Summary Isoprene emissions were studied in one-year old sweetgum (Liquidambar styraciflua L.) seedlings during nine drying--rewatering cycles extending over five months. Each drying cycle lasted to the point of leaf wilting. Growth was essentially stopped in response to the first drying cycle, though seedling survival and capacity to recover turgor on rewatering remained high throughout the entire nine cycles. Photosyn-thetic rates of leaves were inhibited by the drying treatments. Under severe drought, isoprene emission rates of leaves were also inhibited, though isoprene emission was generally less sensitive to drought than photosynthesis. The lower drought sensitivity of isoprene emission compared with photosynthesis resulted in a higher percentage of fixed carbon lost as isoprene as seedlings became more stressed. During the recovery phase of the drying--rewatering cycles, isoprene emission rates in several seedlings were higher than in well-watered control seedlings. Following the ninth drying--rewatering cycle, sus-tained daily watering resulted in recovery of isoprene emission rates to control values within four days. Photosynthetic rates only recovered to 50% of control values after seven days. We conclude that the mechanisms regulating photosynthetic rate and isoprene emission rate are differentially influenced by limited water supplies. The results are consistent with past studies that predict a protective role for isoprene emission during stress, particularly protection from excessive leaf tem-peratures during drought.
Keywords: drought, isoprene synthase, survival, thermopro-tection.
Introduction
Sweetgum (Liquidambar styraciflua L.) is a widely distributed hardwood species in the southeastern United States where water often limits tree growth (Bormann 1953). It has been reported that this species emits both isoprene (2-methyl 1,3-butadiene) and monoterpenes (Rasmussen 1972, Evans et al. 1982), two hydrocarbons that influence the oxidative capacity and chemistry of the continental troposphere (Zimmerman et
al. 1978). In rural and native forested areas, isoprene contrib-utes to the formation of blue haze (Went 1960) and organic acid deposition (Jacob and Wofsy 1988). In urban areas, the oxidation of isoprene in the presence of NOx can lead to the formation of tropospheric ozone, a toxic pollutant and green-house gas (Trainer et al. 1987, Chameides et al. 1988).
The advantage, if any, of isoprene emission to plant function is unclear. Isoprene is synthesized from acetyl-CoA through the mevalonic acid pathway in plants (Sharkey et al. 1991). Controls over isoprene emission include (1) an apparent link-age to photosynthetic processes (Sanadze and Kursanov 1966, Jones and Rasmussen 1975, Tingey et al. 1981, Monson and Fall 1989, Loreto and Sharkey 1990, Delwiche and Sharkey 1993), (2) developmental and seasonal controls by the enzyme isoprene synthase (Monson et al. 1992, Kuzma and Fall 1993, Harley et al. 1994, Monson et al. 1994), and (3) possible control by leaf carbon balance (e.g., the amount of carbon substrate available for isoprene biosynthesis) (Harley et al. 1994, Monson et al. 1994). Recent studies by Sharkey and Loreto (1993) and Sharkey and Singsass (1995) have sug-gested that isoprene emission serves a protective role for plants under stress. According to this hypothesis, isoprene is a mem-brane-soluble compound capable of quenching photooxidative products of photosynthesis or protecting membranes from detrimental phase changes at high temperature, or both.
Tingey et al. (1981) found that isoprene emission from live oak (Quercus virginiana Mill.) was not inhibited during short-term drought (four days), despite substantial reductions in photosynthetic rate. Similarly, withholding water from kudzu (Pueraria lobata (Willd.) Ohwi) leaves for six days caused large declines in photosynthetic rate, whereas isoprene emis-sion rate was only slightly reduced (Sharkey and Loreto 1993). We have extended these studies by using a different tree spe-cies and focusing on long-term drought applied over several drying and wetting cycles to determine the relative re-sponses of isoprene emission rate and photosynthetic rate in a more ecological context. We also assessed (1) the importance of forest isoprene emissions to local atmospheric chemistry during seasonal droughts, and (2) whether isoprene emission
Isoprene emission, photosynthesis, and growth in sweetgum
(
Liquidambar styraciflua
) seedlings exposed to short- and long-term
drying cycles
CHENGWEI FANG,
1RUSSELL K. MONSON
2and ELLIS B. COWLING
11
Department of Forestry, North Carolina State University, Raleigh, NC 27695, USA 2
Department of Environmental, Population and Organismic Biology, University of Colorado, Boulder, CO 80309, USA
Received January 5, 1995
patterns during extended droughts are consistent with its pro-posed role as a thermoprotective agent.
