Summary Vesicular-arbuscular mycorrhizal (M) fungal colonization, growth, and nonstructural carbohydrate status of sour orange (Citrus aurantium L.) seedlings were compared at low- and high-phosphorus (P) supply following inoculation with four Glomus isolates: G. intraradices (Gi, FL208), G. etunicatum (Ge, UT316), G. claroideum (Gc, SC186), and Glomus sp. (G329, FL906). Nonmycorrhizal (NM) seedlings served as controls. At low-P supply, increases in incidence of M colonization, vesicles and accumulation of fungal fatty acid 16:1ω5C in roots were most rapid for G329-inoculated
seed-lings, followed closely by Gi- and Gc-inoculated seedlings. Glomus etunicatum was a less aggressive colonizer and pro-duced lower rates of fungal fatty acid accumulation in seedling roots than the other Glomus species. Nonmycorrhizal and Ge-inoculated seedlings had lower P status and growth rates than seedlings inoculated with Gi or G329. Glomus claroideum increased seedling P status, but growth rate was lower than for seedlings colonized by Gi or G329, suggesting higher below-ground costs for Gc colonization. In P-sufficient roots colo-nized by Gi, Gc, or G329, starch and ketone sugar concentrations were lower than in P-deficient NM and Ge-in-oculated plants.
Under conditions of high-P supply where mycorrhizae pro-vided no P benefit to the seedlings, colonization by Gc, Gi, and G329 was delayed and reduced compared to that at low-P supply; however, the relative colonization rates among Glomus spp. were similar. Colonization by Ge was not detected in roots until 64 days after inoculation. Compared to NM seedlings, growth rates of mycorrhizal seedlings were reduced by the three aggressive fungi but not by the less aggressive Ge. After 64 days, starch and ketone sugar concentrations were lower in fibrous roots colonized by Gc, Gi, and G329 than in NM roots, indicating greater utilization of nonstructural carbohydrates in roots colonized by the aggressive fungi. After 49 days, coloni-zation by the aggressive fungi increased root biomass alloca-tion which may have contributed to the lower growth rate of mycorrhizal seedlings compared to NM seedlings. Thus, Glomus spp. that were aggressive colonizers of roots at low-P supply were also aggressive colonizers at high-P supply, re-sulting in greater belowground C costs and growth depression compared with the less aggressive colonizer, Ge.
Keywords: aggressiveness of mycorrhizal fungi, colonization rate, growth depression, phosphorus status, root carbon allo-cation, root carbohydrate status.
Introduction
In soils with a low phosphorus (P) content, the ability of vesicular-arbuscular mycorrhizal (M) fungi to increase plant growth is explained by increased P acquisition. Generally, the higher the rate of root colonization by M fungi, the greater the growth response as a result of more rapid establishment of external hyphae in the soil and greater P uptake from beyond the P-depletion zones around the root (Abbott and Robson 1977, Sanders et al. 1977). The efficiency of P uptake by M hyphae is strongly affected by the spatial distribution of hy-phae and by M fungal-specific differences in uptake capacity per unit length of hypha (Jakobsen et al. 1992a, 1992b). Al-though growth responses of citrus and subterranean clover appear to be related to the amount of hyphae or the rate of hyphal spread from the roots (Graham et al. 1982, Jakobsen et al. 1992a), often no clear relationship exists between the amount of hyphae and the growth responses of M-colonized plants (Abbott and Robson 1985).
At low-P supply, Glomus intraradices isolate FL208 aggres-sively colonized roots of sour orange and increased P uptake per unit root length over threefold compared with nonmycor-rhizal (NM) roots, resulting in a greatly increased plant growth rate (Eissenstat et al. 1993). However, M colonization also enhanced belowground C partitioning, soil C, and root/soil respiration compared with NM plants of similar P status. Because there was no compensatory increase in shoot C as-similation, the relative growth rate (RGR) of plants inoculated with G. intraradices FL208 was slightly reduced compared with that of NM plants of equivalent P status, consistent with the increase in belowground C expenditure.
