www.elsevier.com / locate / bres
Research report
Dual control of the brainstem on the spindle oscillation in humans
a ,
*
a b cShinobu Kohsaka
, Taeko Sakai , Masako Kohsaka , Noriko Fukuda ,
a
Kunihiko Kobayashi
a
Department of Pediatrics, Hokkaido University School of Medicine, N-15 W-7, Kita-ku, Sapporo 060, Japan
b
Sapporo Hanazono Hospital, Sapporo 064, Japan
c
Department of Laboratory Technology, College of Medical Technology, Hokkaido University, Sapporo 060, Japan Accepted 8 August 2000
Abstract
In human subjects, the excitability change of the brainstem was investigated over the course of the spindle oscillation. The investigation was carried out by a sequential analysis of brainstem auditory evoked potentials (BAEPs) with reference to one sequence of spindle oscillation. The method was based on the characteristics of BAEPs, i.e. far-field evoked potential. The brainstem revealed two types of excitability change: one in the lower ventral brainstem (wave-III components), and the other in the upper dorsal brainstem (wave-V components). The excitability in the dorsal brainstem showed an oscillation with one cycle period of about 1.5 s, whereas in the ventral brainstem, the excitability showed a long-range biphasic (decaying–growing) fluctuation. Both excitability changes in the brainstem preceded the spindle oscillation, and the phase was reversed during the emerging period of spindle oscillation. The results suggest a primary triggering mechanism of the brainstem for the spindle oscillation, which is independent of preceding cortical drives (K-complexes) upon the thalamus. The difference of the excitability change between the spindle oscillation and the paroxysmal discharge (spike-and-wave complex) was also discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behaviour
Topic: Biological rhythms and sleep
Keywords: Sleep spindle; Brainstem; Brainstem auditory evoked potential; Excitability change
1. Introduction upon the cortex [24,32,34]. However, comparing with its established mechanism for the rhythm generation, the Spindle oscillations are the most salient synchronized triggering mechanism for the spindle seems less clear neuronal activities in the cortex during sleep. These except for the hypothesis that widespread hyperpolariza-oscillations are the hallmark of a transitional state from tions in both the cortex and the thalamus might trigger the moderate to deep sleep (slow-wave sleep) on electroence- spindle [8,9].
phalographs (EEGs) [29]. Spindle oscillations appear with Spindle oscillations are also important for the underlying a crescendo–decrescendo shape (waxing–waning) with a mechanism of generalized epileptic seizures with spike-duration of about 0.5–1.0 s, with a repetition rate of 5–6 and-wave complex (SWC). Gloor et al. disclosed a gradual times / min in humans [12,14]. The mechanism for the shift from spindle oscillations to SWC in cats with rhythm generation (12–14 Hz in humans) has been investi- systemic application of penicillin (feline generalized gated in experimental animal models. The results from penicillin epilepsy, FGPE) [13]. Although recent experi-animal models disclosed that the reciprocally connected ments reevaluated the results from FGPE model [35,36], two groups of neurons in the thalamus (reticular thalamic, the question — why patients with absence epilepsy RE; thalamocortical, TC) generate the rhythm by recip- experience their seizures (3-Hz SWC, prototype of SWC) rocal reverberation, and TC neurons project the rhythm even in a preceding vigilant state — has been left unanswered. To solve the problem, we have recently disclosed a functional change in the brainstem, a
long-*Corresponding author. Fax:181-11-706-7898.