Materials and methods
Plant material and experimental design
One-year-old, dormant sweetgum (Liquidambar styraci-flua L.) seedlings were obtained from the North Carolina For-est Service Nursery at Morgenton, NC, in January 1994. Forty-eight seedlings were transplanted to 15-liter plastic pots (20 cm wide × 60 cm tall) containing a 2/2/2/1 (v/v) mix of sand, peat, vermiculite, and perlite. Plants were grown in a greenhouse at the University of Colorado, Boulder. Average midday photosynthetic photon flux density (PPFD) was 1000--1200 µmol m−2 s−1 (400--700 nm) and relative humidity was maintained between 30 and 60%. Average midday temperature was 26 °C (average daytime maximum and nighttime mini-mum temperatures were 32 and 17 °C, respectively. Nighttime minimum temperature was maintained above 15 °C.
Two experiments were conducted. In Experiment 1, pre-viously non-stressed plants were subjected to nine successive drying--rewatering cycles beginning on March 28 and ending on August 4. Photosynthetic and isoprene emission rates were measured at 2- to 3-day intervals during Cycles 4 and 7. Throughout the experiment, control plants were watered at 2-to 3-day intervals depending on weather conditions. In Experi-ment 2, which was conducted during the final 3 weeks of Experiment 1, previously non-stressed plants were subjected to three successive drying--rewatering cycles in the middle of summer, beginning on July 11. The control plants in Experi-ment 2 were watered at 2- to 3-day intervals depending on weather conditions. In Experiment 2, both stressed and control seedlings were measured at 2- to 3-day intervals throughout each of the three cycles. In both experiments, the length of each drying cycle was determined as the time for stressed plants to reach a point where at least half the leaves exhibited obvious wilting. This time ranged from 6 to 22 days, depending on both the greenhouse environment and the number of drying cycles that plants had previously experienced. All seedlings were fertilized with solid, slow-release fertilizer (N/P/K=14,14,14) on May 23 and July 17.
Growth measurements
Shoot elongation (new growth), basal diameter, and number of leaves per seedling were monitored at monthly intervals during the nine cycles of Experiment 1 and every 2 weeks during the three cycles of Experiment 2. Newly emergent leaves were labeled with hanging tags when growth measurements were made in order to follow the age of individual leaves. At the end of the experiment, seedlings were harvested, oven dried at 60 to 70 °C to constant weight, and the biomass of different plant components determined. The biomass of leaves that had died during the experiments, as well as the leaves removed after photosynthesis and isoprene emission measurements, were added to the harvested biomass to determine the total biomass of each seedling.
Photosynthetic gas exchange and isoprene emission measurements
We used the gas exchange and isoprene measurement system described by Monson and Fall (1989) and Hills et al. (1992), with some minor modifications. Gas chromatography was conducted with a Hewlett-Packard gas chromatograph (Model 5890 Series II, Hewlett-Packard) equipped with a flame ioni-zation detector, and modified for vacuum inlet collection and cryogenic enrichment of isoprene samples as described by Hills et al. (1992). Modifications to the gas collection proce-dure were: (1) after each injection of sample gas from the leaf chamber, a stream of pressurized helium was used to back flush the sample loop to minimize contamination between measurements, (2) a temperature program from −25 to 25 °C was used to separate isoprene, and (3) between sample injec-tions, a known isoprene standard (mixed with helium) was used to derive the peak area of a fixed sample volume. Under these conditions, the isoprene retention time was 5.6 min. There was little detectable isoprene accumulation in the Teflon tubing, sampling, or injection systems (< 0.5%) following an 8-h series of measurements.