High-P supply reduced colonization of Volkamer lemon by G. intraradices FL208, but there was still considerable fungal colonization of roots, as revealed by the detection of the fatty acid 16:1ω5C in fungal vesicles in roots 52 days after
inocula-tion (Peng et al. 1993). This colonizainocula-tion neither increased P status nor C assimilation of Volkamer lemon. At 52 days after transplanting, colonization at high-P supply reduced seedling RGR by 9 to 12% as a result of a substantial effect of G. intraradices on root allocation (19% higher root dry weight and 10% higher daily root growth rate). This response partly accounted for an estimated 37% higher root/soil respiration in M seedlings than in NM seedlings. Root construction and respiration estimates identified the building of intraradical vesicles (lipid-rich roots) as 10% of the cost, with 51% of the
Carbon economy of sour orange in response to different
Glomus
spp.
J. H. GRAHAM, D. L. DROUILLARD and N. C. HODGE
Citrus Research and Education Center, University of Florida-IFAS, 700 Experiment Station Road, Lake Alfred, FL 33850-2299, USA
Received October 25, 1995
increase due to greater M root biomass allocation. The remain-ing 39% was presumably required for maintenance of the fungus in the root, and growth and maintenance of hyphae in soil. Even for G. intraradices FL208, which produces a pre-ponderance of intraradical vesicles and chlamydospores, only a small proportion of the respiratory costs associated with M colonization was directly attributable to building lipid-rich roots (Peng et al. 1993).
We predicted that other Glomus species that aggressively colonize citrus roots at low-P supply are capable of stimulating belowground allocation at high-P supply but provide no P benefit or means of C gain aboveground (Graham et al. 1991, Graham and Eissenstat 1994). Here, we define aggressiveness in terms of rate of root colonization because this characteristic is a useful predictor of growth response at low-P supply (Ab-bott and Robson 1985). We evaluated the rate of M coloniza-tion and growth, and the nonstructural carbohydrate status of roots to measure responses of sour orange (Citrus auran-tium L.) at low- and high-P supply to four Glomus species, including G. intraradices FL208 and three Glomus species intensively studied by Sylvia et al. (1993). When the responses of a grass (sorghum) and legume (soybean) host to these three M fungi were evaluated in ten soils in the eastern United States, two of the Glomus isolates were effective and the other was ineffective in stimulating plant growth under a wide range of edaphic conditions. In sorghum, there was a strong relation-ship between colonized root length and growth response, whereas this was not the case in soybean. Thus, the mecha-nisms underlying fungal effectiveness at low-P supply and host responses to these fungi at high-P supply require further study. We hypothesized that Glomus isolates that are aggressive colo-nizers of sorghum and soybean would colonize citrus more rapidly and be more beneficial in terms of P acquisition than less aggressive colonizers. However, at high-P supply, more aggressive colonizers would produce greater belowground C allocation, utilization and growth depression than those pro-duced by less aggressive colonizers.
Materials and methods
Plant inoculation and growth conditions
Sour orange (Citrus aurantium) seeds were germinated in a commercial 1/1/1 (v/v) mix of peat/perlite/bark with starter nutrients and grown in 150 cm3 plastic containers for 3 months (two to four true-leaf stage). The medium was thoroughly washed from roots, and seedlings were transplanted to 150 cm3 of autoclaved (121 °C, 1.1 kg cm−2) Candler fine sand soil
(pH 6.8) with 3.8 mg kg−1 of available P as determined by double-acid extraction (Mehlich 1953). For M treatments, 0.5 g of air-dried Sudan grass (Sorghum sudanense (Staph.) Piper) roots containing from 170 to 350 propagules (deter-mined by most-probable number analysis) of Glomus in-traradices FL208 (Gi), G. etunicatum UT316 (Ge), G. claroideum SC186 (Gc), or Glomus sp. FL906 (G329) were placed below each seedling at transplanting. Soil in the NM treatment received a 5-ml extract of each inoculum passed through a 45-µm sieve to establish similar root microflora in
association with the NM plants. The M and NM plants were grown from May 11 to July 14, 1994 in a shaded greenhouse having a maximum photon flux density of 1000 µmol m−2 s−1.