E-mail address: [email protected] (S. Kohsaka). range fluctuation superimposed with rhythmic oscillations,
104 S. Kohsaka et al. / Brain Research 882 (2000) 103 –111
that precedes 3-Hz SWC in patients with absence epilepsy sampled EEG data; two sequences of spindle appear in Fig. [22]. The investigation was carried out by a simultaneous 1A. We excluded the spindle oscillation with a preceding analysis of far-field evoked potentials (brainstem auditory large negative EEG transient (K-complex or vertex sharp evoked potentials, BAEPs) with reference to the paroxys- transient) from the analysis. An example of the spindle mal discharges (SWC). oscillation with K-complex is also shown in Fig. 1A Here, we investigated the brainstem function over the (marked with ‘1’). K-complexes can be detected easily, course of a spindle oscillation (before and during) with a because they show regular phase reversals between C3–Cz similar analytic method in humans. Probably, spindle and Cz–C4 according to the distribution pattern in the oscillations are more suitable candidates for the analysis EEG field [6].
because of their periodic characteristics rather than in- The method for obtaining sequential BAEPs over one frequent EEG events such as epileptic discharges. spindle oscillation was as follows.
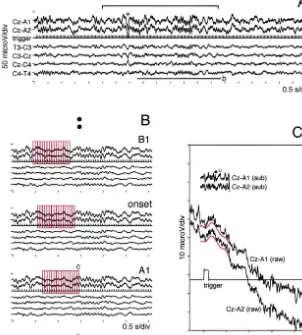
(i) The EEG data just before one spindle oscillation were selected with a series of 16 segments in a manner that
2. Materials and methods the last segment in the series positioned the first spindle wave (red rectangles in Fig. 1B, ‘onset’). BAEPs were 2.1. Data acquisition calculated by averaging these EEG data in Cz–A1 and Cz–A2 (160 average counts; 16 segments in 10 sequences A full description for the recording and the analyzing of spindle), and termed as ‘onset’.
device appeared in our previous study [22]. Six-channel (ii) The average area for ‘onset’ consisting of 16 sleep EEGs (Cz–A1 and Cz–A2 for BAEP recording, 1.5 segments was shifted backward by 2 segments (red rectan-Hz–1.5 kHz for a band-pass filter; T3–C3, C3–Cz, Cz–C4 gles in Fig. 1B, ‘B1’) in 10 sequences of spindle, then the and C4–T4 for EEG monitoring, 1.5–100 Hz for a band- EEG data was averaged. As a segment was defined as a pass filter) were recorded together with one channel of 512-point EEG data with a fixed position of one trigger continuously delivered acoustic trigger signal (80 dB signal (20 times / s repetition rate), the average area was binaural single rectangular pulse with a 0.1-ms width, 20 shifted backward 0.1 s by this procedure. The calculated times / s of repetition rate) from a human subject through a BAEPs were termed as ‘B1’. This averaging procedure multi-modal EEG device. Twelve male volunteers aged was repeated until the BAEPs termed as ‘B18’ were from 20 to 27 years were gathered for this study. The obtained.
procedure was approved by the ethical committee of (iii) The average area for ‘onset’ was shifted forward by Hokkaido University School of Medicine. The EEG data 2 segments (red rectangles in Fig. 1B, ‘A1’), then the EEG were temporarily stored on a magnetic tape through a PCM data was averaged in 10 sequences of spindle. The digital data recorder. The recording lasted until a volunteer calculated BAEPs were termed as ‘A1’. This averaging fell asleep to stage II according to the sleep-stage scoring procedure was repeated until the BAEPs termed as ‘A16’ where sleep spindles appeared constantly [29]. All EEG were obtained.
electrodes were shielded, and the electrode resistance was Following the above procedures, two series (left, Cz– carefully kept below 5 kVthroughout the recording. While A1; right, Cz–A2) of sequentially changing BAEPs, with a the EEG data were replayed, the periods with stable time shift of 0.1 s, were obtained as the spindle wave spindle oscillations were selected and sampled (10 kHz for gradually emerged in the EEGs. The time range for the sampling frequency) through a personal computer (PC) analysis spanned from21.860.4 s (‘B18’; median6range) equipped with an A / D converting board. The sampled to 1.660.4 s (‘A16’) through ‘onset’ (060.4 s). The range EEG data were transferred to the PC memory with a is also shown in Fig. 1A (upper horizontal bar with vertical capacity to store approximately 240 s EEG data for seven limits; from the top segment in ‘B18’ to the last segment in channels. All procedures for the analysis were carried out ‘A16’).
