Solubility examination of palm kernel oil in supercritical
CO
2
and its correlation with solvent
density based model
Wahyu Bahari Setiantoa, Priyo Atmajia and Didi Dwi Anggorob
a Center for Agroindustrial Technology,Agency for the Assessment and Application of Technology
(Badan Pengkajian dan Penerapan Teknologi – BPPT) M.H. Thamrin 8, Jakarta 10340, INDONESIA
b
non-toxic, can be used in food-grade form for food processing. The process is considered as environmental friendly process since it does not use a chemical solvent but utilized CO2 emission as a solvent extraction. Apart from that, the conventional processing method of vegetables oil involved many operation processes i.e. refining degumming, deodorization and bleaching after screw pressing. By using SC-CO2 extraction, the oil can be fractionated and
refined by adjusting the density as a function of pressure and temperature of the CO2. This operation will eliminate
processing such as degumming, bleaching and deodorization.
For developing extraction or other processes using SC-CO2, the knowledge of solubility is essential. The design
of supercritical fluid process requires the solubilities of each component in the supercritical fluid [12]. Many of the solubility/phase equilibrium measurements have been made to meet the need for fundamental data for process design purposes as well as analytical application. The data are important in determining the optimal operating condition, the solvent to feed ratio, and the selectivity of the extracted solute in engineering-scale supercritical fluid extraction. The experimental data may also be used to develope solubility correlation model.
One of the method for determining of solut solubility in SC-CO2 is dynamic method [13, 14]. In the method,
the supercritical fluid is continually swept through the cell using a set of equipment that can ensure the equilibrium condition between supercritical CO2 and the solute. Therefore the measurement involved the formation of a saturated solution by passing the supercritical fluid over the solute in an extraction cell, decreasing the pressure to precipitate the solid or liquid solute, and analysis of the resulting solution is usually accomplished using a gravimetric method. In this research, a solubility of PKO in SC-CO2 was examined using the dynamic method.
The solubility data was fiitted with a solubility model which is based on solvent density.
2. Materials and Methods
2.1. Materials
Sample palm kernel cake (PKC) was collected from palm kernel oil (PKO) industry. The sample impurities was removed using sieved. The sample was also clasified according to the particle size by sieving. Sample with partikel
Abstract
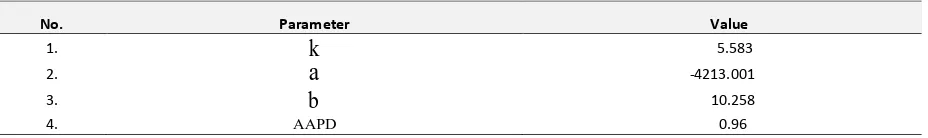
Application of supercritical carbon dioxide (SC-CO2) to vegetable oil extraction became an attractive technique due to its high solubility, short extraction time and simple purification. The method is considered as earth friendly technology due to the absence of chemical usage. Solubility of solute-SC-CO2 is an important data for application of the SC-CO2 extraction. In this work, the equilibrium solubility of the PKO in SC-CO2 has been examined using extraction curve analysis. The examinations were performed at temperature and pressure ranges of 323.15 K to 353.15 K and 20.7 to 34.5 MPa respectively. It was obtained that the experimental solubility were from 0.0160 to 0.0503 g oil/g CO2 depend on the extraction condition. The solubility experimental data was well correlated with a solvent density based model with absolute percent deviation of 0.96.
size 40-80 mesh was used in this experiment. Oil content and moisture content were determined before it were used for extraction. The oil content analysis was performed by soxhlet extraction, resulted 14.25±0.5% oil content (dry basis). Moisture content of the sample needs to be determined for material balance calculation. Carbon dioxide gas with purity of 99.95% was used as rechieved.