Average day/night temperatures were 33/25 °C and relative humidity was 60 to 100%. Seedlings were watered to excess every other day with Hoagland’s solution (Hoagland and Ar-non 1965) containing 0.25 mM (low-P) or 2.0 mM (high-P) P. At 21, 36, 49, and 64 days after transplanting, seedlings from each treatment were harvested. There were five M treatments, two P regimes, four harvests, and five replicate seedlings per treatment per harvest. Seedlings were located in racks in the greenhouse in a split-plot design for fertilizer treatments and relocated on the bench every 3 weeks until harvest. An experi-ment of the same design, with three different harvest dates, was conducted in 1993 with similar results. The results of the 1994 experiment are presented because the 1993 experiment had only three harvests.
Growth analysis and colonization assessment
At each harvest, roots were gently washed free of soil with tap water. Roots (fibrous roots < 2 mm diameter and tap roots > 2 mm), stems, and leaves were dried at 70 °C for 24 h. Rates of plant growth (g day−1) were calculated over the four harvests by regression analysis. Biomass allocation to roots was as-sessed using the ratio of roots (fibrous and tap) to total biomass. At the final harvest (64 days), roots had completely explored the soil in the 150 cm3 containers but were not constricted in the bottoms of the containers.
Total incidence of intraradical (IR) M colonization was estimated as the percentage of 10 1-cm fibrous root segments per plant with vesicles, arbuscules, and hyphae present (Gra-ham et al. 1991), and as the percentage incidence of vesicles only in the same 10 root segments. Fibrous root content of the fungal fatty acid 16:1ω5C, which is not found in host tissue
(Graham et al. 1995), was determined with a Hewlett-Packard 5890 gas-liquid chromatograph after methanol extraction of minced, dried roots (to pass through a 2.0-mm screen), fatty acid saponification, and derivatization to methyl esters. Con-centrations of 16:1ω5C were expressed relative to the highest
value based on the integrated areas of the total fatty acid profile (Peng et al. 1993).
Nonstructural carbohydrate and P analysis
Dried fibrous roots and leaves were ground to pass through a 40-mesh screen (2.0 mm opening size). Root tissue was sus-pended in 15 ml of water, boiled for 2 min, and centrifuged at 800 g for 2 min as previously described (Eissenstat and Dun-can 1992). The supernatant was analyzed for ketone sugars (including sucrose, fructose, and fructans) using resorcinol reagent (Roe et al. 1949). Soluble and insoluble starch were measured using amyloglucosidase after correcting for free glucose (Haissig and Dickson 1979). Glucose was used as the standard for analyses of starch and ketone sugars.
Data analysis
Rates of M colonization and plant growth were subjected to the General Linear Model (GLM) procedure (SAS Institute, Cary, NC) for analysis of variance with time as a repeated measure. Linear contrasts were made in the univariate mode. Nonlinear responses of tissue P and fibrous root carbohydrates and root allocation in the early phase of seedling growth and M coloni-zation were expected (Eissenstat et al. 1993). Thus, data were subjected to ANOVA at individual harvest times when treat-ment effects were most discernable (usually 64 days). Com-parisons among treatments were made with Duncan’s multiple range test.
