See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/277554288
Genetic Diversity of Enhalus acoroides (L.)
Royle from Coastal Waters of Pramuka Island,
Lembongan Island, and Waigeo Island,
Indonesia, Based on Microsatellite DNA
ARTICLE
in
ADVANCED SCIENCE LETTERS · FEBRUARY 2015
Impact Factor: 1.25
READS
23
4 AUTHORS
, INCLUDING:
Made Pharmawati
Udayana University
19
PUBLICATIONS
293CITATIONS
SEE PROFILE
Gusti Ngurah Mahardika
Udayana University
42
PUBLICATIONS
110CITATIONS
SEE PROFILE
Copyright © 2015 American Scientific Publishers All rights reserved
Printed in the United States of America
Advanced Science Letters
Vol. 21, 199–202, 2015
Genetic Diversity of
Enhalus acoroides
(L.) Royle
from Coastal Waters of Pramuka Island,
Lembongan Island, and Waigeo Island,
Indonesia, Based on Microsatellite DNA
Made Pharmawati13∗
, I Nyoman Giri Putra23
, Yuliana Fitri Syamsuni3, and
I Gusti Ngurah Kade Mahardika3
1Biology Departement, Faculty of Mathematics and Natural Sciences, Udayana University, Kampus Bukit Jimbaran, Bali, 80361, Indonesia
2Department of Marine Science and Technology, Faculty of Fisheries and Marine Sciences, Bogor Agricultural University, Jalan Rasamala, Kampus IPB Darmaga, Bogor, 16680, Indonesia 3Indonesian Biodiversity Research Center, Udayana University, Jalan Sesetan Gang Markisa No. 6,
Denpasar, Bali, 80223, Indonesia
Enhalus acoroides (L.) Royle is one of sea grass species that is found in most Indonesian marine waters. A study was conducted to analyze genetic diversity ofE. acoroidescollected from waters of Pramuka Island, Lembongan Island and Waigeo Island, Indonesia using microsatellite DNA markers. Allele frequencies, observed and expected heterozigosities were calculated using GenAlEx 6.501. The average of observed heterozigosity was higher at Pramuka Island compared to the other two sites. In general, analysis of the genetic relationships revealed thatE. acoroidescollected from three sites in Indonesia were grouped based on their habitats although there are some exceptions. There is a possibility of gene flow between the three sampling areas. More samples and microsatellite primers have to be included to further confirmE. acoroidesconnectivity.
Keywords: Enhalus acoroides, Genetic Diversity, Indonesia, Microsatellite DNA.
1. INTRODUCTION
Sea grass bed is one of coastal ecosystem that is dominated by sea grass vegetation. Sea grass bed can be formed by single sea grass species or by mix of two to 12 sea grass species on a substrate.1 Sea grass bed has important ecological functions, for example, as nursery grown, spawning and protection areas for marine biota such as fishes, mollusks, shrimps, sea urchin, and starfish2. Besides that, sea grass is also food for dugong (Dugong dugon) and green turtle (Chelonia mydas).3
Sea grass grows in shallow coastal areas from intertidal zone to sub littoral zone and around rocky islands of Indonesian water.4 There are 60 sea grass species identified in the world5and among them, there are 13 species found in Indonesia.6
One of sea grass species isEnhalus acoroideswhich has bigger size with wider and longer leaf as compared to other species. Its leaves can reach 100 cm long.Enhalus acoroides has high productivity in intertidal zone as food source for marine biota and serves as carbon sequestration. Therefore, it can reduce impact
∗Author to whom correspondence should be addressed.
