Extracelluar signals are transduced to the nucleus through respective signal transduction pathways. Evidence in animals and yeast indicates the importance of regulated nuclear targeting in these processes. Although little is known about plants in this regard, some plant signaling factors have recently been shown to translocate to the nucleus upon receipt of a signal.
Addresses
Department of Botany, Graduate School of Science, Kyoto University, Kitashirakawa, Kyoto 606-8502, Japan;
e-mail: [email protected]
Current Opinion in Plant Biology1998, 1:470–474 http://biomednet.com/elecref/1369526600100470 © Current Biology Ltd ISSN 1369-5266
Abbreviations
ER endoplasmic reticulum
GBF G-box binding factor
HSF heat shock transcription factor
MAPK mitogen-activated protein kinase
NFAT nuclear factor of activated T cells
phyA phytochrome A
phyB phytochrome B
SREBP sterol regulatory element binding protein
STAT signal transducer and activator of transcription
TGFb transforming growth factor β
Introduction
Plant cells change their patterns of gene expression in response to environmental as well as developmental sig-nals. In most cases, extracellular signals are perceived by receptors residing either at the plasma membrane or in the cytoplasm. These receptors then activate signal transduc-tion pathways to influence expression of target genes. Specific transcription factors play important roles in acti-vating these pathways [1]; transcription factors, or factors that modify their activity, translocate from the cytoplasm to the nucleus. In this review, I will first describe how the nuclear targeting of these factors is regulated in yeast and animals because the depth of research is much greater in those systems. Then, I will summarize recent progress in plants. Although it is a relatively new field at present in plant research, it will soon be an important issue.
Mechanisms known in animals and yeast
In animals and yeast, a wide range of signal transduction components is known to be relocalized to the nucleus upon receipt of extracellular signals. These factors are summarized below.Factors that are released from cytoplasmic complexes Nuclear hormone receptors translocate to the nucleus upon hormonal stimuli [2]. At rest, the nuclear hormone receptor is bound to heat shock proteins and retained in
the cytoplasm. Upon the binding of hormonal ligand, the receptors are released from the complex, form a homo- or hetero-dimer, and translocate to the nucleus, where they act independently as transcription factors.
NF-κB is a transcriptional activator that regulates various immune and stress responses in animals [3]. Activity of
NF-κB is regulated by a cytoplasmic inhibitor named IκB. IκB is a kinase with ankyrin-repeat motifs. In a resting state, NF-κB is bound to IκB in the cytoplasm. Upon stimulation, upstream kinases phosphorylate IκB which results in rapid degradation of IκB. Consequently, NF-κB is released from the complex and translocates to the nucleus.
Mitogen-activated protein kinases (MAPKs) act as a key regulator in the MAPK pathways [4]. Extracellular signals are transduced to MAPK through kinase cascades. Upon receipt of signals, MAPK is phosphorylated by MAPK kinase in the cytoplasm. The activated MAPK translocates to the nucleus and phosphorylates target transcription fac-tors [5]. Recently, MAPK kinase has been proposed to act as a cytoplasmic anchoring protein for MAPK [6].
Factors that are cleaved upon stimulation
Sterol regulatory element binding proteins (SREBPs) reg-ulate the expression of sterol-related genes in response to changes in the sterol content [7]. SREBPs are membrane-bound transcription factors. SREBP resides at the endoplasmic reticulum (ER) and nuclear envelope with its amino-terminal and carboxy-terminal domains facing the cytoplasm. Upon the depletion of sterols, the amino-termi-nal domain of the receptor is cleaved out from the other part of the receptor. The domain is then targeted into the nucleus to function as a transcription factor.
Notch/LIN-12 is a class of transmembrane receptors which mediate cell–cell interactions during Drosophila and nema-tode development [8]. Although Notch/LIN-12 resides at the cell surface, a nuclear localization signal is found in its intracellular domain. One possible mechanism of Notch/LIN-12 action is that the intracellular domain is released from the receptor by protein cleavage. Recently, evidence in favor of this model has been reported [9]. The authors fused a strong transcriptional activator to the intra-cellular domain of Notch to show that the domain indeed entered the nucleus.
Other factors
Neither anchoring proteins nor retention domains have been found for some factors. In such cases, alteration in the oligomeric state of the factor apears to play a key role in the regulation of nuclear translation. These factors are described below.
