, and cardiac abnormalities, with consideration of pediat-ric and geriatpediat-ric age groups. The work accompanying the decisions on the new rules showed that, using the R&K system, inter-rater accuracy was highest for REM sleep, followed by stage 2 sleep, and lowest for stage 1 sleep, while reliability for wake and slow wave sleep (SWS) was moderate. Accordingly, scoring rules for stage 1 and SWS required reassessment [5]. Table 1 compares the major sleep staging rules in the two systems.
Compared to the R&K system, the AASM rules extend the recording requirements with specifications that take into full ac-count the current standard techniques for recording and storing biological signal. The visual scoring of sleep now needs to be per-formed on at least three EEG channels, including frontal and occip-ital derivations, besides the classical EOG and EMG channels. This corrects the single central derivation number of channels indicated in the R&K rules and allows a more extensive coverage of scalp areas where significant sleep/wake rhythms and waveforms are better represented, such as K-complexes and delta waves. Even if this choice keeps the costs of polysomnography low to favor its use in large clinical populations, this minimal set of EEG deriva-tions surely does not satisfy research purposes. An alternative with a higher number of EEG channels might have been indicated, with the advantage of standardizing at least some research applications of polysomnography.
It is interesting to notice that each rule of the new AASM man-ual is accompanied by a recommendation level ([RECOMMENDED], [ALTERNATIVE], or [OPTIONAL]), and each recommendation is associated with a procedural note classified into 4 classes, depend-ing on the five levels of evidence contained in the publications which were taken into account to establish each rule[1]. The four classes are [STANDARD] – recommendation based on level 1 evi-dence or overwhelming level 2 evievi-dence; [GUIDELINE] – recom-mendation based on level 2 evidence or a consensus of level 3 evidence; [CONSENSUS] – recommendation with less evidence than guideline for which agreement was reached in a standardized consensus process based on available information; and [ADJUDI-CATION] – recommendation from the steering committee based on all available information; adjudication was only performed (a) when there was insufficient evidence and no consensus agreement or (b) in conjunction with task force leaders on issues regarding minor clarifications and additions to rules. For the visual scoring of sleep, 29 rules were established with only three supported by a recommendation based on a [STANDARD] level procedure, none was classified as [GUIDELINE], the vast majority was supported by a [CONSENSUS] procedure, one was classified as [CONSENSUS/ ADJUDICATION] and only two reached [ADJUDICATION] level. In this section of the manual, these levels seem to be very low and fuel the concerns of the impact of these new rules on the major problem of visual scoring, i.e., inter-rater and intra-rater variabil-ity. This concern is confirmed by a very recent report by Danker-Hopfe et al. [6] on inter-rater reliability of the expert scorings
according to the AASM standard compared with those obtained from R&K rules. These authors have shown that inter-rater reliabil-ity was higher for scorings according to the AASM standard only for stages W, N1 and R, with only a small increase, even if statistically significant; inter-rater reliability was essentially the same for N3/ SWS. On the contrary, inter-rater reliability for N2/S2, the stage in which both normal controls and patients usually spend the majority of their time asleep, was decreased with the new AASM rules compared to that obtained from R&K rules. Moreover, these results were obtained in conditions potentially able to inflate the AASM scoring agreement when compared to R&K, as the same authors also express in their paper. The reasons considered to be at the basis of the decrease in inter-rater reliability for N2/S2 are likely to be connected with the low inter-rater reliability which characterizes the detection of arousals[7], events that have as-sumed a crucial role in determining the end of N2 in the new AASM rules but still with a poor definition (see below). Regarding the introduction of a frontal derivation in the EEG channel set needed for sleep scoring, the inter-rater reliability for N3/SWS has not changed, probably because of the persisting rule of the 75
lV
amplitude for slow waves, a merely conventional value not based on any physiologically sound basis. Finally, it should be noted that, even if inter-rater reliability for N1 was significantly increased, it still remained far below that obtained for other sleep stages, indi-cating a disappointing ‘‘moderate” degree of agreement.Silber et al.[5]stated, ‘‘Scoring rules for stage 1 and SWS sleep needed reassessment”; based on the considerations and results re-ported above, we can affirm that the reassessment proposed by the AASM has not been followed by the expected improvement in in-ter-rater reliability of these stages.
In the new AASM system, the distinction between wakefulness (W), non-REM sleep (N) and REM sleep (R) is maintained, but the non-REM stages are reduced to three: N1 (previous stage 1), N2 (previous stage 2), N3 (previous stages 3 + 4). The new rules are meant to have a major impact on everyday clinical and research practice and will play a topical role in the field of sleep medicine in the next years. Nevertheless, they have been established on a low degree of evidence. This evidence was not present in the scien-tific literature, and a more convincing way to proceed, before the final establishment of rules, should have included a careful multi-center statistical validation. Without these premises, the new rules are simply a stab in the dark and very unlikely to provide better performance; more importantly, they do not support or facilitate a more reliable automatic computer implementation compared to the R&K criteria. The sleep medicine community needs to be supported by new, statistically driven data and procedures which should probably be set in new, large studies that are somewhat free from the errors of the past. Considering studies essentially based on a single system carries the serious danger of creating a vi-cious circle which generates self-similar products (with the same errors), prohibiting new ideas to break through.
information stored in our databases? Are we really ready to throw the baby away with the bath water? Is this information really use-less now? Should we now wait long before we can have a serious
sleep architecture follow-up study? Rescoring old recordings is now impossible if we have not recorded at least one frontal, one central and one occipital channel; thus the new rules limit the
Table 1
Sleep stage scoring rules (simplified).
