Thermally activated iron containing layered double hydroxides as potential
catalyst for N
2
O abatement
q
Tatjana J. Vulic
a,⇑, Andreas F.K. Reitzmann
b, Károly Lázár
caUniversity of Novi Sad, Faculty of Technology, bul. Cara Lazara 1, 21000 Novi Sad, Serbia
bSued-Chemie AG, Research and Development Catalysis and Energy, Waldheimerstr. 13, 83052 Bruckmühl, Germany
cInstitute of Isotopes of the Hungarian Academy of Sciences, Department of Catalysis and Tracer Studies, P.O. Box 77, Budapest H-1525, Hungary
h i g h l i g h t s
"Reduction behavior mainly influences catalytic activity in N2O reduction with NH3.
"Extended M(III) substitution weakens Mg–Al–Fe–O interactions.
"Extended M(III) substitution improves catalytic behavior.
"LDH matrix with M(III) near the limit for incorporation gives the best results.
a r t i c l e
i n f o
Article history: Available online xxxx
Keywords:
Hydrotalcite like materials Mg–Al–Fe mixed oxides Mössbauer spectroscopy TPR
Nitrous oxide
a b s t r a c t
Layered double hydroxides (LDHs) and derived mixed oxides with different Mg/Al/Fe contents were investigated. Two super-saturation precipitation methods were used for the synthesis of LDHs with gen-eral formula [Mg1xM(III)x(OH)2](CO3)x/2mH2O where M(III) presents Al and/or Fe. The content of triva-lent ionsx= M(III)/[M(II) + M(III)], was varied between 0.15 <x< 0.7. Such a wide range of trivalent ions was chosen with the aim to induce the formation of different multiphase mixed oxides. Iron was intro-duced as constituent metal in order to obtain redox properties. LDHs and their derived mixed oxides were characterized with respect to their crystalline structure (XRD), thermal stability (TG/DTA), textural (N2 adsorption), redox (H2TPR) and acid properties (NH3TPD) as well as the nature of the iron species (Mössbauer spectroscopy). Catalytic behavior was studied in two test reactions: N2O decomposition and reduction with NH3. It has been demonstrated that extended M(III) substitution influences the struc-ture and surface properties of Mg–Al–Fe LDHs and derived mixed oxides, weakens Mg–Al–Fe–O interac-tions and improves catalytic behavior correlated with the presence of Fe–O–Fe–O–Fe entities providing possibility for facilitated extraction of oxygen with simultaneous redox Fe3+
MFe2+conversion. The
cat-alytic behavior is mainly determined by redox properties, nature of iron species in mixed oxides and by structural properties of initial LDHs. The best catalytic results were obtained when the amount of M(III) was near the limit for the incorporation into LDH matrix.
Ó2012 Elsevier B.V. All rights reserved.
1. Introduction
Layered double hydroxides (LDHs) and their derived mixed oxides reached growing attention in research and engineering because of possibility to vary a large number of synthesis parame-ters and in that way modify and tailor various properties. Their application ranges from catalysts and catalyst supports in organic synthesis, (photo) degradation of organic wastes, greenhouse gas
control emission and H2production, anion exchangers, adsorbents, fillers (stabilizers for polymers) to medical-pharmaceutical appli-cations by making use of their specific properties[1–6].
LDHs are anionic clay materials and also known as hydrotalcite-like materials have layered structure which consists of Brucite-hydrotalcite-like layers with octahedrally centred M(II) ions. Partial isomorphous substitution of M(II) with M(III) ions creates positive charge which is compensated with different anions present in the interlayer re-gion together with water[3,5,7]. The general formula of LDH is: M(II)1xM(III)x(OH)2(An)x/nmH2O, where M(II) is a divalent cat-ion, M(III) is a trivalent catcat-ion, Anis anion (usually carbonate)
andx= M(III)/[(M(II)+M(III)]. It has been reported that the value of x between 0.2 and 0.4 is optimal for the formation of single
1385-8947/$ - see front matterÓ2012 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.cej.2012.06.152 q
Work was carried out in Karlsruhe Institute of Technology (KIT), Institute of Chemical Process Engineering, Fritz-Haber-Weg 2, D-76131 Karlsruhe, Germany.
⇑ Tel.: +381 21 485 3750; fax: +381 21 450 413.
E-mail addresses:[email protected](T.J. Vulic),andreas.reitzmann@sud-chemie. com(A.F.K. Reitzmann),[email protected](K. Lázár).