All photosynthesis and isoprene emission measurements were made at a leaf temperature of 30 ± 0.3 °C and an incident PPFD of 1000 ± 50 µmol m−2 s−1. Each measurement of isoprene emission rate and photosynthetic rate was made on one leaf per seedling, though the same seedlings were moni-tored on successive days during a particular drying cycle. After each cycle, seedlings that had been subjected to measurement were discarded, and a new set of seedlings that had been treated in parallel were used for the subsequent cycles. All measured leaves were mature, fully expanded, sun-developed leaves on the same position of the crown. In the plants exposed to drought treatments, new leaf emergence was slowed consid-erably, beginning with the first drying cycle. Thus, to obtain similar leaf ages for control and stressed seedlings, we con-ducted measurements on progressively older leaves of both control and stressed seedlings as the drying--rewatering cycles progressed.
Results
Effects of soil drying on growth
Experi-ment 1 (Figure 1). Although basal diameter growth was less sensitive to soil drying than new leaf production and shoot elongation, it was significantly reduced by the drying cycles (Figure 1).
Leaf predawn xylem water potential, which was monitored during the fourth drying cycle of Experiment 1, was, on aver-age, 0.5 MPa lower in the stressed seedlings than in the control seedlings. Most of the decline in water potential occurred during the first two days of the drying cycle (data not shown).
Effects of soil drying on photosynthetic rate and isoprene emission rate
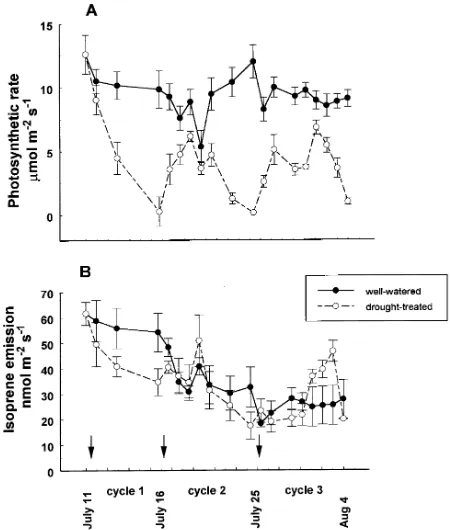
In both Experiments 1 and 2, drought strongly decreased net photosynthetic rate (Figures 2 and 3). Drying to the point of wilting in the fourth and sixth drying cycles in Experiment 1, decreased photosynthetic rates almost to zero (Figure 2). Sto-matal conductance generally decreased in parallel with photo-synthetic rate during all drying cycles (data not shown). In Experiment 2, photosynthetic rate of stressed seedlings de-creased from 13 µmol m−2 s−1 to almost 0 during the first drying cycle (Figure 3). After rewatering, photosynthetic rate recovered to 40% of the pre-stressed value, before declining again in response to the second drying cycle. Photosynthetic rates of control seedlings were relatively constant throughout Experiment 2.
In Experiment 1, isoprene emission rate for stressed seed-lings was not significantly depressed during the fourth cycle, but was slightly depressed during the sixth cycle, compared to that for control seedlings (Figure 2). There was some evidence of a stimulation in isoprene emission rate shortly after rewater-ing durrewater-ing the fourth cycle, but not durrewater-ing the sixth cycle. In Experiment 2, isoprene emission rates declined over time for both control and droughted seedlings, though by the end of each cycle, isoprene emission rate was lowest for the droughted seedlings (Figure 3). In Cycle 2 of Experiment 2, isoprene emission from stressed seedlings recovered after re-watering, exceeding the rate of control seedlings in the middle of the cycle, and then decreasing toward the end of the cycle. After rewatering at the end of Cycle 2, the isoprene emission rate of the previously stressed seedlings increased to a rate 2-fold higher than that of control seedlings, and then decreased to a rate less than that of control seedlings. The amount of carbon lost as isoprene increased from 2% of the CO2 assimi-lation rate in control seedlings to 40--60% of the CO2 assimi-lation rate during the drought treatments.