The heat shock transcription factor (HSF) mediates responses of cells to various stresses [10]. HSF is a tran-scriptional activator that binds to the regulatory element heat shock and stress-inducible genes. HSF in the resting state resides in the cytoplasm as a monomer. Upon expo-sure to stress, HSFs oligomerize to form trimers and translocate to the nucleus.
The signal transducers and activators of transcription (STATs) are key components of the Jak-STAT pathway, which is one of the signaling pathways for cytokines [11]. In the resting state, STAT resides in the cytoplasm in an unphosphorylated form. Binding of cytokines to certain cytokine receptors such as the IL-6 receptors, activates Janus tyrosine kinases (Jaks), which in turn phosphorylate tyrosine-based docking sites on the receptor. STATs are recruited to the docking sites, phosphorylated and released. The released STATs form a dimer and translo-cate to the nucleus.
The transforming growth factor β(TGFβ) regulates
ani-mal cell development. TGFβ receptors are
membrane-spanning serine/threonine kinases [12]. Signals perceived by the TGFβreceptors are transduced MAD-related proteins. Upon binding of the ligands, the receptors rapidly phosphorylate MAD-related proteins that exist as homo-oligomers. The phosphorylated
MAD-related proteins form hetero-oligomers and translocate to the nucleus [13].
The nuclear factor of activated T cells (NFAT) mediates calcium signals to the nucleus during the immune response [14]. At rest, NFAT resides in the cytoplasm in a phosphorylated form. Increase in the intracellular free cal-cium activates a calcal-cium-dependent phosphatase, calcineurin. NFAT dephosphorylated by calcineurin translocates to the nucleus.
A general scheme
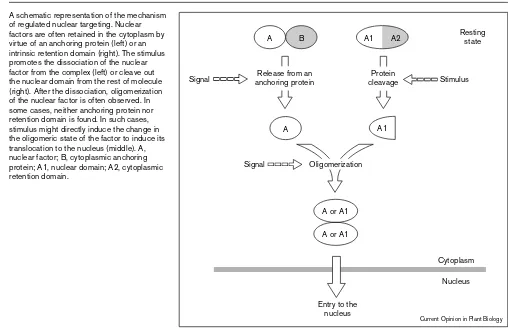
There are a wide variety of ways to regulate nuclear tar-geting; nevertheless, it is possible to draw a general picture (Figure 1). Nuclear factors should be retained in the cytoplasm in the resting state. In some cases, this is attained by virtue of anchoring proteins. The signal mod-ifies the anchoring protein and/or the nuclear factor to dissociate the factor from the complex. In other cases, these two functional ‘components’ are merged into one protein. In such cases, the nuclear domain is cleaved out from the rest of protein upon the receipt of the signal. A drawback of this mechanism should be its irreversibility. The factor cannot be used again once it is activated. Respective anchoring proteins are not found for some fac-tors. This may simply mean that anchoring proteins has not yet been uncovered. Alternatively, anchoring proteins Figure 1
A schematic representation of the mechanism of regulated nuclear targeting. Nuclear factors are often retained in the cytoplasm by virtue of an anchoring protein (left) or an intrinsic retention domain (right). The stimulus promotes the dissociation of the nuclear factor from the complex (left) or cleave out the nuclear domain from the rest of molecule (right). After the dissociation, oligomerization of the nuclear factor is often observed. In some cases, neither anchoring protein nor retention domain is found. In such cases, stimulus might directly induce the change in the oligomeric state of the factor to induce its translocation to the nucleus (middle). A, nuclear factor; B, cytoplasmic anchoring protein; A1, nuclear domain; A2, cytoplasmic retention domain.
A
A or A1 A or A1
A1 A2
A1
A B
Cytoplasm Nucleus
Resting state
Protein cleavage Release from an
anchoring protein Stimulus
Signal
Signal Oligomerization
Entry to the nucleus
may not be required for their cytoplasmic retention. In any case, it is often found that these factors change their oligomeric states upon the stimulation, which could result in exposure of thir nuclear localization signal. Regulation mechanisms of nuclear targeting in yeast and animals are summarized in Table 1.