Contents lists available atSciVerse ScienceDirect
Chemical Engineering Journal
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / c e j
LDH phase and by exceeding this range, hydroxides or other com-pounds may be formed[3]. The nature and amount of M(II) and M(III) ions influences the redox properties of LDHs and derived mixed oxides. After thermal treatment of LDHs, mixed oxides are formed with homogeneous interdispersion of constituting ele-ments, larger surface area, developed porous structure, less diffu-sion resistance than LDHs and abundant acid and basic sites, which makes them more interesting as catalysts or catalyst precur-sors[3,8].
The potential of these materials has also been studied in waste gas catalysis, namely in N2O abatement. Different transition and noble metal containing LHD derived mixed oxides have been re-ported to be active in N2O and NOxdecomposition with different
combinations of divalent (Mg, Co, Ni, Mn, Cu, Ca, Zn) and trivalent (Al, La, Rh) ions[9–14]. Zeolites, exchanged with transition metal, especially with iron, showed high activity in decomposition of N2O [15]. Iron exchanged zeolites exhibit also noticeable efficiencies in the selective catalytic reduction of nitrous oxide with NH3 and with various hydrocarbon reducing agents[16–18]. Therefore, in our investigations iron was chosen as an active component to study its activity in N2O abetment reactions. Fe containing LDH de-rived mixed oxides were reported to be catalytically active in var-ious reactions. Mg–Al–Fe and Mg–Fe mixed oxides were successfully applied in Fischer–Tropsch synthesis[19], in catalytic dehydrogenation of ethylbenzene[20]and in ethylbenzene dehy-drogenation in presence of carbon dioxide [21,22]. In addition, Mg–Al–Fe–LDHs themselves (without calcination to mixed oxides) have been shown to be efficient catalyst for the reduction of 4-nitrotoluene using phenylhydrazine or hydrazine hydrate as reduc-ing agent[23]and in the reduction of aromatic nitro compound with hydrazine hydrate[24].
For the synthesis of Mg–Al–Fe and Mg–Fe layered double hydroxides two coprecipitation methods, low supersaturation, LS, and high supersaturation, HS, were chosen. The content of trivalent ions was varied in a wide range between 0.15 <x< 0.7 with the intention to induce the formation of different LDHs and, after ther-mal treatment, different multiphase mixed oxides. The objective was to study the properties of different iron containing LDHs and derived mixed oxides in correlation to their performance in two re-dox processes: the decomposition of N2O, and the reduction of N2O with ammonia.
2. Materials and methods
2.1. Synthesis
Two different coprecipitation methods, low supersaturation, LS, and high supersaturation, HS, were chosen for the synthesis of LDHs. In the HS method, the solution containing magnesium, alu-minium and/or iron nitrate salts was quickly added to the second solution containing Na2CO3and NaOH. For the LS synthesis, the Mg–Al–Fe containing solution was added drop wise at a constant rate into 1 dm3of distilled water and the pH of the solution was maintained between 9.6 and 9.9 by the simultaneous addition of the second solution containing Na2CO3and NaOH. In both cases, the reaction solution was vigorously stirred, the samples were aged, washed and filtered, dried for 24 h, at 100°C in air, and after-wards calcined for 5 h, at 500°C in air. A detailed explanation of synthesis methods and thermal activation is given elswere[25].
Two series of Mg–Al–Fe LDHs with different Mg/Al ratio, wide range of trivalent ions between 0.15 <x< 0.7 and 5 mol% of iron was prepared. Besides that, Mg–Fe LDHs with higher iron content (30 mol%) and without aluminum were also synthesized.
Samples were denoted according to the synthesis method used (HS or LS) and the initial molar metal ratio. For example,
LS-Mg50Al45Fe5 is the denotation for the sample synthesized by the LS method having following initial metal amounts: 50 mol% of magnesium, 45 mol% of aluminium and 5 mol% of iron.
2.2. Characterization
The X-ray diffraction measurements were performed in a Sie-mens D500 X-ray diffractometer (Cu Ka radiation, =0.154 nm,
45 kV, 25 mA) in 2 range from 3°to 63°. Atomic absorption spec-troscopy, AAS using Hitachi Z-6100 instrument was used for the elemental chemical analysis of constituent metals (Mg, Al and Fe) in calcined samples. A semi-quantitative chemical analysis of calcined sample surface was performed by JEOL, JSM-460 LV instrument equipped with energy dispersive spectroscopy, EDS, Oxford Instruments INCA X-sight system operating at 25 kV.
TG/DTA thermal analysis of all synthesized samples was carried out in Baehr STA503 instrument from ambient temperature to 1000°C with the heating rate of 5°C min1, in static air atmo-sphere. The BET surface areas and pore radius distributions were determined by N2 sorption at 196°C in a Micromeritics ASAP 2010 instrument.