Results
Mycorrhizal colonization responses
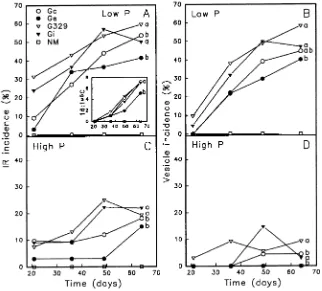
At low-P supply, rates of intraradical (IR) colonization of sour orange by the four Glomus spp. varied significantly (Fig-ure 1A). The rate was highest for G329, intermediate for Gi and Gc, and significantly lower for Ge by 64 days after inocu-lation. Similar trends among Glomus spp. occurred for rates of increase in incidence of IR vesicles (Figure 1B). Differences in rates of colonization between G329, Gi and Gc and the less aggressive Ge were corroborated by accumulation of fungal 16:1ω5C fatty acid in plant roots (Figure 1A inset). This fatty
acid represents about 40 to 60% of the total fatty acid content in the chlamydospores of these fungal species (Graham et al. 1995).
By all measures, colonization by Ge was similar to that of the other three fungi up to Day 35, after which the rate of IR colonization by Ge slowed, whereas the other three fungi continued to colonize at similar rates. However, colonization by Ge continued to convert from hyphae and arbuscules (IR) to vesicles at almost the same rate as the other three fungi, as indicated by the increase in vesicle incidence (Figure 1B) and the accumulation of 16:1ω5C in the roots (Figure 1A inset).
Increased P supply delayed and reduced colonization by all Glomus species, but the relative colonization rates among the Glomus species were similar to those at low-P supply: G329 = Gi > Gc > Ge (Figures 1A and 1C). Colonization by Ge was not detected in roots until Day 64 and Ge failed to form IR vesicles (Figures 1C and 1D). Vesicle incidence in roots colo-nized by G329, Gi, and Gc was low and variable (Figure 1D). At high-P supply, the fungal fatty acid 16:1ω5C was not
de-tected in roots inoculated with any of the fungi in the absence of substantial development of lipid-rich vesicles in the roots.
Phosphorus and plant growth responses
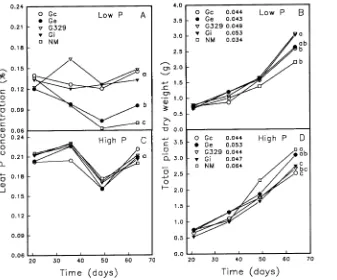
At low-P supply, leaf P concentrations in NM plants and Ge-inoculated plants declined to below P sufficiency (< 0.10%) within 35 days after transplanting, whereas P concen-trations in G329-, Gc-, and Gi-inoculated plants remained above 0.12%, which is within the P-sufficiency range (Fig-ure 2A). Plants colonized by G329 and Gi had significantly higher growth rates than P-deficient NM plants (Figure 2B), whereas growth rates of Gc and Ge-inoculated plants were not significantly greater than those of NM plants.
Figure 1. Mycorrhizal colonization of sour orange roots by four Glomus
spp. as measured by intraradical (IR) incidence (arbuscules, vesicles, and hyphae), vesicle incidence and accu-mulation in fibrous roots of 16:1ω5C
fungal fatty acid expressed in relative units (Panel A inset). Seedlings were grown at low-P supply (Panels A and B) or high-P supply (Panels C and D). Gc = G. claroideum SC186, Ge =
G. etunicatum UT316, G329 =
At high-P supply, leaf P in all plants varied with leaf flushing activity (data not shown), but concentrations were well within the range of P sufficiency (Figure 2C). Nonmycorrhizal seed-lings had the highest growth rate, followed by seedseed-lings inocu-lated with Ge (Figure 2D). Colonization by G329, Gi, and Gc significantly reduced growth rates of sour orange seedlings compared to NM seedlings.