Fig. 1. A method of sequential analysis of BAEPs. Upward positive in both Cz–A1 and Cz–A2 (for the analysis of BAEPs), and upward negative in the other EEGs. (A) A part of an example of sleep EEGs with two sequences of spindle is shown. The acoustic trigger signal (trigger) was delivered continuously (20 times / s) throughout the recording. A K-complex preceding the spindle oscillation, marked with ‘1’, was easily detected by its phase reversal between C3–Cz and Cz–C4. Spindle oscillations with preceding K-complexes were excluded from the analysis. A horizontal bar with limits shows the range for background EEG analysis (refer to text). (B) Three series (‘B1’, ‘onset’ and ‘A1’) are aligned by expanding the EEG data with a horizontal bar labeled with ‘b’ in A. By shifting the average area (a sequence of 16 segments, red rectangles) backward (‘B1’), then forward (‘A1’) from the area of ‘onset’, sequentially changing BAEPs were averaged in 10 sequences of spindle. The average area was shifted by 2 segments (0.1 s, 1 / 20 repetition rate32 segment shift). (C) A segment (512 points) labeled with ‘c’ in B is magnified. To reduce large fluctuations in raw EEG data (Cz–A1(raw) and Cz–A2 (raw)), the trends were subtracted by fitting a 7-order polynomial curve (red tracings, offset by22.5mV for recognition) within the range of BAEP analysis window (128 points). The ‘embryo waves’ for BAEP components (filled circle for wave-III, open circle for wave-V) were discernible even in one trial of acoustic signal in the subtracted EEG data (Cz–A1(sub) and Cz–A2(sub)).
2.3. Background EEG analysis C3–Cz, Cz–C4 and C4–T4 were again averaged across the subject (grand-average).
To correlate the BAEPs’ change with the corresponding EEG activity, background EEGs were also averaged within
the range from the top segment in ‘B18’ to the last 3. Results
segment in ‘A16’ (upper horizontal bar with vertical
limitations in Fig. 1A) in 10 sequences of spindle. The 3.1. Sequential BAEPs change over the spindle background average was carried out by reducing the total oscillation
count of EEG data (84 segments3512 points) to 512-point
106 S. Kohsaka et al. / Brain Research 882 (2000) 103 –111
Fig. 2. Sequential BAEPs over the spindle oscillation from one subject. The left-side BAEP arrays were derived from Cz–A1 (left) and the right-side BAEP arrays were derived from Cz–A2 (right) with a top-to-bottom time course (from ‘B18’ to ‘A16’ with a thick trace of ‘onset’; both upward positive). Wave-III, -IIIn, -V and -Vn were traced around ‘onset’ (red tracings with arrows). It should be noted that wave-III showed a biphasic (decreasing– increasing) contour around ‘onset’, and the acceleration of wave-III was accompanied with deepening of wave-IIIn. Two parameters (amplitude and area) were measured in two component waves of BAEPs (wave-III and -V). The wave-III amplitude was measured as a voltage difference between wave-III and -IIIn, and the wave-III area was measured as a cumulative sum enveloped with wave-IIn, -III and -IIIn; the wave-V amplitude was measured as a voltage difference between wave-V and -Vn, and the wave-V area was measured as a cumulative sum enveloped with wave-IIIn, -V and -Vn (inset). The sum of the left and right parameters was normalized within the range from ‘B18’ to ‘A16’ for further statistical analysis.