Acidity of the calcined samples was determined by temperature programmed desorption (TPD) of ammonia in a Micromeritics
AutoChem 2910 apparatus using 10 vol% NH3 in He and ca.
200 mg of calcined samples, flow rate of gas mixture of 25 cm3min1 and heating rate of 10°C min1 in temperature range from 50°to 600°C. The gases evolved during TPD measure-ments were analyzed using Pfeiffer Vacuum QMS422 Mass Spec-trophotometer. Before TPD measurements the catalysts were preheated in a He gas flow (25 cm3min1) to 500°C and kept at that temperature for 1 h. Then the flow was switched to 10% vol NH3 in He (25 cm3min1) and the temperature was lowered to 50°C (20°C min1). The catalysts were than purged from physi-sorbed ammonia at 50°C in a He gas flow (25 cm3min1) for 1 h. Mössbauer spectra were recorded with a KFKI spectrometer at ambient temperature in constant acceleration mode. The values of isomer shift,IS,are given with respect to metallic
a
-iron. Theestimated accuracy of positional parameters (IS, and quadrupole splitting,QS) is c.a. ±0.03 mm s1. Relative intensity,RI,in% was calculated as relative contribution of the given component to the full area of the spectrum. Intensity per base line,I/BL,represents the total area of spectrum (sum of components) related to the base line. Mössbauer spectra were recorded for each sample in a se-quence of three measurements: (i) as synthesized samples; (ii) samples calcined at 500°C and (iii) after in situ treatment with CO at 340°C for 2 h.
Temperature programmed reduction (TPR) was conducted in a Micromeritics AutoChem 2910 apparatus using ca. 200 mg of cal-cined samples, flow rate of gas mixture (5% vol H2 in N2) of 20 cm3min1and heating rate of 10°C min1in temperature range from 25°to 1000°C. Before the TPR measurements the samples were preheated in a nitrogen gas flow (20 cm3min1) from ambi-ent temperature to 500°C (30°Cmin1) and then cooled to 50°C.
2.3. Catalytic tests
Catalytic properties were studied in a quartz fixed bed flow reactor (ø= 8 mm,L= 19 cm). The experimental conditions were: temperature from 300 to 500°C, pressure 101 kPa, reactant con-centrations of N2O 1000 ppm (vol.) and of NH3 1000 ppm (vol.), modified space velocity, GHSVmod from 2.17 to 6:25 cm
3g1 ðcatÞs1
(NTP).
Before tests, all catalysts were first heated and held 2 h at 500°C in a He stream. The measurements were performed at dif-ferent reaction temperatures starting at 500°C and lowering it
stepwise by 25°C. The temperature and the gas flows were
maintained constant until reaching steady state (waiting peri-od > 1 h). The concentrations were measured at each reaction tem-perature after reaching steady state. The concentrations of gas mixtures N2O, NH3and He, before and after reaction, were mea-sured with nondispersive infrared spectroscope BINOS Leybold Heräus.
3. Results and discussion
3.1. Structural and elemental analysis
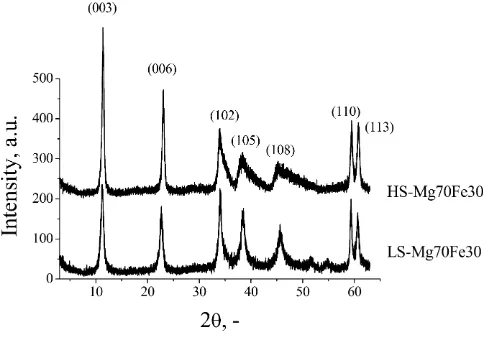
It was reported in our previous work that all Mg–Al–Fe copre-cipitation products have XRD patterns typical for LDH compounds, that HS samples have partially disordered structure, particularly in stacking of the layers and that extended M(III)ion substitution (xP0.5) leads to the formation of additional aluminum hydroxide,
Al(OH)3 – Bayerite phase[25]. Also, after thermal treatment at 500°C layered structure was destroyed and only the presence of Mg,M(III) mixed oxide phase was detected. The XRD patterns of the Mg–Fe as-synthesized samples are presented inFig. 1. Sharp and symmetric reflections from (0 0 3), (0 0 6), (1 1 0) and (1 1 3) planes were observed as well as broad, non-symmetric reflections from (1 0 2), (1 0 5) and (1 0 8) planes. The lattice parameters were calculated for a hexagonal unit cell on the basis of rhombohedral R–3 m symmetry. Basal spacing d0=d003 was calculated as the thickness of one layer constituted of one brucite-like sheet and one interlayer, cation–cation distance within the brucite-like layer asa0= 2d110and lattice parameterc0asc0= 3d003. The phase composition and lattice parameter of Mg–Fe–LDHs are given in Ta-ble 1and are in good agreement with results published by other authors[26–28].