Carbohydrate and root allocation responses
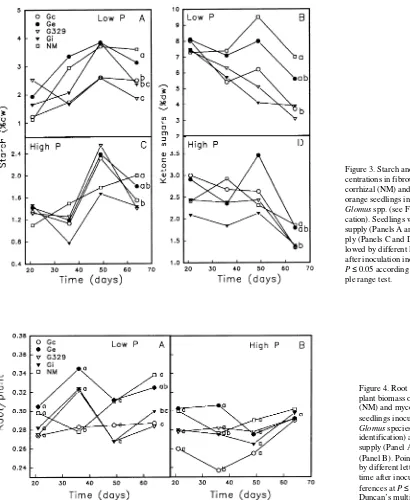
Starch concentrations in M fibrous roots generally reached a peak at 49 days after inoculation and then declined (Figures 3A and 3C), whereas starch concentrations of NM roots either remained constant (low-P) or increased (high-P) after Day 49. Ketone sugars in NM roots increased up to Day 49 (low-P) or Day 36 (high-P) and then declined (Figures 3B and 3D). In roots colonized by G329, Gi and Gc, ketone sugars steadily declined in low-P roots, but remained constant in high-P roots for the first 49 days after inoculation and then declined after the increased colonization rate between Days 36 and 49 (Fig-ure 1D). Although starch and ketone sugar concentrations fluctuated in Ge-colonized roots, concentrations of nonstruc-tural carbohydrates were similar to those in NM roots after Day 64, whereas concentrations of starch and ketone sugars in roots colonized by the other three Glomus spp. were lower than in NM roots (Figures 3A--D).
At low-P supply, C allocation to root growth, as measured by the root biomass/plant biomass ratio, steadily increased over time as the NM plants became P deficient (Figure 4A). In contrast, the overall effect of inoculation with G329, Gi or Gc, all of which enhanced seedling acquisition of P soon after
colonization began, was to maintain a constant root biomass/plant biomass ratio of about 0.29 ± 0.2 (SD) during the course of biomass gain. The less aggressive Ge, which colonized but failed to enhance seedling acquisition of P, stimulated C allocation to roots but did not sustain plant P sufficiency. Hence, the root biomass/plant biomass ratio was higher for Ge-colonized plants than for the other M treatments and the ratio converged with that of NM plants by Day 64.
At high-P supply, the root biomass/plant biomass ratio of P-sufficient NM seedlings did not show a net change over the 64-day study, whereas the ratio increased in G329-, Gi- and Gc-colonized seedlings. In contrast to the three aggressive fungi, the root biomass/plant biomass ratio of Ge-colonized seedlings decreased slightly over time.
Discussion
We predicted that, at low-P supply, Glomus spp. would be aggressive colonizers of sour orange roots and would enhance P acquisition and growth, whereas at high-P supply the fungi would not only be aggressive colonizers but would also result in greater belowground C costs and growth depression than in plants inoculated with less aggressive colonizers.
At low-P supply, two of the four Glomus spp., G329 and Gi, enhanced seedling acquisition of P, resulting in significant increases in growth rate compared to NM seedlings. In contrast to Ge’s high effectiveness on herbaceous hosts (Sylvia et al. 1993), Ge did not promote P acquisition and growth of sour orange seedlings. This Glomus species was the least aggressive colonizer, confirming that colonization rate is a useful
predic-Figure 2. Leaf P concentration and plant growth rate (g day−1) of nonmy-corrhizal (NM) and mynonmy-corrhizal sour orange seedlings inoculated with four
tor of fungal effectiveness under P-deficient conditions (Ab-bott and Robson 1977, Sanders et al. 1977). Among the Glomus spp. studied, Gc was intermediate in aggressiveness; colonization by Gc increased P acquisition of the seedlings but did not produce a seedling growth response greater than the relatively poor colonizer Ge. This may indicate greater carbon expenditure on colonization by Gc than by the other Glomus spp. including Gi, which has been previously identified as capable of increasing belowground allocation and reducing growth rate of sour orange (Eissenstat et al. 1993). Pearson and Jakobsen (1993) observed a greater belowground carbon
par-titioning and a lower mycorrhizal efficiency (calculated as 14C use per unit 32P transported) for Scutellospora calospora than for two Glomus spp., suggesting that C--P exchange varies considerably among M fungi.