Section 4. In every subject, two parameters (amplitude and 3.2. Wave-III and -V components change before and area) of the component waves (wave-III and -V) were during spindle oscillation
measured in each left and right BAEP tracings from ‘B18’
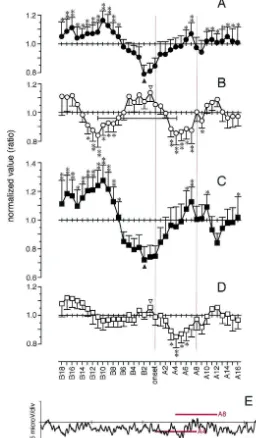
to ‘A16’. Wave-III amplitude was measured as the am- Results are shown in Fig. 3 (A, wave-III amplitude; B, plitude difference between wave-III and -IIIn (negative wave-V amplitude; C, wave-III area; D, wave-V area; and component of wave-III); and wave-V amplitude was mea- E, grand-average). The results are aligned according to the sured as the amplitude difference between wave-V and -Vn same temporal course from ‘B18’ to ‘A16’. The back-(negative component of wave-V). Wave-III area was mea- ground EEG (Fig. 3E) is proportionally expanded because sured as the amplitude accumulation enveloped by wave- of the duration of time (16 segments) for the average. IIn, -III and -IIIn; and wave-V area was measured as the Three horizontal red bars in Fig. 3E show the average area amplitude accumulation enveloped by wave-IIIn, -V and of ‘onset’, ‘A4’ and ‘A8’. It should be noted that ‘onset’ -Vn (Fig. 2, inset). contains no substantial spindle oscillation, ‘A4’ contains a A part of tracks of wave-III, -IIIn, -V and -Vn is also half amount of spindle, and in ‘A8’ the spindle oscillation shown including the period of ‘onset’ (red line with an fully develops in the EEGs.
arrow in Fig. 2). The sum of the left and right parameters The time course difference was significant in wave-III were normalized across the time series from ‘B18’ to amplitude (Fig. 3A; F11,2451.92, P,0.01; from ‘B12’ to ‘A16’. The time course difference of the normalized value ‘A12’), wave-V amplitude (Fig. 3B; F11,3451.70, P,0.05; was tested by one-way repeated measures of ANOVA from ‘B18’ to ‘A16’) and wave-III area (Fig. 3C; F11,345
Fig. 3. Time course change of wave-III and -V over the spindle oscillation in 12 subjects. All figures are aligned according to the same time course from ‘B18’ to ‘A16’. Wave-III amplitude (A; filled circle, mean1S.E.) and wave-III area (C; filled rectangle, mean1S.E.) showed biphasic fluctuations with the minimums at ‘B2’ preceding the spindle oscillation. Wave-V amplitude (B; open circle, mean2S.E.) showed an oscillatory change with the maximum at ‘B1’. The one-cycle period was measured 1.5 s from ‘B11’ to ‘A4’ (a horizontal bar with limits) whereas, in wave-V area (D; open rectangle, mean2S.E.), the first cycle of the oscillation was not apparent. The grand-average of background EEGs (E, averaged from C4–T4) shows the time course reference for one sequence of spindle oscillation. Three horizontal red bars indicate the average area for ‘onset’, ‘A4’ and ‘A8’. During this period the spindle gradually emerges in the EEGs. The phase of the parameters was reversed between wave-III (A,C) and wave-V (B,D) before and during the spindle oscillation. The paired difference within the time course was tested in comparison with the minimum (A,C) or the maximum (B,D) by contrast method; the significant difference is shown with a single asterisk (P,0.05) or double asterisks (P,0.01).