2.2. Solubility examination
“olu ilit easure e t a ordi g to d a i ethod a e arried out through a like e tra tio pro ess
method. The principles of SC-CO2 extraction apparatus are shown in the Figure 1. In the extraction process, firstly,
the CO2 was liquefied in the cooler and then its pressure is elevated using the pump to above its critical pressure
according to the required condition. Then, the pressurized CO2 is flown to a high pressure vessel. The vessel was
heated in a jacket heater, and the temperature is adjusted at above the critical temperature according to the required condition. The CO2, which is in a supercritical condition, then flows to the sample inside the vessel and
form a mixture/solution with the analyte in the sample matrices. The mixture then flows to the expansion valve. Due to the depressurization, the analyte will be separated from the CO2 and it was collected in a trap. The mass of
CO2 that used in the extraction is measured using a gas meter.
Figure 1. The principle of SC-CO2 extraction apparatus [15]
A set of supercritical fluid extraction apparatus with 30 mL high pressure vessel (SFT-100, Supercritical Fluid Technologies, Inc) was utilized for the experiments. Approximately 30 g of the palm kernel cake sample were loaded into the extraction cell. The solvent CO2 was saturated with the PKO by flowing it through a PKC sample
bed with 0.5-0.6 cm3/min flow rate. The solu ilit ’s were easured at te peratures fro 313.15 to 353.15 K and at pressures from 20.7 to 34.5 MPa. Mass of the extracted oil was obtained gravimetrically at certain time interval of the extraction. The weight of extracted oil at a certain interval time of extraction was measured. Experimental data were plotted as total carbon dioxide used versus grams of extracted oil. Solubility values at each temperature and pressure are equilibrium concentrations that are indicated by linear portion of the extraction curve. A linear regression was performed at the constant extraction rate (CER) for each condition. The solubility at each pressure and temperature was obtained from the slope of the fitted line on the experimental data.
3. Results and Discussion
3.1. Experimental Solubility
In the solubility examination the supercritical CO2 is allowed to flow through the solute in the cell. The flow
rate of the CO2 must be very low to ensure the saturation of CO2 with solute. Mass of the extracted solute versus
the total mass of supercritical CO2 used to dissolve the solute are plotted. In the extraction process, the extraction
system is achieved. The extract concentration at the exit of the extractor represents the solubility of the solute. Therefore, the slope of the CER is themeasured solubility at the temperature and pressure of the solute in SC-CO2
at the correspondence operation condition [13, 14, 17-19].
Solubility examination data is presented at Figure 2. At the Figure 2, It can be observed that at pressures between 24.3 and 34.5 MPa the oil solubility increases with temperature. However, increasing temperature from 313.15 to 353.15 K at lower pressure (below about 24 MPa), lowers the solubility. The trend created a solubility cross-over zone in the region of the experiment. The cross-over zone obviously could be observed by plotting the solubility against pressure (Fig. 2). The over zone occurred at the pressure of about 24 MPa. At the cross-over zone, the solubility of the oil is almost independent of temperature. However, the solubility can slightly increase or decrease with temperature depending on the pressure condition within the zone.
0.00
Figure 2. Plotting pressure-solubility of PKO - SC-CO2 . Itshows cross-over region where PKO solubility decreases with increasing
temperature at the pressure of below cross-over zone and where solubility PKO increases with increasing temperature at the pressure of above cross-over zone.
The solubility cross-over phenomenon often happen in the phase of supercritical and have been noticed by previous researchers observed effect of temperature on the solute solubility in SC-CO2. For example,
Asghari-Khiavi et al. (2004) [20] reported that cross-over zones of medroxyprogesterone and cyproterone acetate to be about 22 and above 30 MPA respectively. Vatanara et al. (2005) [21] also reported that beclomethasone diproprionate and budesonide showed the zones at 24.3 MPa. Furthermore, Setianto et al. (2009) [6] calculated phase behavior of of CNSL and CO2 system using Peng-Robinson EOS. It was reported that the cross-over zone
appeared at pressures between 12 to 20 Mpa. The inverse effect on solubility occurred possibly due to the combined effect of solvent solvating power and solute vapor pressure that are affected by temperature. In the isobaric experiment set, effect of increasing temperatures increases the solute vapor pressure and decreases of the solvent solvating power. These two competing factors letting the cross-over zone occurs in the SC-CO2-solute
systems. The zone varies depend on typical of the solutes.