of climate change.7 Enhalus acoroidesis sea grass species that is mostly found in almost all types of marine water and grow well in shallow sea water and in open area during low tide.8
In Indonesia,E. acoroidesis distributed throughout Indonesia, such as Java, Bali, Kalimantan, Sumatra, Papua, Nusa Tenggara, Sulawesi, Ambon and Papua.69 Seribu archipelago is located
off Jakarta’s Northern Coast and consists of 110 islands.10Here, E. acoroidesis reported as one of common sea grass species.1112
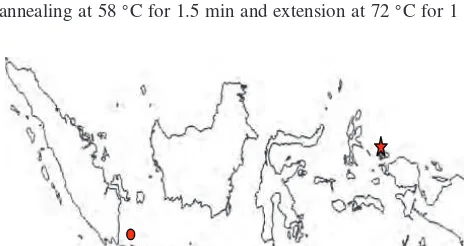
One of the islands in Seribu archipelago is Pramuka Island. This island was chosen as one study area, representing west of Indone-sia. In central Indonesia, Lembongan Island which is a small island in east of Bali Island is famous for its marine biota includ-ingE. acoroides. Lembongan Island water was the second sam-pling location for this study. In Papua, Raja Ampat archipelago is place whereE. acoroidesis also found.13 Weigo Island which is located in Raja Ampat area was the third sampling location that represents eastern part of Indonesia.
R E S E A R C H
A R T I C L E
Adv. Sci. Lett. 21, 199–202, 2015Table I. Location of sample collection and number of sample.
Location (ISLAND) GPS position No. of sample
Pramuka S 0545’06” 12
E 10636’44”
Lembongan S 0839’978” 28
E 11528’116”
Waigeo S 0026’11.05” 11
E 13046’37.45”
waters of Pramuka Island, Nusa Lembongan and Waigo Island by using microsatellite data. Microsatellite DNA markers have been widely used in genetic diversity and molecular ecology studies both in plants and animals. Microsatellite marker has sev-eral advantages as compared to other DNA markers. Microsatel-lites have high mutation rate, hence, high polymorphism can be detected, becoming high informative molecular markers.14 Information of E. acoroides genetic diversity is important for ecosystem conservation, population management and biodiversity maintenance.
2. MATERIALS AND METHODS
2.1. Sample CollectionSamples were collected during low tide in three areas of Indone-sia (Table I, Fig. 1). Samples were taken from the shoreline up to a perpendicular distance of 100 m–200 m. Young leaf blades of E. acoroideswere taken from individuals that separated at least 5 m apart. Leaves were cleaned, dried, cut about 1.5 cm long and kept in silica gel in plastic bag. The samples were then trans-ferred to laboratory for further analysis.
2.2. DNA Extraction and PCR
DNA was extracted from 0.1 g leaf using Qiagen Plant Mini kit according to manufacturer instruction. DNA was quantified using comparison with known concentration of lambda DNA in 1% agarose gel electrophoresis. PCR was conducted using five primer pairs (Eaco1, Eaco9, Eaco19, Eaco51, Eaco55).15
The PCR reaction consisted of 1×PCR buffer, 2.5 mm MgCl, 200 m dNTP, 1U taq polymerase (Amplitaq Gold-Life
Tech-nologies), 0.75 m each forward and reverse primers, 50 ng
template DNA and sterile water to make up 20l PCR reaction.
The PCR cycles were as follows: pre denaturation at 95C for
15 min, followed by 32 cycles of denaturation at 94C for 30 s,
annealing at 58C for 1.5 min and extension at 72C for 1 min.
Fig. 1. Map of location of samples collection, Pramuka Island (•), Nusa Lembongan (), Weigeo Island (⋆).
Final extension was done at 60C for 30 min. The PCR products
were sent to UC Berkeley, Department of Molecular and Cell Biology Sequencing Facility, USA for fragment identification.
2.3. Data Analyses
Data were analyzed using GenAlEx 6.501 software and presented as allele frequencies, expected heterozigosity (He) and observed heterozigosity (Ho). A dendrogram showing genetic relationships of samples from three regions was developed by MVSP Software using UPGMA based on Euclidean distance matrix.