Rechtschaffen and Kales[3]
Waking 50% of the page (epoch) consists of alpha (8 13 Hz) activity or low voltage, mixed (2 7 Hz) frequency activity
Stage 1 50% of the epoch consists of relatively low voltage mixed (2 7 Hz) activity, and <50% of the epoch contains alpha activity. Slow rolling eye movements lasting several seconds often seen in early stage 1
Stage 2 Appearance of sleep spindles and/or K-complexes and <20% of the epoch may contain high voltage (>75lV, <2 Hz) activity. Sleep spindles and K-complexes each must last >0.5 s
Stage 3 20 50% of the epoch consists of high voltage (>75lV), low frequency (<2 Hz) activity Stage 4 >50% of the epoch consists of high voltage (>75lV) <2 Hz delta activity
Stage REM Relatively low voltage mixed (2 7 Hz) frequency EEG with episodic rapid eye movements and absent or reduced chin EMG activity
Iber et al.[1]
Stage W A. Score epochs as stage W when more than 50% of the epoch has alpha rhythm over the occipital region B. Score epochs without visually discernable alpha rhythm as stage W if any of the following are present
(1) Eye blinks at a frequency of 0.52 Hz (2) Reading eye movements
(3) Irregular conjugate rapid eye movements associated with normal or high chin muscle tone
Stage N1 A. In subjects who generate alpha rhythm, score stage N1 if alpha rhythm is attenuated and replaced by low amplitude, mixed frequency activity for more than 50% of the epoch
B. In subjects who do not generate alpha rhythm, score stage N1 commencing with the earliest of any of the following phenomena (1) Activity in the range 4–7 Hz with slowing of background frequencies byP1 Hz from those of stage W
(2) Vertex sharp waves (3) Slow eye movements
Stage N2 A. The following rule defines the start of a period of stage N2 sleep
(1) Begin scoring stage N2 (in absence of criteria for N3) if l or both of the following occur during the first half of that epoch or the last half of the previous epoch
a. One or more K-complexes unassociated with arousals b. One or more trains of sleep spindles
B. The following rule defines continuation of a period of stage N2 sleep
(1) Continue to score epochs with low amplitude, mixed frequency EEG activity without K-complexes or sleep spindles as stage N2 if they are preceded by
(a) K-complexes unassociated with arousals or (b) sleep spindles
(1) End stage N2 sleep when 1 of the following events occurs a. Transition to stage W
b. An arousal (change to stage N I until a K-complex unassociated with an arousal or a sleep spindle occurs)
c. A major body movement followed by slow eye movements and low amplitude mixed frequency EEG without non-arousal associated K-complexes or sleep spindles
d. Transition to stage N3 e. Transition to stage R
Stage N3 Score N3 when 20% or more of an epoch consists of slow wave activity (waves of frequency 0.5–2 Hz and peak-to-peak amplitude >75lV, measured
over the frontal regions), irrespective of age
Stage R A. Score stage R sleep in epochs with all the following phenomena a. Low amplitude, mixed frequency EEG
b. Low chin EMG tone c. Rapid eye movements
B. The following rule defines the continuation of a period of stage R sleep
Continue to score stage R sleep, even in the absence of rapid eye movements. For epochs following 1 or more epochs of stage R as defined in A above, if the EEG continues to show low amplitude, mixed frequency activity without K-complexes or sleep spindles and the chin EMG tone remains low C. The following rule defines the end of a period of stage R sleep
(1) Stop scoring stage R sleep when 1 or more of the following occur a. There is a transition to stage W or N3
b. An increase in chin EMG tone above the level of stage R is seen and criteria for stage N1 are met c. An arousal occurs followed by low amplitude, mixed frequency EEG and slow eye movements
d. A major body movement followed by slow eye movements and low amplitude mixed frequency EEG without non-arousal associated K-complexes or sleep spindles
e. One or more non-arousal associated K-complexes or sleep spindles are present in the first half of the epoch in the absence of rapid eye movements, even if chin EMG tone remains low
D. Score epochs at the transition between stage N2 and stage R as follows
(1) In between epochs of definite stage N2 and definite stage R, score an epoch with a distinct drop in chin EMG in the first half of the epoch to the level seen in stage R as stage R if all of the following criteria are met, even in the absence of rapid eye movements
a. Absence of non-arousal associated K-complexes b. Absence of sleep spindles
(2) In between epochs of definite stage N2 and definite stage R, score an epoch with a distinct drop in chin EMG in the first half of the epoch to the level seen in stage R as stage N2 if all of the following criteria are met
a. Presence of non-arousal associated K-complexes or sleep spindles b. Absence of rapid eye movements
(3) In between epochs of definite stage N2 with minimal chin EMG tone and definite stage R without further drop in chin EMG tone, score epochs as stage R if all of the following are met, even in the absence of rapid eye movements
use of previous recordings, and completely new databases need to be collected. Preliminary studies show significant differences be-tween sleep parameters derived from visual sleep scoring on the basis of the R&K rules and those based on the new AASM criteria. In particular, the time spent in N1 and N3 increases, while N2 de-creases significantly. Moreover, wake after sleep onset is pro-longed, while sleep latency, REM latency, total sleep time and sleep efficiency seem to be little affected[12].
Finally, it is necessary to spend some words on the effects of sleep fragmentation into epochs. It has been clear for years that each sleep epoch of 30 s encompasses several short-time events which carry important information not showing up in the classical sleep staging reports. These features (K-complexes, sleep spindles, delta bursts, etc.) have been grouped under the label of sleep pha-sic events, and the most comprehensive method for their analysis and report is the so-called Cyclic Alternating Pattern (CAP)[13]. CAP cannot be measured without having scored sleep stages in ad-vance; however, it is not limited to epoch fragmentation and spans over long periods of NREM sleep. Perhaps the sleep community is seeking a breath of fresh air with a serious discussion on alterna-tive methods which overcome the boundaries of rigid epochs. The new system should start from the consideration that sleep is a continuous function[4,13], including stages and cycles, but with-out restricting a complex dynamic process into a static sequence of 30-s artificial segments.
There are many studies in the literature attempting to over-come the limitations of the fixed-length epoch segmentation based on different approaches, such as those proposing to represent NREM sleep with a continuous function[14–16]and those based on variable-length segmentation techniques and followed by clus-ter analysis[17–22]. Again, none of these methods has ever been tested in large numbers of sleep recordings and cannot be consid-ered as valid alternatives until this type of validation is carried out; however, it is also important to reemphasize here that the newly proposed AASM rules are not supported by any validation study.
Thus, it seems reasonable to propose that the new AASM rules should not be used for sleep staging in replacement of the R&K rules because they have failed to introduce significant improve-ments, and, for this reason, they do not justify the necessary tech-nical, economic, clinical and scientific sacrifices that are needed to pass from one system to the other. This does not imply that the R&K system should be kept for a long time, but points to the urgent need for serious international effort for a reliable, neurophysiologi-cally-based, data-driven, computer-compatible system for sleep scoring.
3. Arousal rule: is something missing?
It must be recognized that the new AASM manual dedicates extensive attention to REM sleep. An epoch can be scored as R only with the following coexisting phenomena: low amplitude mixed frequency EEG, low chin EMG tone, and rapid eye movements. After a clear cut onset of REM sleep, the following epochs are scored as R if the EEG remains unmodified and muscle tone is not restored in the first half of the epoch regardless of the occur-rence of rapid eye movements. This definition seems to overcome some of the previous constraints, such as the 3-min absence of ra-pid eye movements (the period of time between 2 spindles or K-complexes with a low voltage mixed EEG was previously scored as stage 2 if the interval was <3 min and without rapid eye move-ments[3]).