Regulated targeting to the nucleus in plants
Basically, nuclear import machinery in plants is similar to that of other organisms [15,16]. Signaling molecules such as G-proteins, calmodulin and kinases have been implicat-ed in plant signal transduction pathways (for example, see [17,18,19,20•,21•]). Hence, regulated nuclear targetingof signaling components should play important roles in plants as well, but our knowledge is still very limited. Recent progress in the plant field is summarized below.
COP1: a repressor of photomorphogenesis
Plants change their developmental patterns according to the light environment. In the light, plants develop in a way that photosynthesis is maximized. In contrast, plants in darkness try to reach the light using the storage materials. These two distinct developmental strategies are referred to as ‘photomorphogenesis’ and ‘skotomorphogenesis’, respectively. COP1 is a repressor of photomorphogenesis [22]. Loss of its function results in constitutive expression of photomorphogenic genes in darkness. TheCOP1gene encodes a novel protein with a RING finger and WD-40 repeat motifs which are found in various signaling mole-cules. These motifs are suggested to be involved in protein–protein interactions. The COP1 protein is local-ized to the nucleus in darkness [23]. Hence, it is speculated that COP1 acts as a repressor of photomor-phogenic genes in the nucleus. Interestingly, irradiation of the seedling with light results in relocalization of COP1 protein in the cytoplasm [23,24••]. This is consistent with
the proposed function of COP1, because the function of COP1 should be disabled in the light. Hence, nucleocyto-plasmic partitioning of COP1 appears to play a regulatory role in developmental transition from skotomorphogenesis to photomorphogenesis [23]. The slow kinetics of COP1
relocalization (about 24 hours), however, suggests that it plays a role in maintaining a committed developmental fate rather than in causing the commitment [24••].
COP1 has been shown to interact with two other nuclear proteins, CIP7 [25••] and HY5 [26••]. Both of these
pro-teins, however, are localized to the nucleus irrespective of light conditions[25••,27••]. Hence, it is not clear whether
or not these proteins contribute to nucleocytoplasmic par-titioning of COP1. COP1 is also known to interact with a cytoplasmic cytoskeletal protein, CIP1 [28]. CIP1 might be acting as a cytoplasmic anchoring protein for COP1.
GBF: a transcription factor mediating light-dependent gene expression
Expression of various genes is regulated by light [29]. G-box binding factors (GBFs) are a basic-leucine zipper class of transcription factors that bind to DNA elements called G-Boxes in the promoter regions of light-regulated genes. Using evacuolated parsley protoplasts, Harter and colleagues examined subcellular localization of GBFs [30]. The results indicate that, in darkness, GBFs are located primarily in the cytoplasm. Furthermore, irradiation of par-tially permeabilized protoplasts enhances translocation of GBFs to the nucleus.
Light-dependent nuclear localization of a GBF has been further confirmed recently [31••]; nuclear localization of
GBF2-GUS fusion protein in soybean protoplasts is enhanced by blue light. Interestingly, red light did not affect the localization, even though both blue and red light effectively induce light-dependent genes. Hence, GBFs might be involved in a signal transduction pathway specif-ic to the blue light receptors. Deletion analysis of GUS-GBF fusion proteins suggests that the region between amino acids 112 and 167 is required for cytoplas-mic retention of GBF [31••].
MAPK: a signal transduction kinase
A number of homologues of MAPKs have been reported in various plant species [32,33]. Protein kinases with Table 1
Factors that translocate to the nucleus upon stimulation.
Nuclear factor DNA binding Stimulus Regulation mechanism of nuclear targeting Nuclear hormone receptor Yes Steroid hormones Release from Hsps, dimerization
HSF Yes Heat shock Trimerization
SREBP Yes Sterol depletion Protein cleavage, dimerization
MAPK No Growth factors, stresses etc. Phosphorylation by MAPKK, release from MAPKK
NF-κB Yes Stresses Release from I-κB, dimerization
STAT Yes Cytokines Phosphorylation by Jaks, dimerization
NFAT Yes Ca2+ Dephosphorylation by calcineurin
Notch/LIN-12 No Cell–cell interactions Protein cleavage
properties of MAPK are activated or induced by various stimuli such as wounding, elicitor treatment, ABA treat-ment and auxin starvation. Ethylene and cytokinin receptors are known to be two-component receptors [34] and in yeast, signals perceived by such receptors are transduced to the MAPK pathways [35]. Furthermore, a homologue of MAPK kinase, CTR1, is implicated in the ethylene signal transduction pathway [36•].