The less aggressive Ge differed from the other Glomus spp. in its effect on C allocation to roots at low-P supply. In the absence of increased seedling acquisition of P during early colonization by Ge, the biomass of the root fraction increased compared to that of the M-colonized plants in which enhanced P uptake promoted C allocation to shoot growth. Similarly, Eissenstat et al. (1993) concluded that, in P-deficient NM Figure 3. Starch and ketone sugar con-centrations in fibrous roots of nonmy-corrhizal (NM) and mynonmy-corrhizal sour orange seedlings inoculated with four
Glomus spp. (see Figure 1 for identifi-cation). Seedlings were grown at low-P supply (Panels A and B) or high-P sup-ply (Panels C and D). Points (n = 5) fol-lowed by different letters at 64 days after inoculation indicate differences at
P ≤ 0.05 according to Duncan’s multi-ple range test.
Figure 4. Root biomass per total plant biomass of nonmycorrhizal (NM) and mycorrhizal sour orange seedlings inoculated with four
plants, C allocation for root growth increased with time, thereby increasing P acquisition.
At low-P supply, NM and Ge-colonized plants had higher concentrations of starch and ketone sugars than plants colo-nized by the other Glomus spp., possibly as a result of greater C allocation to P-deficient root tissues. Alternatively, because M fungi are obligate biotrophs that derive all of their C from the host (Harley and Smith 1983), they probably readily utilize available pools of sugars transported to roots, such as ketone sugars and sucrose (Patrick 1989). Greater utilization of non-structural carbohydrates in roots colonized by aggressive fungi is likely a result of higher belowground costs associated with additional construction costs and maintenance respiration of roots and intra- and extraradical fungal structures (Peng et al. 1993).
Although the confounding effects of P nutrition on C pro-duction and allocation are minimized under conditions of high-P supply (Eissenstat et al. 1993), this condition delays and reduces the frequency of colonization in sour orange (Menge et al. 1978). We found that the aggressive fungi pro-duced appreciable colonization under high-P supply, whereas the less aggressive Ge did not. At high-P supply, concentra-tions of starch and ketone sugars in roots of P-sufficient NM plants increased and decreased, respectively. In contrast, pools of nonstructural carbohydrates fluctuated in roots of Glomus -colonized plants, presumably in response to M-induced changes in C allocation to roots with time. After 64 days, pools of starch and ketone sugars in roots colonized by the three aggressive Glomus spp. declined compared with pools in NM roots, suggesting greater utilization of nonstructural carbohy-drates by these fungi. The less aggressive Ge had the least effect on these pools after 64 days.
The aggressive Glomus spp., G329, Gi, and Gc, reduced growth rate of sour orange, whereas the less aggressive fungus Ge had little impact on growth. At high-P supply, the reduction in growth rate by the aggressive fungi was indicative of high belowground costs of colonization, as previously demon-strated for Gi in Volkamer lemon seedlings (Peng et al. 1993). Because Gi is highly vesicular and directly converts from intraradical vesicles to chlamydospores in the root, the high belowground costs of colonization incurred by this species may not be a general phenomenon of Glomus spp. However, both G329 and Gc, which are capable of aggressive coloniza-tion at low-P supply, colonized roots at high-P supply and reduced seedling growth and affected C allocation to roots and C utilization in the same way as did Gi. In contrast, coloniza-tion by the less aggressive Ge at high-P was insufficient to affect C costs and growth rate of the sour orange seedlings.
Of the four Glomus species examined, G329 was the most effective growth promoter at low-P supply and the most effec-tive growth inhibitor at high-P supply. Sylvia et al. (1993) reported similar findings of the growth promotory effects of this fungus based on studies with sorghum and soybean in low-P soils. Reduction of woody plant growth by M fungal inoculation at high-P supply has been reported for sweetgum (Kiernan et al. 1983) and apple (Miller et al. 1985). In both cases, several species of Glomus were screened and those
isolates that colonized aggressively and increased growth most at low-P supply were the fungi that colonized most extensively at high-P supply. Similarly, isolates of G. etunicatum and G. claroideum were the most aggressive colonizers of sweet-gum in the high fertility treatment and both species produced growth depression. Another species, G. fasciculatum, colo-nized at a moderate to low rate at both high and low fertility and produced a smaller reduction in growth of sweetgum at high fertility than the aggressive colonizers.