3. EXPERIMENTAL RESULTS
The number of alleles from five loci used in this study varied from 6 to 18 (data not shown). The highest number of allele was at locus Eaco51 which had 18 alleles while locus Eaco1 and locus Eaco19 had the lowest number of allele (6 alleles). Locus Eaco9 had 10 alleles and there were 15 alleles at locus Eaco55. The number of total alleles at Pramuka was 26 while there were 40 alleles from Lembongan and 27 alleles from Waigeo. Lem-bongan had the highest number of alleles with low frequency of each allele.
Allele 254 bp at locus Eaco51 was a common allele at Pra-muka Island and Nusa Lembongan while allele 205 bp had the highest frequency at Waigeo Island.
Analysis of heterozigosity showed thatE. acoroidesfrom Pra-muka Island had the highest value of observed heterozigosity which was 0.767. Observed heterozigosity at population at Nusa Lembongan was 0.436 which was slightly lower than that in Waigeo ie. 0.582 (Table II). The high levels of observed het-erozigosity at water of Pramuka Island may be due to scattered individual distribution ofE. acoroides. At study site,E. acoroides do not form continuous sea grass bed.
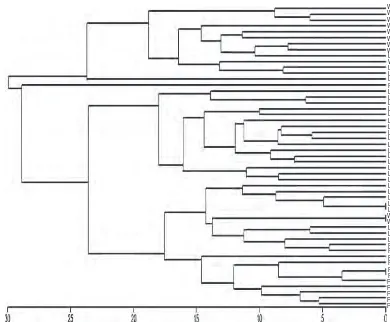
A dendrogram using UPGMA based on Eucliden distance showing genetic relationships of a total of 51E. acoroides sam-ples from waters of Pramuka, Lembongan and Waigeo Islands is presented in Figure 2.
The dendrogram divided all samples into four groups which were group A, B, C and D. There is no previous study on genetic relationship ofE acoroidesfrom Indoneian waters, therefore, this is the first report on its genetic relationships from three loca-tions in Indonesia. In general, the samples were grouped into their growing locations, although there are some exceptions. Sev-eral individuals from Lembongan Island water were grouped with E. acoroidesfrom Waigeo water (Group A). Group C consisted of individuals from waters of Pramuka, Lembongan and Waigeo Islands.
Table II. Heterozigosity ofE. acoroidesin Pramuka Island, Nusa Lem-bongan and Waigeo Island.
Location
Pramuka Island Lembongan Island Waigeo Island
Locus He Ho He Ho He Ho
Eaco1 0.152 0167 0.438 0.429 0.454 0636
Eaco9 0.761 1 0.676 0.463 0.849 1
Eaco19 0.577 1 0.406 0.143 0.298 0364
Eaco51 0.667 0833 0.850 0.964 0.835 0909
Eaco55 0.712 0833 0.867 0.909 0.806 0909
Average 0.574 0767 0.647 0.436 0.648 0582
WO
Fig. 2. Dendrogram showing genetic relationships ofE. acoroidescollected from waters of Pramuka, Lembongan and Waigeo Islands.
Genetic structure of marine species in Indonesia is also affected by oceanic factors such as ocean current of Indonesian through flow current that convey water from Pacific to Indian Oceans.18This may facilitate gene flow ofE. acoroidesbetween locations. There is also a dynamic seasonally reversing current in Java sea18 which may contribute to the mixed position of E. acoroidesfrom Pramuka Island water, Lembongan and Waigeo waters (Group C). More samples and microsatellite primers are needed to further elucidate the grouping and connectivity of E. acoroidesfrom these three areas.
It is believed that there is genetic divergence between popu-lations of Indian and Pacific Oceans.16 However, several stud-ies showed that there was genetic exchange between oceans.17 This may explain the grouping of some E. acoroides individu-als from Waigeo and Lembongan waters. Similar situation was reported for pearl oyster species (Pinctada maxima) from West Papua and Bali where there was genetic exchange between the two populations.18
4. CONCLUSIONS
Enhalus acoroidesfrom coastal waters of Pramuka Island, Lem-bongan Island and Waigeo Island have high heterozigosity. The samples were clustered into four groups and in general they were grouped based on their growing location although in group A and group C, there were some individuals from different locations.