MAPKs are known to translocate from the cytoplasm to the nucleus upon stimulation in other organisms (see above). Recently, Ligterink and colleagues identified a novel MAPK that is activated in response to fungal elicitor stim-ulation in parsley cells [37••]. The authors examined
intracellular localization of this kinase and demonstrated that it rapidly translocated to the nucleus upon stimula-tion, as expected.
HSF: a transcription factor mediating heat shock responses
HSFs translocate to the nucleus upon exposure to stress (see above). In plants, multiple HSF genes have been cloned [38] and recently, one of the tomato HSFs has been shown to translocate to the nucleus upon heat shock [39••].
It is known in other organisms that trimerization of HSFs occurs before translocation, but change in oligomeric state of the tomato HSFs was not observed [39••]. This may
indicate that translocation of plant HSFs to the nucleus is regulated differently.
phyB: a photoreceptor mediating light responses Phytochrome is a soluble chromo-protein that mediates various responses of plants to red and far-red light [40]. Phytochrome is encoded by a small multigene family. Among the members of the family, phytochrome A (phyA) is the most abundant in dark-grown seedlings. PhyA resides in the cytoplasm and is rapidly degraded upon light irradiation [41]. In contrast, phytochrome B (phyB) is light-stable. Recent analysis of mutants deficient in either phyA or phyB indicates that phyB rather than phyA mediates typical red/far-red reversible responses [42].
Intracellular localization of phyB has been examined using GUS reporter fusion proteins [43]. The results from this study indicated that a functional nuclear localization signal resides in the carboxy-terminal region of phyB. Nuclear localization of intact phyB was further confirmed by immunoblot analysis of isolated nuclei. Interestingly, dark-adaptation of the plants reduced the level of phyB in the nucleus, suggesting that phyB nuclear localization is not only light stable, but also light-dependent.
Effects of light on phyB nuclear localization has been examined in pea seedlings (A Nagatani, unpublished data). PhyB was not detected in the nuclei isolated from dark-grown seedlings. Treatment of the seedlings with a pulse of red light, however, induced accumulation of phyB in the nucleus. The level reached a plateau after a few hours, and
PhyB remained detectable in the nucleus for at least 12 hours in darkness. Far-red light exhibited an opposite effect — it accelerated disappearance of phyB from the nucleus. These observations suggest that the nuclear tar-geting of phyB is Pfr-dependent.
NIM1/NPR1: a protein involved in systemic pathogen resistance
Recently, a gene involved in the signal transduction path-way for systemic pathogen resistance responses has been cloned in Arabidopsis [44,45]. The npr(nonexpresser of PR genes) and the nim (noninducible immunity) mutants of Arabidopsis are impaired in systemic pathogen resistance. Recently, the genes mutated in the npr1 and the nim1 mutants have been identified [44,45]. Since the two genes are identical, its product is referred to as NIM1/NPR1 pro-tein in this review. Interestingly, the encoded propro-tein, NIM1/NPR1, shows homology to Iκ-B. Iκ-B binds to a transcription activator NF-κB to inhibit its entry to the nucleus (see above). Hence, NIM1/NPR1 might regulate nuclear targeting of transcription factors in plants.
Conclusions
The importance of regulated targeting to the nucleus in plant signal transduction has never been explored thor-oughly. Increasing evidence, however, suggests similarity between plant and animal signal transduction mechanisms. Rapid progress in the genome projects in Arabidopsis [46] will aid further identification of signal transduction mole-cules in plants. Hence, the subject will soon be an important issue in the plant field as well. It should also be noted that novel regulation mechanisms could emerge through such studies. For example, phytochrome is the first photoreceptor that is suggested to translocate to the nucleus upon light stimulation.
Acknowledgements
I thank A Amey for editorial assistance. Completion of this review is partly supported by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Hill C, Treisman R: Transcriptional regulation by extracellular signals: mechanisms and specificity.Cell1995, 80:199-211. 2. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono
K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM: The nuclear receptor superfamily: the second decade.Annu Rev Med
1995, 46:443-453.