The results for sweetgum and citrus indicate that, for woody tree species, there is a relationship between aggressiveness of Glomus spp. at low- and high-P supply and the potential for growth depression at high-P. Although fungi with an aggres-sive colonization phenotype commonly occur in M fungal populations in the field (Abbott and Robson 1991, Brundrett 1991), the functional significance of their effects on plant growth in the field remains to be determined (Abbott and Gazey 1994). Tobacco stunt disease is associated with the occurrence of G. macrocarpum in heavily fertilized Kentucky field soils (Modjo and Hendrix 1986). Citrus soils in Florida commonly contain G. intraradices and other intraradically sporulating Glomus spp. (Graham and Fardelmann 1986). De-spite high-P fertility (160 µg g−1 soil), these M fungi colonize
citrus roots of mature trees to a high level of incidence (60 to 80%) (Graham et al. 1991). We have evidence (Graham and Eissenstat, unpublished observations) that benomyl treatment controls M colonization and depresses P acquisition of young citrus trees grown with high-P supply under orchard condi-tions, indicating a fungitoxic effect of benomyl (Fitter and Nichols 1988). No other soil-borne fungi pathogenic to citrus roots are present in this field site. Control of the M fungal colonization apparently relieves young citrus trees of below-ground expenditure of C for use in abovebelow-ground processes and, as a result, the growth rate of young Valencia orange trees on four different rootstocks treated with benomyl increased by approximately 10%.
We conclude that if aggressive fungi are present in popula-tions of indigenous M fungi, they have the potential to colonize at high-P supply and affect crop productivity. A strategy for management of crops under high-P fertility conditions would be to lower soil P availability to concentrations at which M fungi function as mutualists rather than as parasites. For exam-ple, the Glomus spp. G329, Gc and Ge promoted growth of sorghum and soybeans in six soils with less than 10 µg ex-tractable P kg−1 soil, but not in four soils with greater than 20 µg kg−1 (Sylvia et al. 1993). This strategy assumes that symbiotically effective fungi exist within each diverse field population. Unfortunately, the relevance of species (or isolate) diversity to the functioning of mycorrhizae in the field is not yet known (Abbott and Gazey 1994).
Acknowledgments
References
Abbott, L.K. and C. Gazey. 1994. An ecological view of the formation of VA mycorrhizas. In Management of Mycorrhizas in Agriculture, Horticulture and Forestry. Eds. A.D. Robson, L.K. Abbott and N. Malajczuk. Kluwer Academic Publishers, Dordrecht, pp 66--78. Abbott, L.K. and A.D. Robson. 1977. Growth stimulation of
subterra-nean clover with vesicular-arbuscular mycorrhizas. Aust. J. Agric. Res. 28:639--649.
Abbott, L.K. and A.D. Robson. 1985. Formation of external hyphae in soil by four species of vesicular-arbuscular mycorrhizal fungi. New Phytol. 99:245--255.
Abbott, L.K. and A.D. Robson. 1991. Factors influencing the occur-rence of vesicular-arbuscular mycorrhizal fungi. J. Agric. Ecosyst. Environ. 35:121--150.
Brundrett, M. 1991. Mycorrhizas in natural ecosystems. Adv. Ecol. Res. 21:171--313.
Eissenstat, D.M. and L.W. Duncan. 1992. Root growth and carbohy-drate responses in bearing citrus trees following partial canopy removal. Tree Physiol. 10:245--257.
Eissenstat, D.M., J.H. Graham, J.P. Syvertsen and D.L. Drouillard. 1993. Carbon economy of sour orange in relation to mycorrhizal colonization and phosphorus status. Ann. Bot. 71:1--10.