In contrast, the role of the homeostatic process ‘‘S” is unexpect-edly omitted in the new methodological framework. Stages 3 and 4 are now lumped together in a single stage N3, in contrast to the nocturnal development of the process ‘‘S,” characterized by decreasing peaks across the night, according to the decreasing
power of the EEG delta band[23]. Instead of including enriching information on the power of the EEG signal, the adopted solution is a reductive simplification of the previous sleep stages; obliterat-ing the exponential profile of process ‘‘S,” the restorative function of slow wave sleep and its direct relationship with the waking activities are shadowed.
The presentation of arousal confirms the definition established in 1992 by the American Sleep Disorders Association (ASDA; since named the AASM) [24]. According to the original framework, arousals are markers of sleep disruption representing a detrimen-tal and harmful feature for sleep. For this reason they were ex-cluded from the conventional staging procedures. Several papers have been written in the last 16 years, since the scoring rules for arousal were established[24], but no substantial update has been provided.
A number of studies have established that spontaneous arousals are natural guests of sleep and undergo a linear increase along the life span following the profile of maturation and aging[25–27]. Moreover, the spectral composition of arousals [28] and their ultradian distribution throughout the sleep cycles reveal that arousals are endowed within the texture of physiological sleep
[29–31]under the biological control of REM-on and REM-off mech-anisms[32].
According to these indications, arousal scoring is now consid-ered a fundamental process in staging classification as well as spin-dles and K-complexes. However, in the R&K system [3], a K-complex, with or without an arousal, was unmistakably a marker of sleep stage 2. According to the AASM manual, if a K-complex is associated with an arousal the epoch is scored as N1. In other words, the R&K approach privileged the role of EEG synchrony in the scoring process, while the AASM manual enhances the impact of EEG desynchrony.
If the brain is able to produce a K-complex this means that sleep has reached the threshold of what is arbitrarily classified as stage 2. A K-complex can be followed by other K-complexes, and, in that case, stage 2 tends towards consolidation; or it can be followed by an arousal which reflects a regression towards a shallower neuro-physiological condition. Most arousals (87%), however, are pre-ceded by a K-complex or delta burst[28], indicating that sleep can become transiently lighter without necessarily changing stage. This is exactly what happens with major body movements, which are scored by the AASM manual as N2 if the previous epoch is N2 and there are no slow eye movements [1]. The methodological weakness of the AASM system is confirmed by the lack of scoring instructions on the occurrence of an arousal in an epoch with at least 20% of slow wave activity. Shall such an epoch be classified as N3 or N1? Perhaps, a simple distinction between consolidated and unstable sleep would have been a more appropriate solution
[33].
Further problems are raised by the definition[1]: ‘‘Begin scoring N2 if one or more K-complexes not associated with arousals occur during the first half of the epoch or the last half of the previous epoch.” The split of each epoch in first half and second half frankly appears as an arbitrary time-consuming procedure, which proba-bly tries to attenuate the rigid division in 30 s segments.
Finally, the original scoring of an arousal during REM sleep re-quired a concurrent increase in submental EMG amplitude. In the new AASM manual[1], the increase in submental EMG amplitude must last for at least one second. This is because EMG increase can provide better scoring reliability during all sleep stages, particu-larly comparing REM sleep vs. light sleep [34]. But data were collected from OSA patients where the respiratory-driven muscle activation in REM sleep can differ from normal conditions.
morphology can be organized into sequences[35]. This extensive interpretation of arousals and their non-random distribution into periodic sequences converges on CAP, which represents a struc-tured organization of sleep beyond the static stage setting
[36,37]. In the review chapter on the scoring of the AASM manual
[7], the authors state that ‘‘reliable scoring of arousals is a difficult and time-consuming process.” Nevertheless, identification of arousals has become mandatory in the new classification. In the same chapter, the authors state that ‘‘measurement of CAP is more time consuming than scoring of ASDA arousals,” although ‘‘it can be argued that the statistical relationship between certain sub-types of CAP activity and the occurrence of ASDA arousals makes those measures similar.” Indeed, a highly significant correlation (p< 0.0001) has been ascertained between subtypes A2 and A3 of CAP and ASDA-scored arousals[38], and a Kendall W inter-rater coefficient of concordance value of 0.90 for various CAP parameters in normal adults has also been reported[39]. The over 120 PubMed articles published on CAP suggest that this scoring method could provide integrative information to what is supplied by the simple counting of arousals (Table 2) because CAP is not a measure of sleep fragmentation, but quantifies the amount of unstable, non-consolidated sleep. Moreover, the CAP framework not only recov-ers all the information provided in NREM sleep by AASM arousals, but also integrates single or repetitive arousals with other EEG events endowed with activation properties[26,38]. A number of studies have shown that both K-complex sequences and delta bursts (either evoked or spontaneous) are regularly accompanied by activation of autonomic functions, i.e., heart rate, blood pres-sure, muscle sympathetic activity[40], which are generally weaker compared to what accompanies an AASM arousal, but may be equally effective[41–43]. In a recent investigation carried out in normotensive subjects with chronic insomnia, higher night time blood pressure and blunted blood pressure dipping was associated with brain cortical activation during sleep in the absence of arousal rate changes[44]. This implies that scoring of single arousal events can be enriched by integrative sleep parameters, including the more general concept of oscillating activation. Moreover, in an era that has overcome most of the time-consuming constraints due to paper recording and that can rely on computer-assisted memory attention, can be extended beyond the static framework of 30-s epochs in search of boundless EEG patterns.
4. Movement thresholds: time for computer-assisted quantification
For the first time, the new AASM Manual has introduced, in a comprehensive manner, the scoring criteria for different types of
movements. Besides periodic limb movements (PLMs), scoring cri-teria have also been established for the more frequent bruxism, REM sleep Behavior Disorder (RBD), and Rhythmic Movement Dis-order (RMD), and for other less common movement disturbances, such as Alternating Leg Movement Activation (ALMA), Hypnagogic Foot Tremor (HFT), and Excessive Fragmentary Myoclonus (EFM).