3.2. Solubility Modeling
The solubility of a solute in supercritical fluid is probably the most important thermophysical property that must be determined and modeled in order to design effective supercritical fluid processes. The pressure and temperature, therefore the density of the supercritical fluid, dependence of solubility must be understood. This will allow the engineer to specify the operating conditions of unit operations such as extractors, separators, transfer line, valves and process controllers.
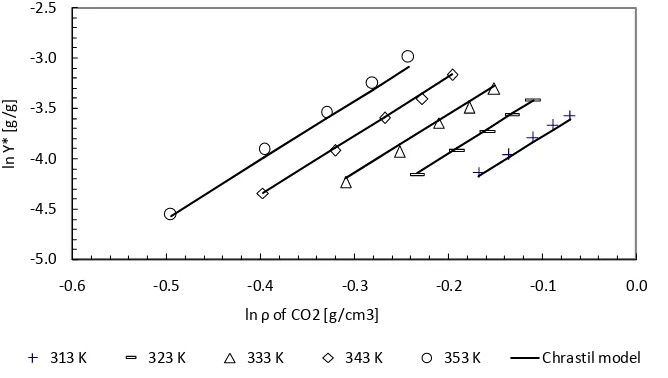
The Chrastil model is given by :
Y
= solute solubility in solvent [g/g].
= CO2 density [g/cmThe correlation considers the system as complex solution of solutes under SC-CO2 condition. The equation is based
on the fact that in an isothermal condition, plotting the natural logarithm of solute solubility in the solvent (ln
*
Y
) against natural logarithm of solvent density (ln
) yields a straight line with slopek
, which is an association constant related to the total number of molecules in the complex mixture.-5.0
Figure 3. Correlation of PKO solubility in SC-CO2 experimental data with Chrastil model.
The parameters were determined by searching the minimum error of the fitting. Average absolute percent deviation
(
AAPD
)
was used as an objective function (OF) (Equation 2).. exp.
Y = calculated solubility using the model
exp.
*
Y = experimental solubility data
n = number of data point
Table 1. Parameters of the Chrastil model for palm kernel oil solubility in supercritical CO2
Solubility of the PKO in supercritical carbon dioxide has been examined using dynamic method and has been well-correlated using empirical density based model. The oil-CO2 system showed a cross-over phenomenological.
Acknowledgement
The research funding from the Ministry of Research and Technology (Kemenristek) of the Republic of Indonesia in the Fundamental Research Incentive Program is greatly acknowledged.
References
[1] Reverchon E. 1997Supercritical fluid extraction and fractionation of essential oils and related products. The Journal of Supercritical Fluids.10(1):1-37.
[2] Reverchon E, Marrone C. 2001Modeling and simulation of the supercritical CO2 extraction of vegetable oils. The Journal of
Supercritical Fluids.19:161-75.
[3] Westerman D, Santos RCD, Bosley JA, Rogers JS, Al-Duri B. 2006. Extraction of Amaranth seed oil by supercritical carbon dioxide. The Journal of Supercritical Fluids.37(1):38-52.
[4] Zaidul ISM, Norulaini NAN, Omar AKM, Sato Y, Smith JRL. 2007. Separation of palm kernel oil from palm kernel with supercritical carbon dioxide using pressure swing technique. Journal of Food Engineering.81(2):419-28.
[5] Ramsey E, Sun Q, Zhang Z, Zhang C, Gou W. 2009. Mini-Review: Green sustainable processes using supercritical fluid carbon dioxide. Journal of Environmental Sciences. 21(6):720-6.