At the end of the ninth drying cycle in Experiment 1,
Table 1. The partitioning of dry biomass and leaf area of sweetgum seedlings harvested at the end of nine drying cycles in Experiment 1.
Treatment Leaf biomass Stem biomass Root biomass Total biomass Shoot/root Leaf area
(g) (g) (g) (g) biomass (m2 seedling−1)
Well-watered (n = 10) 42.6 ± 2.31 78.0 ± 4.6 70.3 ± 2.9 190.9 ± 9.1 1.72 0.85 ± 0.02 Drought (Expt. 1, n = 10) 10.2 ± 0.4 17.0 ± 0.5 23.4 ± 0.6 51.1 ± 1.0 1.14 0.21 ± 0.01 Drought (Expt. 2, n = 10) 29.8 ± 1.8 46.7 ± 2.8 45.4 ± 3.9 121.9 ± 7.6 1.69 0.66 ± 0.03
1 Differences among all treatments were significant (P < 0.01). All values are means ± SE; there were no significant differences for any biomass
or leaf area measurements prior to establishing the drought or control treatments.
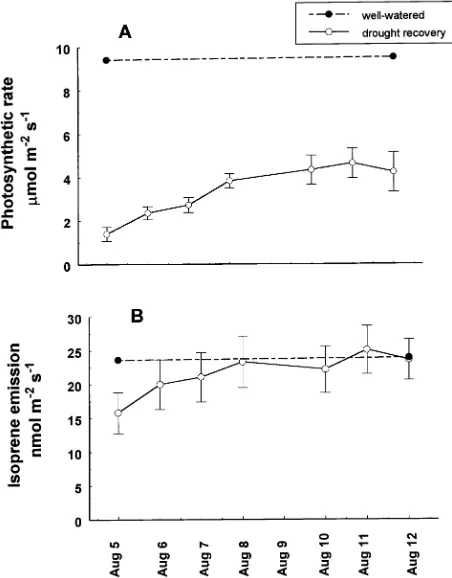
stressed seedlings were watered on a daily basis to monitor recovery of the photosynthetic rate and isoprene emission rate (Figure 4). After seven days of recovery, photosynthetic rate had only recovered to 50% of that of the controls, whereas
isoprene emission rate had fully recovered within four days.
Discussion
Sweetgum seedlings exhibited a high capacity for tolerating chronic drought. After nine drying cycles, each progressing to the point of wilting, survival of sweetgum seedlings remained high (95%) and plants continued to regain turgor on rewater-ing. Although drought tolerance and the capacity to recover from drought remained high, growth essentially ceased during the first drying cycle and did not resume for the rest of the drying--rewatering cycles (Figure 1). This tendency to cease growth may contribute to sweetgum’s capacity to reduce its water use and tolerate extended dry periods. Pezeshki and Chambers (1986) observed that sweetgum seedlings exhibited ‘‘effective and rapid stomatal closure resulting in avoidance of leaf desiccation’’ during exposures to artificially induced dry-ing cycles. Sweetgum is known to inhabit a variety of dry sites in the southeastern United States, and is often exposed to severe droughts during the growing season (Bormann 1953, Dixon et al. 1965).
Isoprene emission rate was substantially less sensitive to drought than photosynthetic rate (Figures 2 and 3). Similar findings have been obtained from studies of the effects of short-term acute drying on photosynthesis and isoprene emis-sion (Tingey et al. 1982, Sharkey and Loreto 1993). Our results extend these findings to plants exposed to numerous drought cycles lasting over several months.