4.1. Limb movements
The R&K manual[3]contained only rules for sleep stage scoring. In 1993, in sleep medicine’s pre-computer era, the Atlas Task Force of the American Sleep Disorders Association[45]established some cri-teria, reassembling some previous data collected by Coleman et al. in 1982[46]and based on a group of polysomnographic studies re-corded on paper, and it never changed over the years. The new crite-ria indicated for PLMs have been mostly advocated from the standards for recording and scoring PLMs in sleep (PLMS) and wake-fulness (PLMW) developed by World Association of sleep Medicine (WASM) and International Restless Legs Syndrome Study Group (IRLSSG) [47]. Briefly, the definition of minimum and maximum duration, amplitude onset and offset of a leg movement (LM) event, minimum number of consecutive LMs with a period length to define a PLM series, as well as the criteria to determine bilateral or unilate-ral LM have been detailed. The novelties are the inclusion of longer LM (from 0.5 to 10 s in duration), the shorter movement inter-val (offset-to-onset 0.5 s) to determine the separation between LMs, despite the fact that clinical scoring for the minimum period length to include them in a PLM series remains 5 s, and the separation of less than 5 s to determine the bilaterality of two LMs (and counted as a single LM). Also the relationship with other events (arousals and respiratory events), as well as calibration, detection and recording criteria have been settled according to the recommendations and standards coming from a detailed statistical analysis of computer-ized sleep recordings in patients with RLS-PLMS, controls and differ-ent pathologies presdiffer-enting with PLMs [48–50]. The criteria for considering LMs associated with respiratory events and arousals have been established in detail. These events are not discarded from the motor activity report but, tabulated and scored apart, should be considered different from the other ‘‘true” PLMS.
The new AASM rules stem from a process of review and analysis of different studies and of the WASM/IRLSSG standards, in particu-lar, reaching a high level of agreement with these standards be-cause of the fact that the scoring of PLMS has evolved and moved towards more advanced procedures and because there is some le-vel of evidence for these new criteria and their reliability when used by experts[51].
Unfortunately, the new atlas rules miss some important charac-teristics of PLMs, such as PLMW which are sometimes as sensitive and specific as PLMS[51], the Suggested Immobilization Test (SIT), generally used in clinical trials of drugs for RLS-PLMS and thought to be a sensitive indicator of RLS severity[49,52], and the relation-ship with CAP[53]. Sleep recordings of patients affected by Rest-less Legs Syndrome, PLMS or other conditions generally contain a significant amount of non-periodic LMs[54–57], and LM activity during wakefulness in the same patients is essentially non-periodic
[57]. Perhaps, the new AASM rules should have better defined this type of activity and how to evaluate it, differentiating isolated LMs from those occurring with intervals shorter or longer than PLMs, as these types of activities seem to be represented in different amounts in different groups of patients. Adopting the entire WASM/IRLSSG standards[47]would probably be simpler and clearer.
The next step, not mentioned in this Atlas, should be a better system and software to automatically analyze the candidates for LMs and PLMs and to use a computerized scoring method that may provide reliability, variability and validity as good as visual analysis, with less effort and better accuracy[47].
Table 2
Major clinical applications of CAP.
Insomnia (increase of CAP rate and of all phase A subtypes; reduction of CAP
rate and of subtypes A1 and A2 following acute drug treatment)
Narcolepsy (reduction of CAP rate and of subtypes A1)
Periodic Limb Movements (increase of CAP rate and of subtypes A2 and A3;
motor events related to phase A)
Sleep Apnea Syndrome (marked increase of CAP rate and of subtypes A3,
reduction of subtypes A1, respiratory events related to phase B; decrease of CAP rate, reduction of subtypes A3 and partial recovery of subtypes A1 fol-lowing treatment with CPAP)
Upper airway resistance syndrome (increase of CAP rate and subtypes A1) Depression (increase of CAP rate and of subtypes A2)
Sleep Deprivation (increase of CAP rate during morning sleep recovery and
decrease of CAP rate during night sleep recovery)
Sleepwalking and night terrors (increase of CAP rate)
Bruxism (normal CAP rate with selective increase of subtypes A3) Prion diseases (Creutzfeldt-Jakob disease: increase of CAP rate)
Epilepsy (generally increase of CAP rate and interictal EEG events related to
phase A)
4.2. REM sleep Behavior Disorder
Among the other sleep-related movement disorders or para-somnias, the scoring rules for bruxism, RBD and RMD reached the ‘‘recommended” level. For RBD, it is the first time that rules are written in a formal consensus setting with semiquantitative definition, although they still reflect relatively old criteria, based on the visual analysis of polysomnography[58]. During REM sleep the 30-s epochs are considered for both tonic and phasic activities, while a division in 3-s mini-epochs is recommended for the phasic activity. Either or both the features must be present for the poly-somnographic scoring of RBD, together with the audio-video docu-mentation of motor episodes or the typical clinical history. Although the reliability of these criteria is still not completely ex-plored and validated[51], they are a step towards the use of poly-somnographic criteria in clinical practice to diagnose both clinical and instrumental RBD, starting from the seminal paper of Lapierre and Montplaisir in 1992[58]and applying the ICSD-2 indications
[59]. Thus, despite the crucial relevance of increased chin EMG activity for the diagnosis of RBD, the reliability of polysomnograph-ic criteria for REM without atonia is probably largely unexplored because of the use of paper recordings in sleep research for dec-ades. In these studies, no clear mathematical and quantitative def-initions, in terms of amplitude and duration, have been provided for the elements taken into account: atonia, phasic and tonic EMG activations. This is also reflected in the notes of the new scor-ing rules for RBD: it is difficult to quantitatively differentiate tran-sient normal muscle activities and twitching in small or large groups of muscles during REM sleep from the pathological twitch-ing and phasic activities of RBD without a cut-off threshold. More-over, the absolute amplitude of EMG activity is a very difficult quantity to pick up visually because of its continuous intraindivid-ual changes and its very large interindividintraindivid-ual variability; again, a computer-analysis with quantitative data of digital EMG recording is more than welcome for their real quantification, overcoming the semiquantitative data obtained by the currently used visual scoring.
Practically, a quantification of the whole sleep period (with the exclusion of very few artefact epochs) and the use of mini-epochs long enough to allow a reliable estimation of the background EMG mean amplitude and short enough to point out short-lasting phasic activities could be useful, as well as an index of atonia to better cat-egorize differences of muscle atonia during REM sleep in controls and RBD patients[60].
4.3. Bruxism
The scoring rules for bruxism have been established as mini-mum burst amplitude, duration and interval criteria to correctly classify most of bruxers and controls, as tested in a limited number of controlled studies[61]: a minimal number of three elevations of chin EMG burst activity in a regular sequence or sustained eleva-tion of chin EMG activity longer than 2 s (separated by a stable EMG activity of at least 3 s) constitutes the minimal criteria to de-fine an episode of bruxism. Also in this case, it is the first time that rules for bruxism scoring are written, based on a few studies inves-tigating reliability and sensitivity/specificity of these criteria. Among these studies we have to recognize the expertise and effort of the group led by Lavigne in Montreal, who quantified and deter-mined specific polysomnographic features to recognize sleep brux-ism, based not just on video or audio recordings, (considered no longer sufficient to diagnose sleep bruxism if used alone)[51].