oscillat-108 S. Kohsaka et al. / Brain Research 882 (2000) 103 –111
ory characteristics of wave-V amplitude change (asterisks has been extensively investigated with animal models in in Fig. 3B). The points of inflection, the minimums of vivo [32] or with slice preparations in vitro [2,10,24]. By wave-III components (filled triangles in Fig. 3A,C) and the intracellular recordings, reciprocally connected RE and TC maximums of wave-V components (open triangles in Fig. neurons in the thalamus generate the rhythm with excitat-3B,D), precedes the period of ‘onset’. Furthermore, the ory postsynaptic drives (EPSPs) from TC to RE and, in increasing limb of wave-III components and the decreasing return, with inhibitory postsynaptic drives (IPSPs) from limb of wave-V components corresponded to the emerging RE to TC [24]. In TC neurons, the IPSP-induced hyperpo-period of the spindle oscillation (from ‘onset’ to ‘A4’). larization triggers bursts of action potentials (APs) by an activation of the cascade of intrinsic inward currents [25], and the cycle resumes, forming the synchronized spindle
4. Discussion oscillation [24,34]. In addition to this cycle-to-cycle re-ciprocal synaptic connection between RE and TC neurons, 4.1. Anatomy of BAEP component waves there are slow membrane fluctuations underlying one sequence of spindle oscillation [32]: a slowly depolarizing Since Jewett et al. first discovered BAEPs [18], the envelope in RE neurons and a slowly hyperpolarizing method has been widely used to investigate the brainstem envelope in TC neurons. The EPSPs in RE neurons and the function in various neurological disorders [7]. BAEPs are IPSPs with subsequent APs in TC neurons superimpose on classified as ‘far-field evoked potentials’ because, with these slow membrane fluctuations. Since the characteristic their very short latencies (usually below 7 ms), individual of spindle oscillation, i.e. waxing–waning, goes parallel evoked potentials (EPs) generated in the brainstem by an with these fluctuations [32], these reversed membrane acoustic trigger signal are transmitted to the scalp elec- fluctuations are considered as a substrate for one sequence trodes by volume conduction [19]. That is what the term of the spontaneously occurring spindle oscillation. The ‘far-field’ implies, and therefore, BAEPs are independent mechanism for the termination of spindle has been investi-of the level investi-of cortical activities. These characteristics are gated with slice preparations; a gradual decay of the shown in Fig. 1C. Although individual EPs are embedded membrane hyperpolarization in TC neurons, due to tonic in a large EEG fluctuation (spindle oscillation), major activation of the intrinsic current, suppresses the spike component waves were discernible with an appropriate generating mechanism in TC neurons [2,10], then the method of trend-subtraction even in one trial of acoustic spindle is terminated. However, the triggering mechanism stimulation. Our previous study has also shown these for spindle oscillation, the mechanism for generating the characteristics of BAEPs even in a seizure state of human reversed membrane fluctuations between RE and TC absence epilepsy [22]. neurons, is still left ambiguous.
The topography of the generator for two major com- RE neurons are endowed with an ability to self-sustain ponent waves (wave-III and -V) has been investigated, and the spindle rhythm. The RE neurons, even in the isolated a consensus has been gained on their location: the genera- reticular thalamic nucleus, still exhibit the oscillation tor of wave-III is located in superior olivary complex (SO) within the spindle range in the animal model in vivo [31]. in the ventral part of the lower brainstem; and the The ability is ascribed to the mutual inhibitory connections generator of wave-V is considered to be located around between RE neurons within reticular thalamic nucleus inferior colliculus (IC) in the dorsal part of the upper [10]. However, this autonomous characteristic of RE brainstem [17,40]. Between SO and IC the ascending route neurons does not exclude a possibility that other inputs to of BAEPs travels dorsolaterally through lateral lemniscus, the thalamus might trigger the spindle oscillation. In fact, generating wave-IIIn and -IV [40]. As the neuronal the frequency, the repetition rate and the duration of the substrate for BAEP component waves are the sum of spindle from the isolated RE neurons were not entirely the axonal potentials [26], wave-III and -V represent a gross same as intact ones [31].