[6] Setianto WB, Yoshikawa S, Smith Jr RL, Inomata H, Florusse LJ, Peters CJ. 2009. Pressure profile separation of phenolic liquid compounds from cashew (Anacardium occidentale) shell with supercritical carbon dioxide and aspects of its phase equilibria. The Journal of Supercritical Fluids.48(3):203-10.
[7] Nik Norulaini NA, Setianto WB, Zaidul ISM, Nawi AH, Azizi CYM, Omar AKM. 2009. Effects of supercritical carbon dioxide extraction parameters on virgin coconut oil yield and medium-chain triglyceride content. Food Chemistry.116(1):193-7. [8] Comim SRR, Madella K, Oliveira JV, Ferreira SRS. 2010. Supercritical fluid extraction from dried banana peel (Musa spp.,
genomic group AAB): Extraction yield, mathematical modeling, economical analysis and phase equilibria. The Journal of Supercritical Fluids. 54(1):30-7.
[9] Prado JM, Prado GHC, Meireles MAA. 2011. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. The Journal of Supercritical Fluids. 56(3):231-7.
[10] Ansari K, Goodarznia I. 2012. Optimization of supercritical carbon dioxide extraction of essential oil from spearmint (Mentha spicata L.) leaves by using Taguchi methodology. The Journal of Supercritical Fluids.67(0):123-30.
[11] Patinha DJS, Domingues RMA, Villaverde JJ, Silva AMS, Silva CM, Freire CSR, et al. 2013. Lipophilic extractives from the bark of Eucalyptus grandis x globulus, a rich source of methyl morolate: Selective extraction with supercritical CO2.
Industrial Crops and Products. 43(0):340-8.
[12] Prausnitz JM, Lichtenthaler RN, de Azevedo EG. Molecular Thermodynamics of Fluid-Phase Equilibria. 3 ed. Upper Saddle River, NJ: Prentice-Hall, Inc.; 1999.
[13] Sovova H. 2005. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. The Journal of Supercritical Fluids.33(1):35-52.
[14] Danielski L, Campos LMAS, Bresciani LFV, Hense H, Yunes RA, Ferreira SRS. 2007. Marigold (Calendula officinalis L.) oleoresin: Solubility in SC-CO2 and composition profile. Chemical Engineering and Processing.46(2):99-106.
[15] Smith Jr RL, Malaluan RM, Setianto WB, Inomata H, Arai K. 2003. Separation of cashew (Anacardium occidentaleL.) nut shell liquid with supercritical carbon dioxide. Bioresource Technology. 88(1):1-7.
[17] Favati F, King JW, Mazzanti M. 1991. Supercritical carbon dioxide extraction of evening primrose oil JAOCS, Journal of the American Oil Chemists' Society. 68:422-7.
[18] Sovova H. Rate of the vegetable oil extraction with supercritical CO2--I. 1994. Modelling of extraction curves. Chemical
Engineering Science.49(3):409-14.
[19] Ferreira SRS, Nikolov ZL, Doraiswamy LK, Meireles MAA, Petenate AJ. 1999. Supercritical fluid extraction of black pepper (Piper nigrun L.) essential oil. Journal of Supercritical Fluids, The.14(3):235-45.
[20] Asghari-Khiavi M, Yamini Y, Farajzadeh MA. 2004. Solubilities of two steroid drugs and their mixtures in supercritical carbon dioxide. The Journal of Supercritical Fluids. 30:111-7.
[21] Vatanara A, Najafabadi AR, Khajeh M, Yamini Y. 2005. Solubility of some inhaled glucocorticoids in supercritical carbon dioxide. The Journal of Supercritical Fluids. 33:21-5.
![Figure 1. The principle of SC-CO2 extraction apparatus [15]](https://thumb-ap.123doks.com/thumbv2/123dok/1128607.652879/2.595.145.461.307.455/figure-principle-sc-co-extraction-apparatus.webp)