With respect to previous criteria for scoring bruxism during sleep, the novelty of the Atlas resides in the possibility to score this movement disorder by combining audio recording with polysom-nography (at least two audible tooth grinding episodes per night).
The addition of masseter EMG recordings is left (as a note) at the discretion of examiners. This may be a limit, because when the clinical/anamnestic relevance is unknown, this type of recording allows the detection and recognition of subtle forms of bruxism, with persistence in different sleep stages, and permits the differen-tiation from other similar EMG activities, i.e., rhythmic masticatory muscle activity and oro-mandibular myoclonus[62].
4.4. Rhythmic Movement Disorder
The scoring criteria for RMD are very simple and clear because all the studies are case series or case reports with no controlled data. The rules do not clarify whether the movements have to be exclusively present during sleep, rather than during wakefulness or in sleep-wake transition, but in the notes is it specified that vi-deo-polysomnography is necessary for the diagnosis. This should be better defined, since RMD are often present both in wakefulness and sleep (NREM and REM) and also because the movements dur-ing wake periods may interfere with sleep duration and quality.
4.5. Hypnagogic Foot Tremor, Excessive Fragmentary Myoclonus, Alternating Leg Muscle Activation
For the scoring of the other movement-related phenomena dur-ing sleep, such as HFT, EFM and ALMA, criteria and rules are de-fined as ‘‘optional.” Perhaps, in order to go more deeply into the definition and detection of these motor events and understand bet-ter their clinical significance (if any), we should wait for more data to be produced. So far, we have no real evidence that these move-ment disorders are separate nosological and clinical entities. In particular, for HFT and ALMA, the few reports available are obser-vational or brief case series coming from a few sleep labs describ-ing them as possible variations of PLMs or RLS. Frequently in clinical practice it is possible to encounter patients with PLMs that have, during the recording night, periods of different periodic leg or foot movements, such as ALMA or HFT, but without specific clinical correlates and without particular anamnestic features.
5. Respiratory events
The AASM Manual for the Scoring of Sleep and Associated Events introduces a systematic approach and scoring criteria for evaluating different types of respiratory events[1]. Previous scor-ing criteria, in both the 1999[63]and 2005[64]versions, raised a number of clinical problems, mainly due to different methods used to measure or identify the ‘‘target reduction” in respiratory signals and the definition of hypopnea and respiratory-effort-re-lated arousals (RERA). In particular, two different definitions of hypopnea were proposed, one for the clinical setting and the other for research purposes. Worldwide, however, many other defini-tions of hypopnea have been presented in the scientific literature
[65].
The new AASM rules represent a significant step towards resolving these problems and enable a common background for analyzing clinical and research data. The manual dedicates much attention to the technical issues involved in obtaining valid, repro-ducible traces. Alternative methods to obtain valid respiratory sig-nals are extensively described, with the aim of minimizing technical problems that may limit the validity of single studies and improving the diagnostic approach in individual patients.
For the first time, the rules for event duration, for both apnea and hypopnea, are precisely described, with specific recommenda-tions on the beginning and end of events, so that the intra-scorer, inter-scorer, and night-to-night reliability should be improved.
5.1. Definition of hypopnea
As in the 2005 AASM practice parameters, two definitions of hypopnea are still proposed [64]. It is not clearly specified why one definition should be used rather than the other. Data reported in many studies, extensively reviewed by the AASM Task Force on Respiratory Scoring, showed that the inter-scorer, intra-scorer and night-to-night reliability is good when hypopnea is defined by any oxyhemoglobin desaturation. The reliability is, however, lower when using arousals in the event definition or when events are scored without regard to associated desaturation or when more subtle flow limitation is used for event identification[65].
As reported by the Task Force, using discernible amplitude cri-teria with a criterion of 3% desaturation provides an estimate of AHI equivalent to that obtained using a 50% amplitude reduction, irrespective of desaturation, but it is more reliable and reproduc-ible. However, the level of associated desaturation used in the rec-ommended definition of hypopnea isP4%, so fewer episodes of
hypopnea will be recorded. The decision to use 4% rather than 3% was essentially based on cross-sectional data obtained in the Sleep Heart Health Study, showing that AHI (hypopneas defined as dis-cernible reductions in amplitude plus aP4% desaturation) is
sig-nificantly associated with blood pressure, cardiovascular disease, sleepiness and impaired quality of life [65]. Recently, Punjabi et al., analyzing data from the Sleep Heart Health Study to deter-mine the definition of hypopneas that would be best correlated with cardiovascular disease, found that hypopneas with a 4% or more decrease in oxyhemoglobin saturation are associated with prevalent cardiovascular disease[66]. But from a clinical point of view, differences in odds ratios for prevalent cardiovascular dis-eases derived for using the hypopnea index with P4% orP3%
desaturation thresholds were minimal.
On the other hand, the clinical setting is quite different from the epidemiological point of view. The above mentioned studies showed an increasing risk of cardiovascular diseases in subjects with very low AHI values, independently of daytime symptoms re-lated to sleep-disordered breathing. In routine practice, we usually evaluate patients who are being investigated to explain the signs and symptoms they experience. Thus, a more sensitive definition of hypopnea is needed since most of the events may not be prop-erly classified until the related desaturation is below 4%. The de-gree of desaturation is, however, dependent on many factors, such as the duration of apnea, lung volumes, deranged lung mechanics, presence of sub-clinical pulmonary hypertension, pre-existing lung diseases, age, obesity, control of breathing, etc., which suggest the use of such a lower cut-off in the clinical setting. On the other hand, repetitive episodes of hypopnea, even in the absence of relevant desaturations, may determine other morbidities such as cognitive dysfunction, insomnia, depression and others, as stated in the ‘‘Diagnostic and Coding Manual of the International Classifi-cation of Sleep Disorders”[59].
Cross-sectional epidemiological data and follow-up studies have shown that OSA is a strong risk factor for death and cardio-vascular diseases in younger patients[67–69]. CPAP-treated pa-tients who were compliant with treatment had mortality rates similar to those recorded in the general population[70], whereas an excess of fatal cardiovascular events occurred in patients aged 50 years who refused CPAP treatment[70,71]. In fact, the major determinant of long-term outcome was compliance with CPAP treatment rather than severity of OSAS at diagnosis[72]. As a con-sequence, much effort should be given to diagnosing this condition early and treating it well.