of spindle obliteration. The cycle of this spontaneous Hz) of the widespread hyperpolarizations, K-complexes as oscillation was measured about 1.5 s (Fig. 3B). The cortical components, is considered to provide the overall experiment on feline model has already shown a similar sculpturing effect on the synchronized sleep EEG activities spontaneous oscillation (around 1.0 s one-cycle period) in froms,a-band (spindle) to d-band (slow-wave) frequency the discharging rate of cholinergic neurons in the dorsal [34]. Although spectrum analyses on both s- and d-band brainstem during the transition from wakefulness to slow- frequency in humans have supported the hypothesis [37], wave sleep [33], while its relation to the spindle has not the temporal distributions of both frequencies differed been investigated. It is apparent, however, that the sponta- during a one-cycle sleep period in humans [11]. In the neous excitability oscillation in the dorsal brainstem is not present study, we excluded the sequence of spindle pre-the primary driving mechanism for spindle oscillation. The ceded by K-complex (Fig. 1A), consequently none of such recurrence rate of spindle is more prolonged [12,14], and large negative EEG activities were observed in the back-the background EEG did not exhibit a spindle oscillation ground EEGs (Fig. 3E; K-complex has an amplitude during the preceding cycle of wave-V amplitude change several times larger than spindle). Therefore, the results (Fig. 3E). exclude a possibility for the preceding K-complex to The present results also disclosed a long-range fluctua- trigger the spindle with a spontaneous waxing–waning tion of the wave-III components corresponding to the pattern.
spindle oscillation. This biphasic (decaying–growing) fluc- The wave-V components’ change is not attributed to tuation preceded the spindle with its minimum at ‘B2’. The some disinhibitory or disfacilitatory effects from the lower growing slope corresponded to the emerging period of brainstem (wave-III), because we did not find any simulta-spindle oscillation from ‘onset’ to ‘A8’ (Fig. 3A,C,E); neous wave-V components’ change during the period there were no actual oscillations in ‘onset’, the spindle where wave-III exhibited profound oscillations during, and emerged a half amount in ‘A4’ and finally the spindle even before, the seizure state in human absence epilepsy became full-blown in ‘A8’. Probably, this long-range [22]. The spindle was triggered only when the excitability fluctuation of wave-III underlies the primary triggering change of both dorsal and ventral brainstem exhibited a mechanism for the spontaneous spindle oscillation in reversed phase. During this period, the growing excitability humans. The wave-III components’ change was achieved in the ventral brainstem together with the decaying ex-by deepening of wave-IIIn in every case (Fig. 2, right, for citability in the dorsal brainstem corresponded to the an example), therefore the excitability change probably emergence of spindle oscillation (from ‘onset’ to ‘A4’ in takes place slightly rostral than in SO in the ventral Fig. 3). The result suggests a dual brainstem control on the brainstem. The effect of the increasing excitability in the spontaneous spindle oscillation in humans. It is intriguing ventral brainstem is still hypothetical because of a relative to assume that the growing excitability provides the paucity of physiological investigations, compared to the depolarizing envelope in RE neurons and the decaying established connection between the cholinergic neurons in excitability provides the hyperpolarizing envelope in TC the dorsal brainstem and TC neurons [15,16]. However, neurons, which considered as a neuronal substrate of one histological studies have proved the ascending inputs to sequence of spindle oscillation. However, the former RE neurons from midbrain reticular formation (MRF), mechanism needs to be clarified.
which are considered direct or indirect through
intralami-nar nucleus in the thalamus [5,23,28,38]. One physiologi- 4.3. Difference between spindle and 3-Hz SWC cal study disclosed an excitatory depolarizing input to RE
110 S. Kohsaka et al. / Brain Research 882 (2000) 103 –111
[10] A. Destexhe, T. Bal, D.A. McCormick, T.J. Sejnowski, Ionic
As we predicted in the previous study on human absence
mechanisms underlying synchronized oscillations and propagating
epilepsy, the method applied here was capable of
inves-waves in a model of ferret thalamic slices, J. Physiol. 76 (1996)
tigating the underlying mechanism of the brainstem for the 2049–2070.
periodic event (sleep spindle) in sleep EEGs. The ex- [11] D.-J. Dijk, B. Hayes, C.A. Czeisler, Dynamics of electroence-phalographic sleep spindles and slow wave activity in men: effect of
citability of the brainstem could be investigated with a
sleep deprivation, Brain Res. 626 (1993) 190–199.
more precise temporal resolution than that of 3-Hz SWC
[12] D.-J. Dijk, EEG slow waves and sleep spindles: Window on the
because of its periodicity compared to the rarity of the sleeping brain, Behav. Brain Res. 69 (1995) 109–116.
latter. There are two major differences between the two [13] P. Gloor, Electrophysiology of generalized epilepsy, in: P.A. Schwartzkroin, H. Wheal (Eds.), Electrophysiology of Epilepsy,
highly synchronized EEG patterns (spindle vs. 3-Hz
Academic Press, London, 1984, pp. 107–136.