Several studies have provided evidence for metabolic links between photosynthesis and isoprene biosynthesis (Ras-mussen and Jones 1972, Monson and Fall 1989, Loreto and Sharkey 1990 1993). However, other studies have provided evidence that these two processes respond independently to
Figure 2. Mean rates of net photo-synthesis and isoprene emission of sweetgum as a function of date during the 4th and 6th dry-ing cycles of Experiment 1. Error bars represent one standard error of the mean (n = 4).
changes in several environmental conditions and developmen-tal states (Grinspoon et al. 1991, Monson et al. 1992, Kuzma and Fall 1993, Sharkey and Loreto 1993, Harley et al. 1994, Monson et al. 1994). Our findings support the latter view. The cause of the differential responses of photosynthetic rate and isoprene emission rate to drought was not established. The two processes could differ in their responses to stomatal closure. Photosynthetic responses to drought in L. styraciflua are domi-nated by stomatal limitations (Pezeshki and Chambers 1986), whereas isoprene emission rate is independent of stomatal dynamics (Fall and Monson 1992). Alternatively, unlike pho-tosynthesis, isoprene biosynthesis may be unaffected by de-clining plant water potential.
The maintenance of high rates of isoprene biosynthesis during drought may reflect a protective role for this process as has been suggested by Sharkey and coworkers (Sharkey and Loreto 1993, Sharkey and Singsass 1995). Leaf temperatures are often excessively high during drought when transpirational cooling is reduced. Sharkey and Singsass (1995) have recently shown that isoprene can protect membrane-bound photosyn-thetic reactions from high-temperature stress. The observation that isoprene emission rates remain high, even during extended drought, indicates that the capacity for isoprene biosynthesis is retained during those times when leaf temperatures are likely to reach their maximum.
The persistence of isoprene emissions during prolonged
droughts could have important implications for the contribu-tion of biogenic hydrocarbon emissions to urban tropospheric O3 formation (Monson et al. 1995). Chameides et al. (1988) showed that biogenic isoprene emissions contribute up to 40% of the elevated O3 concentrations in urban Atlanta. The time of greatest likelihood for prolonged drought in the southeastern United States is during the hot, late summer months. This is when climatic conditions favor the formation of stagnant air masses that accumulate photochemically generated O3. Iso-prene emissions during this time would create a substantial hydrocarbon stock available for reaction with hydroxyl radical in the presence of NOx, contributing to high O3 episodes.
Acknowledgments
This research was supported by Southern Oxidant Study, a collabora-tive university, government, and private industry study to improve scientific understanding of the accumulation and effects of photo-chemical oxidants. Financial and in-kind support for SOS research is provided by the Environmental Protection Agency, National Oceanic and Atmospheric Administration, Department of Energy, Tennessee Valley Authority, Electric Power Research Institute, The Southern Company, Coordinating Research Council, Duke Power Company, and the States of Alabama, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Texas. Although the research described in this article has been funded wholly or in part by the Environment Protection Agency under Agreement No. CR817766 to North Carolina State University, it has not been sub-jected to the Agency’s peer and administrative review, and therefore may not necessarily reflect the views of the Agency and no official endorsement should be inferred. The authors thank Dr. Theodore K. Raab, University of Colorado, for suggestions on the manuscript. Thanks are also due to Marcy Litvak, Erica Kelly, and Tracy Lynn for assistance with the gas chromatography measurements.
References
Bormann, F.H. 1953. Factors determining the role of loblolly pine and sweetgum in early old-field succession in the Piedmont of North Carolina. Ecol. Monogr. 23:339--358
Chameides, W.L., R.W. Lindsay, J. Richardson and C.S. Kiang. 1988. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 241:1473--1475
Delwiche, C.F. and T.D. Sharkey. 1993. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 16:587--591
Dixon, R.E., J.F. Hosner and N.W. Hosley. 1965. The effects of four water regimes upon the growth of four bottomland tree species. For. Sci. 11: 299--305
Evans, R.C., D.T. Tingey, M.L. Gumpertz and W.F. Burns. 1982. Estimates of isoprene and monoterpene emission rates in plants. Bot. Gaz. 143:304--310
Fall, R. and R.K. Monson. 1992. Response of isoprene emission to changes in stomatal conductance as influenced by abscisic acid. Plant Physiol. 100:987--992
Grinspoon, J., W.D. Bowman and R. Fall. 1991. Delayed onset of isoprene emission in developing velvet bean (Mucuna sp.) leaves. Plant Physiol. 97:170--174
Harley, P.C., M.E. Litvak, T.D. Sharkey and R.K. Monson. 1994. Isoprene emission from velvet bean leaves. Interaction between nitrogen availability, growth photon flux density and leaf develop-ment. Plant Physiol. 105:279--285
Hills, A.J., R. Fall and R.K. Monson. 1992. Methods for the analysis of isoprene emission from leaves. In Modern Methods of Plant Analysis, New Series. Eds. H.F. Linskens and J.F. Jackson. Vol. 13, Plant Toxin Analysis, pp 297--315
Jacob, D. and S. Wofsy. 1988. Photochemistry of biogenic emissions over the Amazon forest. J. Geophys. Res. 93:1477--1486. Jones, C.A. and R.A. Rasmussen. 1975. Production of isoprene by leaf
tissue. Plant Physiol. 55:982--987.