One could argue that an episode of obstructive hypopnea with a desaturation <4% could easily be named as a RERA (respiratory-ef-fort-related arousal), but the scoring of RERA is an option and not a recommendation; furthermore, RERA cannot be scored using type
2 portable monitors. The identification of respiratory events in practice, however, may be more complicated. Indeed, Cracowski et al.[73]performed a study in a population of patients with mod-erate to severe OSAS with the aim of characterizing the proportion of different types of obstructive non-apneic events (hypopneas and RERAs) using the AASM 1999 criteria[63]. They found that 61.8% of these events were hypopneas (>30% reduction of baseline flow) with associated cortical arousal, but desaturation was <3%; 14.8% did not precisely fulfill the criteria for both hypopneas and RERAs. As a consequence, the new criteria for hypopnea may lower the sensitivity of the diagnostic test or may result in an underestima-tion of AHI. Indeed, Ruehland et al. recently demonstrated that using different standard hypopnea (including the new AASM stan-dards) leads to marked differences in AHI in patients who under-went polysomnographic evaluation for clinical suspect of OSA[74]. The longitudinal data on progression of sleep apnea over time, albeit scarce, have shown that sleep disordered breathing worsens progressively[75,76]. Consequently, the question is: Is it better to quickly identify patients with nocturnal or daytime complaints and a hypopnea with 3% desaturation, or to wait a long time, until the OSA worsens and the degree of sleep hypoxia is greater?
The problem of sensitive and specific criteria for identifying hypopnea, mainly obstructive, has not been adequately assessed, especially when portable monitors are used instead of standard polysomnography. As stated by the AASM, portable monitors should only be used for the diagnosis of OSA in adulthood in pa-tients with a high pre-test probability of moderate to severe OSA. But very recently, while analyzing differences in clinical features between patients with upper airway resistance syndrome, pri-mary snoring and OSA (hypopnea defined using the so-called ‘‘Chicago Criteria”), Stoohs et al. found that the patients with upper airway resistance syndrome had the greatest impairment of daily functioning and worst perception of sleep quality [77]. In other words, patients with upper airway resistance syndrome do not differ clinically from patients with OSAS. As a conse-quence, using portable monitors without EEG channels leads to a high risk of performing in-lab polysomnography for patients with upper airway resistance syndrome and ‘‘detectable hypopne-as,” but with desaturation <4%.
Finally, two different diagnostic aspects must be resolved in the clinical setting: first, the diagnosis must be made, and, secondly, the severity of the disease must be determined. This second aspect is relevant since the therapeutic algorithm is based on quite a dif-ferent definition of events from that currently proposed.
5.2. Identification of respiratory events during O2-therapy
Polysomnography is usually performed in a clinical setting in patients suffering from diseases other than OSA that may require O2-therapy (patients with respiratory or thoracic diseases, patients with chronic heart failure, etc.). No rules are available for the iden-tification of hypopnea in these patients.
5.3. Identification of respiratory events during CPAP therapy
No mention is made in the manual or in the recent guidelines for CPAP titration of the criteria to identify hypopnea during CPAP therapy, either during titration or in follow-up studies[78]. It is known that desaturations may not be present or may be lower dur-ing sub-optimal CPAP therapy so that some events could go unrec-ognized[79].
6. Final considerations
According to Thomas Kuhn, ‘‘Normal science does not aim at novelties of fact or theory” [80]. This striking view challenges
the popular picture of the constant critical scrutiny of accepted scientific belief. Kuhn’s response to the fact that normal science does not aim at novelty is that scientists are entrenched within a certain way of seeing things, and this clouds their vision. In other words, they tend to see what they expect to see. The impression that emerges reading the new AASM criteria for anal-ysis of stages and arousals is a sleep field locked in a defensive tactic that rejects a number of alternative approaches, especially those that fail to yield the desired result. This is typical of normal science that strives to protect the dominating paradigms and brings theory and fact into close agreement, even in the absence of clear evidence[80]. In particular, without statistical validation using an appropriate amount of PSG recordings, it will be difficult to ascertain the superior advantage of the new visual scoring rules for sleep stages compared to the R&K framework. Prelimin-ary comparisons have been recently carried out[12], while AASM is organizing several training initiatives to improve acquaintance with the new manual. Unfortunately, the methodological imprint-ing widely consolidated in the sleep labs all over the world inter-feres with an easy, smooth shift towards the updated rules. While it will be relatively fast to overlap slow wave sleep and stage N3, complications will certainly derive from the classification of any epoch of NREM sleep, including an EEG arousal as N1. The issue becomes even more laborious when we are asked to establish whether arousal (with or without an associated K-complex) oc-curs during the first half of the epoch or the last half of the pre-vious one. Paradoxically, it would have been preferable to modify the framework radically (e.g., eliminating the fixed 30-s epochs), allowing a completely renewed visual or automatic scoring ap-proach. At the moment, only young sleep fellows with no previ-ous experience in PSG investigation will be able to apply the new rules without excessive problems. Unfortunately, several years will pass before they become a robust army when perhaps the current criteria will be extensively amended.
Credit must be given to the rapid planning and accomplishment of the new scoring rules. While outside the U.S. the interval be-tween programs and facts can last decades, the AASM steering committee and the single sessions were able to carry out a huge amount of work in a relatively short period. Exclusion of non-US scientific boards and societies certainly allowed AASM to acceler-ate the final outcome; however, a discussion of the agreed criteria with other international organizations (e.g., World Association of Sleep Medicine, European Sleep Research Society, etc.) should be encouraged. Two articles by Schulz recently published in the Jour-nal of Clinical Sleep Medicine[4,81]are in tune with this opening
spirit as well as the present contribution by the Italian Association of Sleep Medicine. We are glad to verify that the new AASM rules have incorporated a number of suggestions raised by the Italian school on the neurophysiological function of EEG arousals in the structural organization of sleep. This issue has been so deeply ac-cepted that arousals are now scored as stage markers of N1. The AASM rules for limb movements reflect the WASM/IRLSSG stan-dards for recording and scoring periodic leg movements in sleep and wakefulness as well[47]. Unfortunately, a similar inclusive drive was not extended to CAP, although a process for revision has been created to incorporate this technique in future updates. Among the weaknesses, a more sensitive definition of hypopnea is needed as well as a more accurate definition of RERA. It has been clearly demonstrated that respiratory events in patients with OSA can be terminated, even in the absence of an ASDA arousal, by EEG patterns which meet the criteria of phase A1 subtypes of CAP[43]. This should leave the door open to the recovery of CAP as an inte-grative and non-antagonistic methodology. The same potential can be extended to delta sleep analysis. In other words, without any distortion of the basic AASM rules, CAP and slow wave activity will certainly be exploited by sleep specialists due to their clinical added value and also because their quantification is independent from the rigid staging procedure based on 30-s epochs.