Fitter, A.H. and R. Nichols. 1988. The use of benomyl to control infection by vesicular-arbuscular mycorrhizal fungi. New Phytol. 110:201--206.
Graham, J.H. and D.M. Eissenstat. 1994. Host genotype and the formation and function of VA mycorrhizae. Plant Soil. 159:179--185.
Graham, J.H., D.M. Eissenstat and D.L. Drouillard. 1991. On the relationship between a plant’s mycorrhizal dependency and rate of vesicular-arbuscular mycorrhizal colonization. Funct. Ecol. 5:773--779.
Graham, J.H. and D. Fardelmann. 1986. Inoculation of citrus with root fragments containing chlamydospores of the mycorrhizal fungus
Glomus intraradices. Can. J. Bot. 64:1739--1744.
Graham, J.H., N.C. Hodge and J.B. Morton. 1995. Fatty acid methyl ester profiles for characterization of Glomalean fungi and their endomycorrhizae. Appl. Environ. Microbiol. 61:58--64.
Graham, J.H., R.G. Linderman, and J.A. Menge. 1982. Development of external hyphae by different isolates of mycorrhizal Glomus spp. in relation to root colonization and growth of Troyer citrange. New Phytol. 91:183--189.
Haissig, B.E. and R.E. Dickson. 1979. Starch measurement in plant tissue using enzymatic hydrolysis. Physiol. Plant. 47:151--157. Harley, J.L. and S.E. Smith. 1983. Mycorrhizal symbiosis. Academic
Press, London, 483 p.
Hoagland, D.R. and revised by D.I. Arnon. 1965. The water-culture method for growing plants without soil. University of California, Agricultural Experiment Station Circular 347, Berkeley, CA. Jakobsen, I., L.K. Abbott and A.D. Robson. 1992a. External hyphae
of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 120:371--380.
Jakobsen, I., L.K. Abbott and A.D. Robson. 1992b. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 2. Hyphal transport of 32P over defined distances. New Phytol. 120:509--516.
Kiernan, J.M., J.W. Hendrix and D.M. Maronek. 1983. Fertilizer-in-duced pathogenicity of mycorrhizal fungi to sweetgum seedlings. Soil Biol. Biochem. 15:257--262.
Mehlich, A. 1953. Determination of P, Ca, Mg, K, Na, NH4 by the
North Carolina Soil Testing Laboratory. North Carolina State Uni-versity, Raleigh.
Menge, J.A., D. Steirle, D.J. Bagyaraj, E.L.V. Johnson and R.T. Leonard. 1978. Phosphorus concentration in plant responsible for inhibition of mycorrhizal infection. New Phytol. 80:575--578. Miller, D.D., D.A. Domoto and C. Walker. 1985. Colonization and
efficacy of different endomycorrhizal fungi with apple seedlings at two phosphorus levels. New Phytol. 100:393--402.
Modjo, H.S. and J.W. Hendrix. 1986. The mycorrhizal fungus,
Glomus macrocarpum as a cause of tobacco stunt disease. Phy-topathology 76:668--691.
Patrick, J.W. 1989. Solute efflux from the host at plant--microorganism interfaces. Aust. J. Plant Physiol. 16:53--57.
Pearson, J.N. and I. Jakobsen. 1993. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhi-zal fungi. New Phytol. 124:481--488.
Peng, S., D.M. Eissenstat, J.H. Graham, K. Williams and N.C. Hodge. 1993. Growth depression in mycorrhizal citrus at high phosphorus supply: Analysis of carbon costs. Plant Physiol. 101:1063--1071. Roe, J.H., J.H. Epstein and N.P. Goldstein. 1949. A photometric
method for the determination of insulin in plasma and urine. J. Biol. Chem. 178:839--845.
Sanders, F.E., P.B. Tinker, R.L.B. Black and S.M. Palmerley. 1977. The development of endomycorrhizal root systems. I. Spread of infection and growth-promoting effects with four species of vesicu-lar-arbuscular endophyte. New Phytol. 78:257--268.