3. Ghosh S, May MJ, Kopp EB: NF-kB and REL proteins:
evolutionarily conserved mediators of immune responses.Annu Rev Immunol1998, 16:225-260.
4. Robinson MJ, Cobb MH: Mitogen-activated protein kinase pathways.Curr Opin Cell Biol1997, 9:180-186.
5. Treisman R: Regulation of transcription by MAP kinase cascades.
Curr Opin Cell Biol1996, 8:205-215.
6. Fukuda M, Gotoh Y, Nishida E: Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of
nucleocytoplasmic transport of MAP kinase.EMBO J1997,
7. Brown MS, Goldstein JL: The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor.Cell1997, 89:331-340.
8. Kimble J, Simpson P: The LIN-12/Notch signaling pathway and its regulation.Annu Rev Cell Dev Biol1997, 13:333-361.
9. Struhl G, Adachi A: Nuclear access and action of Notch in vivo.
Cell1998, 93:649-660.
10. Wu C: Heat stress transcription factors.Annu Rev Cell Biol1995,
11:441-469.
11. Leonard WJ, O’Shea JJ: Jaks and STATs: biological implications.
Annu Rev Immunol1998, 16:293-332.
12. Massagu J: TGFbsignaling: receptors, transducers, and Mad proteins.Cell1996, 85:947-950.
13. Wrana J, Pawson T: Mad about SMADs.Nature1997, 388:28-29. 14. Rao A, Luo C, Hogan PG: Transcription factors of the NFAT family:
regulation and function.Annu Rev Immunol1997, 15:707-747. 15. Hicks GR, Raikhel NV: Protein import into the nucleus: an
integrated view.Annu Rev Cell Dev Biol1995, 11:155-188. 16. Merkle T, Nagy F: Nuclear import of proteins: putative import
factors and development of in vitro import systems in higher plants.Trends Plant Sci1997, 2:458-464.
17. Ma H: GTP-binding proteins in plants: new members of an old family.Plant Mol Biol1994, 26:1611-1636.
18. Millar AJ, McGrath RB, Chua NH: Phytochrome phototransduction pathways.Annu Rev Genet1994, 28:325-349.
19. Jonak C, Heberle-Bors E, Hirt H: MAP kinases: universal multi-purpose signaling tools.Plant Mol Biol1994, 24:407-416. 20. Wilson I, Vogel J, Somerville S: Signaling pathways: a common • theme in plants and animals? Curr Biol1997, 7:175-178. This review illustrates how similar plant and animal signal transduction path-ways are.
21. Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP: Signaling in • plant-microbe interactions. Science1998, 276:726-733.
This review highlights recent identification of various signaling molecules involved in the disease resistance of plants.
22. von Arnim A, Deng XW: Light control of seedling development.
Annu Rev Plant Physiol Plant Mol Biol1996, 47:215-243. 23. von Arnim A, Deng XW: Light inactivation of Arabidopsis
photomorphogenic repressor COP1 involves a cell-type-specific modulation of its nucleocytoplasmic partitioning.Cell1994,
79:1035-1045.
24. von Arnim A, Deng XW: Genetic and developmental control of •• nuclear accumulation of COP1, a repressor of
photomorphogenesis in Arabidopsis.Plant Physiol1997,
114:779-788.
Further analysis of light-dependent nucleocytoplasmic partitioning of COP1.
25. Yamamoto YY, Matsui M, Ang LH, Deng XW: Role of a COP1 •• interactive protein in mediating light-regulated gene expression in
Arabidopsis.Plant Cell1998, 10:1083-1094.
Functions and intracellular localization of a COP1 interactive protein, CIP7, are described. The paper suggests that CIP7 is involved in the activation of light-dependent genes.
26. Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, •• Deng XW: Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development.
Mol Cell1998, 1:213-222.
Direct interactions between two possible regulators of photomorphogenesis are demonstrated both in vivo and in vitro. Investigation of biochemical inter-actions between the signaling components identified through the analysis of mutants has just started. This paper is the first successful example of such efforts. Research in this direction is important for elucidation of the molecu-lar mechanism of light signal transduction.
27. Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei, N: Arabidopsis •• bZIP protein HY5 directly interacts with light-responsive
promoters in mediating light control of gene expression.Plant Cell1998, 10:673-684.