The Italian school, which has played a topical role in the history of PSG also thanks the pioneering studies of Elio Lugaresi and co-workers[82,83], is grateful to the AASM members who contributed to the achievement of the new rules for sleep. Every effort that tries to push forward a scientific discipline is welcome and we believe this was the spirit that animated the project participants. But weaknesses and imperfections are endowed in all human activi-ties. We wish to be a friendly voice that respects the challenge but warmly addresses topical issues that need to be discussed and clarified without any cultural or geographical prejudice. The common final aim is to overcome obsolete paradigms and identify the smartest way to exploit all the information available in a stan-dard PSG recording.Table 3summarizes our main issues of dis-agreement for discussion.
Disclaimer
The comments and opinions in this article reflect the views of the authors of the article and the Italian Association of Sleep Med-icine and in no way indicate an endorsement by the editorial board ofSleep Medicineor any other organization. The final judgement rests with the readers of the journal.
Table 3
Main issues for discussion.
Sleep staging and arousals
1. No validation of the new scoring rules for sleep stages 2. Static approach to sleep dynamics by means of 30-s epochs 3. Limited comparability between R&K and AASM scored records 4. Excessive flattening of SWA determined by the merging process of N3
5. Puzzling role of arousals regarding sleep staging in relation to their occurrence within the 30-s epoch 6. Neglect of alternative non-conflicting methods of sleep analysis reflecting sleep instability
Movement events
1. Missing rules on PLMs (periodic limb movements) during wakefulness, on the Suggested Immobilization Test and on the relationship with sleep microstructure such as CAP (cyclic alternating pattern)
2. No differentiation between isolated LMs (limb movements) and those occurring with intervals shorter or longer than PLMs 3. No indications provided for automatic analysis of candidates for LMs and PLMs
4. Lacking index of atonia to better categorize differences of muscle atonia during REM sleep controls and RBD (REM Behaviour Disorder) patients.
Respiratory events
1. Conflict between sensitive and specific criteria for identifying hypopnea, mainly obstructive
2. No mention of the criteria to identify hypopnea during CPAP therapy, either during titration or in follow-up studies
References
[1] Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007.
[2] American Academy of Sleep Medicine. Review articles for the AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. J Clin Sleep Med 2007;3:99–246.
[3] Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington: Washington Public Health Service, US Government Printing Office; 1968. [4] Schulz H. Rethinking sleep analysis. J Clin Sleep Med 2008;4:99–103. [5] Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger M,
Hirshkowitz M, et al. The visual scoring of sleep in adults. J Clin Sleep Med 2007;3:121–31.
[6] Danker-Hopfe H, Anderer P, Zeithofer J, Boeck M, Dorn H, Gruber G, et al. Interrater for sleep scoring according to the Rechtschaffen and Kales and the new AASM standard. J Sleep Res 2009;18:74–84.
[7] Bonnet MH, Doghramji K, Roehrs T, Stepanski EJ, Sheldon SH, Walters AS, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med 2007;3:133–45.
[8] Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum Press; 1990.
[9] Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci 2000;23:793–842. [10] Massaquoi SG, McCarley RW. Extension of the limit cycle reciprocal interaction model of REM cycle control. An integrated sleep control model. J Sleep Res 1992;1:138–43.
[11] Koella WP. A modern neurobiological concept of vigilance. Experientia 1982;38:1426–37.
[12] Moser D, Anderer P, Gruber G, Parapatics S, Loretz E, Boeck M, et al. Sleep classification according to AASM and Rechtschaffen & Kales: effects on sleep scoring parameters. Sleep 2009:139–49.
[13] Terzano MG, Parrino L, Smerieri A, Chervin R, Chokroverty S, Guilleminault C, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med 2001;2:537–53.
[14] Kemp B. A proposal for computer-based sleep/wake analysis. Consensus report. J Sleep Res 1993;2:179–85.
[15] Haustein W, Pilcher J, Klink J, Schulz H. Automatic analysis overcomes limitations of sleep stage scoring. Electroencephalogr Clin Neurophysiol 1986;64:364–74.
[16] Pardey J, Roberts S, Tarassenko L, Stradling J. A new approach to the analysis of the human sleep/wakefulness continuum. J Sleep Res 1996;5:201–10. [17] Agarwal R, Gotman J. Digital tools in polysomnography. J Clin Neurophysiol
2002;19:136–43.
[18] Amir N, Gath I. Segmentation of EEG during sleep using time-varying autoregressive modeling. Biol Cybern 1989;61:447–55.
[19] Kaplan A, Röschke J, Darkhovsky B, Fell J. Macrostructural EEG characterization based on nonparametric change point segmentation: application to sleep analysis. J Neurosci Methods 2001;106:81–90.
[20] Gath I, Bar-On E. Computerized method for scoring of polygraphic sleep recordings. Comput Prog Biomed 1980;11:217–23.
[21] Krajcˇa V, Petránek S, Patáková I, Värri A. Automatic identification of significant graphoelements in multichannel EEG recordings by adaptive segmentation and fuzzy clustering. Int J Biomed Comput 1991;28:71–89.
[22] Krajcˇa V, Petránek S, Paul K, Matousek M, Mohylova J, Lhotska L. Automatic detection of sleep stages in neonatal EEG using the structural time profiles. IEEE Eng Med Biol Soc 2005;6:6014–6.
[23] Borbely AA. Two process model of sleep regulation. Hum Neurobiol 1982;1:195–204.
[24] American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15: 173–184.
[25] Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep 1998;21:351–7.
[26] Terzano MG, Parrino L, Rosa A, Palomba V, Smerieri A. CAP and arousals in the structural development of sleep: an integrative perspective. Sleep Med 2002;3:221–9.
[27] Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med 2007;3:271–4.
[28] De Carli F, Nobili L, Beelke M, Watanabe T, Smerieri A, Parrino L, et al. Quantitative analysis of sleep EEG microstructure in the time–frequency domain. Brain Res Bull 2004;63:399–405.
[29] Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. J Sleep Res 2004;13:1–23.
[30] Terzano MG, Parrino L, Boselli M, Smerieri A, Spaggiari MC. CAP components and EEG synchronization in the first three sleep cycles. Clin Neurophysiol 2000;111:283–90.
[31] Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev 2000;4:101–23.
[32] Terzano MG, Parrino L, Smerieri A, De Carli F, Nobili L, Donadio S, et al. CAP and arousals are involved in the homeostatic and ultradian sleep processes. J Sleep Res 2005;14:359–68.
[33] Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 2005;28: 1151–61.