Acknowledgments: This work was supported by USAID under NAS Sub-Grant No. PGA-2000003438. We would like to
thank Andri Kuncoro from Papua University for helping us with sample collection at Waigeo Island.
References and Notes
1. W. Kiswara and Winardi, Sebaran lamun di Teluk Kuta dan Teluk Gerupuk, Lombok, Pusat Penelitian Oseanografi, Jakarta: LIPI(1999).
2. R. C. Phillips and E. G. Menez, Seagrass, Washington: Smithsonian Institute Press(1998).
3. J. Lanyon, C. J. Limpus, and H. Marsh, Dugongs and turtles; Grazers in the sea grass system, edited by A. W. D. Larkum, A. J. McComb, and S. A. Sheperd, Biology of Australian sea grasses, An Australian perspective, Ams-terdam: Elsevier(1989).
4. P. H. Nienhuis, J. Coosen, and W. Kiswara,Neth. J. Sea Res.23, 197(1989).
5. I. H. Supriyadi, Pemetaan Kondisi lamun dan bahaya ancamannya dengan menggunakan citra satelit Alos di Pesisir Selatan Bitung-Manado, Sulawesi Utara. Oseanologi dan Limnologi di Indonesia 34, 445(2008).
6. T. E. Kuriandewa, Tinjaun tentang lamun di Indonesia, Lokakarya Nasional I Pengelolaan Ekosistem Lamun, Jakarta: Sheraton Media(2009).
7. S. L. Williams,Ecol. Appl.11, 1472(2001).
8. W. Kiswara, Kondisi padang lamun (seagrass) di Perairan Teluk Banten 1998-2001, Jakarta: Lembaga Penelitaian Oseanografi, Lembaga Ilmu Penge-tahuan Indonesia(2004).
9. W. Kiswara and M. Hutomo, Habitat dan sebaran geografik lamun, Oseana 10, 21(1985).
10. Z. Arifin, Local millennium ecosystem assessment: Condition and trend of the Greater Jakarta Bay ecosystem, Jakarta: Research Center for Oceanograpy, LIPI(2004).
11. O. S. Christon, Djunaedi, and N. P. Purba, Pengaruh tinggi pasang surut terhadap pertumbuhan dan biomassa daun lamun Enhalus acoroides di Pulau Pari Kepulauan Seribu Jakarta, Jurnal Perikanan dan Kelautan 3, 287
R E S E A R C H
A R T I C L E
Adv. Sci. Lett. 21, 199–202, 201512. N. Ekaningrum, Ruswahyuni, and Suryanti,J. Manage. Aquat. Resour.1, 1
(2012).
13. M. Hutomo and M. K. Moosa,Indian J. Mar. Sci.34, 88(2005).
14. Q. H. Wan, H. Wu, T. Fujihara, and S. G. Fang, Which genetic marker for which conservation genetics issue?Electrophoresis25, 2165(2004).
15. Y. Nakajima, Y. Matsuki, C. Lian, M. D. Fortes, W. H. Uy, W. L. Campos, M. Nakaoka, and K. Nadaoka,Conservative Genetic Resources4, 515(2012).
16. J. A. H. Benzie, Major genetic differences between crown-of thorns starfish (Achantaster placi) populations in the Indian and Pacific Oceans. Evolution 53, 1782(1999).
17. H. A. Lessios, J. Kane, and D. R. Robertson,Evolution57, 2026(2003).
18. C. E. Lind, Population genetics, phytogeography and the effects of aquacul-ture on genetic diversity of the silver-lipped pearl oysterPinctada maxima
(Jameson), Ph.D. Thesis, Australia: James Cook University(2009).
Received: 29 September 2014. Accepted: 25 October 2014.