SWC). First, there are no simultaneous excitability
[14] M. Guazzelli, I. Feinberg, M. Aminoff, G. Fein, T.C. Floyd, C.
changes in the upper dorsal brainstem in 3-Hz SWC where Maggini, Sleep spindles in normal elderly: Comparison with young the excitability in the lower ventral brainstem exhibits adult patterns and relation to nocturnal awakening, cognitive func-profound oscillations before and during the paroxysms tion and brain atrophy, Electroencephalogr. Clin. Neurophysiol. 63
(1986) 526–539.
[22]. Second, in 3-Hz SWC, the paroxysmal discharge is
[15] B. Hu, M. Steriade, M. Deschenes, The effects of brainstem
triggered during the long-range decaying period of the
peribrachial stimulation on perigeniculate neurons: The blockage of
excitability change in the ventral brainstem [22], whereas spindle waves, Neuroscience 31 (1989) 1–12.
in spindle, this physiological EEG synchronization is [16] B. Hu, M. Steriade, M. Deschenes, The effects of brainstem peribrachial stimulation on neurons of the lateral geniculate nucleus,
triggered during the growing period of the excitability
Neuroscience 31 (1989) 13–24.
change in the ventral brainstem (Fig. 3A,C,E). Despite all
[17] J.R. Hughes, J.J. Fino, A review of generators of the brainstem
of these differences, it should also be emphasized that both auditory evoked potential: Contribution of an experimental study, J. EEG synchronizations are controlled by the functional Clin. Neurophysiol. 2 (1985) 355–381.
[18] D.L. Jewett, M.N. Romano, J.S. Williston, Human auditory evoked
change in the brainstem, especially in the lower ventral
potentials: Possible brain stem components detected on the scalp,
area.
Science 167 (1970) 1517–1518.
[19] D.L. Jewett, J.S. Williston, Auditory-evoked far fields averaged from the scalp of humans, Brain 94 (1971) 681–696.
Acknowledgements [20] Y. Kayama, I. Sumitomo, T. Ogawa, Does the ascending cholinergic projection inhibit or excite neurons in the rat thalamic reticular nucleus?, J. Neurophysiol. 56 (1986) 1310–1320.
We express special thanks to the newcomers to our
[21] P. Kellaway, J.D. Frost, J.W. Crawley, Time modulation of
spike-department (Department of Pediatrics in Hokkaido Uni- and-wave activity in generalized epilepsy, Ann. Neurol. 8 (1980) versity School of Medicine) who were willing to partici- 491–500.
pate in this study as volunteers. [22] S. Kohsaka, S. Mizukami, K. Uetake, T. Sakai, M. Kohsaka, Brainstem triggers absence seizures in human generalized epilepsy, Brain Res. 837 (1999) 277–288.
[23] C.I. Kolmac, J. Mitrofanis, Patterns of brainstem projection to the
References thalamic reticular nucleus, J. Comp. Neurol. 396 (1998) 531–543.
[24] M. Krosigk, T. Bal, D.A. McCormick, Cellular mechanisms of a [1] F. Amzica, M. Steriade, The K-complex: Its slow (,1 Hz) synchronized oscillation in the thalamus, Science 261 (1993) 361–
rhythmicity and relation to delta waves, Neurology 49 (1997) 364.
952–959. [25] D.A. McCormick, H. Pape, Properties of a hyperpolarization-acti-[2] T. Bal, D.A. McCormick, What stops synchronized thalamocortical vated cation current and its role in rhythmic oscillation in thalamic
oscillations?, Neuron 17 (1996) 297–308. relay neurons, J. Physiol. 431 (1990) 291–318.