[34] Drinnan MJ, Murray A, Griffiths CJ, Gibson GJ. Interobserver variability in recognizing arousal in respiratory sleep disorders. Am J Respir Crit Care Med 1998;158:358–62.
[35] Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. PNAS 2004;101:5053–7.
[36] Terzano MG, Mancia D, Salati MR, Costani G, Decembrino A, Parrino L. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep 1985;8:137–45.
[37] Terzano MG, Parrino L, Spaggiari MC. The cyclic alternating pattern sequences in the dynamic organization of sleep. Electroencephalogr Clin Neurophysiol 1988;69:437–47.
[38] Parrino L, Smerieri A, Rossi M, Terzano MG. Relationship of slow and rapid EEG components of CAP to ASDA arousals in normal sleep. Sleep 2001;24:881–5. [39] Ferri R, Bruni O, Miano S, Smerieri A, Spruyt K, Terzano MG. Inter-rater
reliability of sleep cyclic alternating pattern (CAP) scoring and validation of a new computer-assisted CAP scoring method. Clin Neurophysiol 2005;116:696–707.
[40] Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol 2000;111:1611–9.
[41] Ferri R, Parrino L, Smerieri A, Terzano MG, Elia M, Musumeci SA, et al. Cyclic alternating pattern and spectral analysis of heart rate variabilità during normal sleep. J Sleep Res 2000;9:13–8.
[42] Ferini-Strambi L, Bianchi A, Zucconi M, Oldani A, Castronovo V, Smirne S. The impact of cyclic alternating pattern on heart rate variabilità during sleep in healthy young adults. Clin Neurophysiol 2000;111:99–101.
[43] Thomas RJ. Arousals in sleep-disordered breathing: patterns and implications. Sleep 2003;26:1042–7.
[44] Lanfranchi PA, Pennestri M-H, Fradette L, Dumont M, Morin CM, Montplaisir J. Night time blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep 2009;32:760–6.
[45] American Sleep Disorders Association. Recording and scoring leg movements. The Atlas Task Force. Sleep 1993;16:748–759.
[46] Coleman, RM, Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor, Sleeping and waking disorders: indications and techniques. Menlo Park: Addison-Wesley;1982. p. 265–295. [47] Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official
World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med 2006;7:175–83.
[48] Garcia-Borreguero D, Larrosa O, de la Llave Y, Granizo JJ, Allen R. Correlation between rating scales and sleep laboratory measurements in restless legs syndrome. Sleep Med 2004;5:561–5.
[49] Allen RP, Dean T, Earley CJ. Effects of rest-duration, time-of-day and their interaction on periodic leg movements while awake in restless legs syndrome. Sleep Med 2005;6:429–34.
[50] Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep 2006;29:759–69.
[51] Walters AS, Lavigne G, Hening W, Picchietti DL, Allen RP, Chokroverty S, et al. The scoring of movements in sleep. J Clin Sleep Med 2007;3:155–67. [52] Aksu M, Demirci S, Bara-Jimenez W. Correlation between putative indicators
of primary restless legs syndrome severity. Sleep Med 2007;8:84–9. [53] Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The
cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol 1996;13:314–23. [54] Manconi M, Ferri R, Zucconi M, Oldani A, Fantini ML, Castronovo V, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med 2007;8:491–7.
[55] Ferri R, Zucconi M. Heart rate and spectral EEG changes accompanying periodic and isolated leg movements during sleep. Sleep 2008;31:16–7. [56] Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate
and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol 2007;118:438–48.
[57] Pennestrì M-H, Whittom S, Benoit A, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: Prevalence and interval distribution. Sleep 2006;29:1183–7.
[58] Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology 1992;42:1371–4. [59] American Academy of Sleep medicine. The international classification of sleep
disorders. 2nd ed. Westchester: American Academy of Sleep Medicine; 2005. [60] Ferri R, Manconi M, Plazzi G, Bruni O, Vandi S, Montagna P, et al. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J Sleep Res 2008;17:89–100.
[61] Lavigne GJ, Rompre PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res 1996;75:546–52.
[63] American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–68.
[64] Kushida CA, Littner MR, Morgentaler T, et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep 2005;28:499–521.
[65] Redline S, Budhiraja R, Kapur V, et al. Reliability and validity of respiratory event measurement and scoring. J Clin Sleep Med 2007;3:169–200. [66] Punjabi NM, Newman AB, Young TB, et al. Sleep-disordered breathing and
cardiovascular disease. Am J Respir Crit Care Med 2008;177:1150–5. [67] Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea
syndrome: declining mortality rates with age. Eur Respir J 2005;25: 514–20.
[68] Marin JN, Carrizo SJ, Vicente E, Agust AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–53.
[69] Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 2007;176:1274–80.
[70] Veale D, Chailleux E, Hoorelbeke-Ramon A, et al. Mortality of sleep apnoea patients treated by nasal continuous positive airway pressure registered in the ANTADIR observatory. Association Nationale pour le Traitement A Domicile de l’Insuffisance Respiratoire chronique. Eur Respir J 2000;15:326–31. [71] Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/hypopnoea
syndrome patients: impact of treatment. Eur Respir J 2002;20:1511–8. [72] Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in
obstructive sleep apnea–hypopnea patients treated with positive airway pressure. Chest 2005;128:624–33.
[73] Cracowski C, Pepin JL, Wuyam B, Levy P. Characterization of obstructive non apneic respiratory events in moderate sleep apnea syndrome. Am J Respir Crit Care Med 2001;164:944–8.
[74] Ruehkand WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thomton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009;32:150–7.
[75] Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–39. [76] Redline S, Larkin E, Schluchter M, et al. Incidence of sleep disordered breathing
(SDB) in a population-based sample. Sleep 2001;24:511.
[77] Stoohs RA, Knaack L, Blum HC, et al. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/ hypopnea syndrome. Sleep Med 2008;9:121–8.
[78] Kushida CA, Chediak A, Berry RB, et al. Positive Airway pressure titration task force of the American academy of sleep medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 2008;4:157–71.
[79] Montserrat JM, Ballester E, Olivi H, et al. Time-course of stepwise CPAP titration. Behavior of respiratory and neurological variables. Am J Respir Crit Care Med 1995;152:1854–9.
[80] Kuhn T. The structure of scientific revolutions. 2nd ed. The University of Chicago Press; 1970. p. 1–210.
[81] Schulz H. Phasic or transient? Comment on the terminology of the AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2007;3:752.
[82] Lugaresi E, Coccagna G, Mantovani M, Lebrun R. Some periodic phenomena arising during drowsiness and sleep in man. Electroecphalograph clin Neurophysiol 1972;32:701–5.