Functions and intracellular localization of HY5 are described. The paper sug-gests direct interaction of HY5 with light-responsive promoters for the first time. Although several genes involved in photomorphogenic responses have
been identified, their biochemical functions remain unclear with the excep-tion of HY5.
28. Matsui M, Stoop CD, von Arnim AG, Wei N, Deng XW: Arabidopsis
COP1 protein specifically interacts in vitro with a cytoskeleton-associated protein, CIP1.Proc Natl Acad Sci USA1995,
92:4239-4243.
29. Terzaghi WB, Cashmore AR: Light-regulated transcription.Annu Rev Plant Physiol Plant Mol Biol1995, 46:445-474.
30. Harter K, Kircher S, Frohnmeyer H, Krenz M, Nagy F, Schäfer E:
Light-regulated modification and nuclear translocation of cytosolic G-box binding factors in parsley.Plant Cell1994,
6:545-559.
31. Terzaghi WB, Bertekap RL, Cashmore AR: Intracellular localization •• of GBF proteins and blue light induced import of GBF2 fusion
proteins into the nucleus of cultured Arabidopsis and soybean cells.Plant J1997, 11:967-982.
Further analysis of light-dependent nucleocytoplasmic partitioning of GBF2 is described. Among known transcription factors that bind to the light-responsive promoters, GBF2 is the only one for which regulated nuclear tar-geting has been demonstrated.
32. Hirt H: Multiple roles of MAP kinases in plant signal transduction.
Trends Plant Sci1997, 2:11-15.
33. Mizoguchi T, Ichimura K, Shinozaki K: Environmental stress response in plants: the role of mitogen-activated protein kinases.
Trends Biotechnol1997, 15:15-19.
34. Schaller GE: Ethylene and cytokinin signaling in plants: the role of two-component systems.Essays Biochem1997, 32:101-111. 35. Wugler-Murphy SM, Saito H: Two-component signal transducers
and MAPK cascades.Trends Biochem Sci1997, 22:172-176. 36. Clark KL, Larsen PB, Wang X, Chang C: Association of the • Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS
ethylene receptors.Proc Natl Acad Sci USA1998, 95:5401-5406. This work suggests the similarity between plant and animal signal transduc-tion pathways. Recent papers on ethylene signal transductransduc-tion including this one highlight the strength of molecular genetic approaches in this field.
37. Ligterink W, Kroj T, zur Nieden U, Hirt H, Scheel D: Receptor •• mediated activation of a MAP kinase in pathogen defense of
plants.Science1997, 276:2054-2057.
Stimulus-induced nuclear targeting of MAPK in plants is demonstrated for the first time. It suggests that animal and plant MAPK activates target genes in similar ways. Since quite a few MAPK genes are known to exist in plants, similar analysis on those gene products is awaited.
38. Nover L, Scharf KD, Gagliardi D, Vergne P, Czarnecka-Verner E, Gurley WB: The Hsf world: classification and properties of plant heat stress transcription factors.Cell Stress Chap1996, 1:215-223. 39. Lyck R, Harmening U, Höhfeld I, Scharf KD, Nover L: Intracellular •• distribution and identification of the nuclear localization signals of
two tomato heat stress transcription factors.Planta1997,
202:117-125.
Detailed analysis of nuclear localization of plant HSFs. The results indicate that the mechanism of the regulation of HFS nuclear localization may differ between plants and animals, although heat-shock responses are highly con-served phenomena.
40. Fankhauser C, Chory J: Light control of plant development.Annu Rev Cell Dev Biol1997, 13:203-229.
41. Clough RC, Vierstra RD: Phytochrome degradation.Plant Cell Environ1997, 20:713-721.
42. Furuya M, Schäfer E: Photoperception and signaling of induction reactions by different phytochromes.Trends Plant Sci1996,
1:301-307.
43. Sakamoto K, Nagatani A: Nuclear localization activity of phytochrome B.Plant J1996, 10:859-868.
44. Cao H, Glazebrook J, Clarke JD, Volko S, Dong X: The Arabidopsis
NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats.Cell1997, 88:57-63. 45. Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY,
Johnson J, Delaney TP, Jesse T, Vos P, Uknes S: The Arabidopsis
NIM1 protein shows homology to the mammalian transcription factor inhibitor I k–b.Plant Cell1997, 9:425-439.