Kuzma, J. and R. Fall. 1993. Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol. 101:435--440.
Loreto, F. and T.D. Sharkey. 1990. A gas-exchange study of photosyn-thesis and isoprene emission in Quercus rubra L. Planta 182:523--531.
Loreto, F. and T.D. Sharkey. 1993. On the relationship between iso-prene emission and photosynthesis metabolites under different en-vironmental conditions. Planta 189:420--424.
Monson, R.K. and R. Fall. 1989. Isoprene emission from aspen leaves. The influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 90:267--274.
Monson, R.K., C.H. Jaeger, W.W. Adams, E. Driggers, G. Silver and R. Fall. 1992. The relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol. 98:1175--1180.
Monson, R.K., P.C. Harley, M.E. Litvak, M. Wildermuth, A.B. Guen-ther, P.R. Zimmerman and R. Fall. 1994. Environmental and devel-opmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 99:260--270.
Monson, R.K., M.T. Lerdau, T.D. Sharkey, D.S. Schimel and R. Fall. 1995. Biological aspects of constructing biogenic hydrocarbon emission inventories. Atmosph. Environ. 29:2987--3002.
Pezeshki, S.R. and J.L. Chambers. 1986. Stomatal and photosynthetic response of drought-stressed cherrybark oak (Quercus falcata var.
pagodaefolia) and sweetgum (Liquidambar styraciflua). Can. J. For. Res. 16:841--846.
Rasmussen, R.A. 1972. What do the hydrocarbons from trees contrib-ute to air pollution. J Air Pollut. Control Assoc. 22:537--543. Rassmussen, R.A. and C.A. Jones. 1972. Emission of isoprene from
leaf discs of Hamamelis. Phytochemistry 12:15--19.
Sanadze, G.A. and A.L. Kursanov. 1966. On certain conditions of the evolution of the diene C5H8 from poplar leaves. Sov. Plant Physiol. 13:184--189.
Sharkey, T.D. and F. Loreto. 1993. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 95:328--333.
Sharkey, T.D., F. Loreto and C. F. Delwiche. 1991. The biochemistry of isoprene emission from leaves during photosynthesis. In Trace Gas Emission from Plants. Eds. T.D. Sharkey, E.A. Holland and H.A. Mooney. Academic Press, San Diego, pp 153--184.
Sharkey, T.D. and E. Singsass. 1995. Why plants emit isoprene. Nature 374:769.
Tingey, D.T., R. Evans and M. Gumpertz. 1981. Effects of environ-mental conditions on isoprene emission from live oak. Planta 152:565--570.
Trainer, M., E.J. Williams, D.D. Parrish, M.P. Buhr, E.J. Allwine, H.H. Westberg, F.C. Fehsenfeld and S.C. Liu. 1987. Models and obser-vations of the impact of natural hydrocarbons on rural ozone. Nature 329:705--707.
Went, F.W. 1960. Blue haze in the atmosphere. Nature 187:641--643 Zimmerman, P.R., R.B. Chatfield, J. Fishman, P.J. Crutzen and P.L.