[3] M. Bazhenov, I. Timofeev, M. Steriade, T.J. Sejnowski, Self- [26] T. Nakanishi, Action potentials recorded by fluid electrodes, Elec-sustained rhythmic activity in the thalamic reticular nucleus me- troencephalogr. Clin. Neurophysiol. 53 (1982) 343–345.
diated by depolarizing GABA receptor potentials, Nat. Neurosci. 2A [27] G. Oakson, M. Steriade, Slow rhythmic rate fluctuations of cat (1999) 168–174. midbrain reticular neurons in synchronized sleep and waking, Brain [4] J.S. Bendat, A.G. Piersol (Eds.), Random Data, Wiley-Interscience, Res. 247 (1982) 277–288.
New York, 1986, pp. 361–368. [28] D. Pare, Y. Smith, A. Parent, M. Steriade, Projections of brainstem [5] D.J. Berry, P.T. Ohara, A.R. Lieberman, Are there connections core cholinergic and non-cholinergic neurons of cat to intralaminar
between the thalamic reticular nucleus and the brainstem reticular and reticular thalamic nuclei, Neuroscience 25 (1988) 69–86. formation?, J. Comp. Neurol. 243 (1986) 347–362. [29] A. Rechtschaffen, A. Kales (Eds.), A Manual of Standardized [6] R. Broughton, J. Hasan, Quantitative topographic eletroencephalo- Terminology, Techniques and Scoring System for Sleep Stages of graphic mapping during drowsiness and sleep onset, J. Clin. Human Subjects, Public Health Service in U.S. Government Printing Neurophysiol. 12 (1995) 372–386. Office, Washington, DC, 1968.
[7] K.H. Chiappa (Ed.), Evoked Potentials in Clinical Medicine, Raven, [30] S. Sato, F.E. Dreifuss, J.K. Penry, The effect of sleep on spike-wave New York, 1990, pp. 223–305. discharges in absence seizures, Neurology 23 (1973) 1335–1345. [8] D. Contreras, M. Steriade, Cellular basis of EEG slow rhythms: A [31] M. Steriade, L. Domich, G. Oakson, M. Deschenes, The
deaf-study of dynamic corticothalamic relationships, J. Neurosci. 15 ferented reticular thalamic nucleus generates spindle rhythmicity, J.
(1995) 604–622. Neurophysiol. 57 (1987) 260–273.
[33] M. Steriade, S. Datta, D. Pare, G. Oakson, R. Curro Dossi, Neuronal chronology in corticothalamic networks, Sleep Res. Online 1 (1998) activities in brain-stem cholinergic nuclei related to tonic activation 1–10.
processes in thalamocortical systems, J. Neurosci. 10 (1990) 2541– [38] R.P. Vertes, G.F. Martin, Autoradiographic analysis of ascending
2559. projections from the pontine and mesencephalic reticular formation
[34] M. Steriade, D.A. McCormick, T.J. Sejnowski, Thalamocortical and the median raphe nucleus in the rat, J. Comp. Neurol. 275 oscillations in the sleeping and aroused brain, Science 262 (1993) (1988) 511–541.
679–685. [39] S. Wada, S. Matsuoka, E. Urasaki, C. Yadomi, Quantitative analysis [35] M. Steriade, D. Contreras, Relations between cortical and thalamic of reversible dysfunction of brain-stem midline structures cased by cellular events during transition from sleep patterns to paroxysmal disturbance of basilar artery blood flow with the auditory brain-stem activity, J. Neurosci. 15 (1995) 623–642. responses, Electroencephalogr. Clin. Neurophysiol. 69 (1988) 148– [36] M. Steriade, D. Contreras, Spike-wave complexes and fast com- 159.
ponents of cortically generated seizures. I. Role of neocortex and [40] S. Wada, Present status and problems of auditory brainstem evoked thalamus, J. Neurophysiol. 80 (1998) 1439–1455. potentials, Jpn. J. EEG EMG 22 (1994) 245–255.