Elemental chemical analysis of constituent metals of the Mg– Al–Fe samples is also reported in our previous work[25]and the results of the Mg–Fe samples are given inTable 1. The AAS analysis showed good agreement between initial amounts of Mg and Fe and the bulk metal amount. Nevertheless, the surface-enhanced information about metal composition obtained by the EDS analysis revealed higher presence of iron on the surface of sample HS-Mg70Fe30 when compared with the sample LS-Mg70Fe30.
3.2. Thermal analysis
A detailed thermal analysis of Mg–Al–Fe samples is already re-ported[25]. The data from TG/DTA analysis of Mg–Fe samples in comparison to the Mg–Al–Fe samples having the same M(III)ion amount is given inTable 2. Samples have two endothermic
transi-tions with corresponding mass losses typical for the LDHs, the first from the loss of physisorbed and interlayer water and the second due to the loss of hydroxyl groups and interlayer anions. A third endothermic transition without significant mass loss is also ob-served at temperatures higher that 700°C indicating stoichiome-tric spinel phase and single magnesium-oxide phase formation
[25]. Both Mg–Fe samples have lower temperatures of first two endothermic transitional stages than corresponding Mg–Al–Fe samples, indicating lower thermal stability of Mg–Fe LDHs, and also smaller mass losses, indicating smaller amount of physisorbed and interlayered water in Mg–Fe samples. Nevertheless, sample HS-Mg70Fe30 has to some extent higher thermal stability than sample LS-Mg70Fe30 since it has higher temperatures of both transitional stages.
3.3. Textural analysis
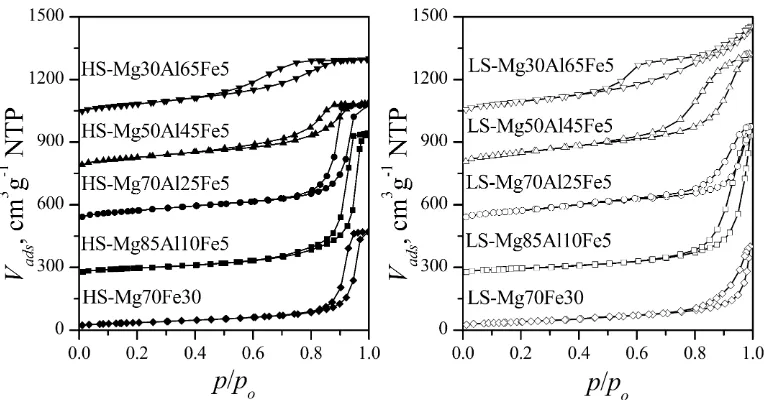
Nitrogen adsorption isotherms of all Mg–Al–Fe samples cal-cined at 500°C are presented in Fig. 2. The isotherms of Mg–Fe samples correspond to the isotherms of Mg–Al–Fe samples iso-therms with same M(III) amount and the same synthesis method, only the amount of nitrogen adsorbed is smaller in case of Mg– Fe samples. In accordance to IUPAC classification[29], all HS sam-ples have adsorption isotherms of the Type IV, with the hysteresis loop and the plateau at high p/po, typical for mesoporous oxide
catalysts and supports. The samples Mg85Al10Fe5, HS-Mg70Al25Fe5 and HS-Mg70Fe30 have hysteresis loop Type H1 rep-resentative of an adsorbent with a narrow distribution of relatively uniform cylindrical mesopores. The samples HS-Mg50Al45Fe5 and HS-Mg30Al65Fe5, have somewhat different shape of hysteresis loop, belonging to Type H2 associated with a more complex pore structure with narrow slit-shaped pores, probably due to the for-mation of additional Bayerite phase in these samples.
All LS samples have adsorption isotherms of the Type II and hys-teresis loop H3 with no limiting adsorption at high relative pres-sure and no well-defined mesopore structure, typical for micro and mesoporous materials with plate like aggregates and nonuni-form slit-shaped pores, such as clays. The sample LS-Mg30Al65Fe5 has similar but somewhat different shape of hysteresis loop, com-pared to other LS samples. It has the combination of H2 and H3 hysteresis loop which could be explained with the presence of the additional Bayerite phase similar as in case of multiphase HS samples.
The most commonly used procedure for determination of mesopore size distribution is the BJH method, proposed by Barrett, Joyner and Halenda, based on the notional emptying of the pores – desorption branch. The steep region of the desorption branch for the hysteresis loops Type H2 and H3 (obtained for the samples with extended M(III) substitution) is a feature depending on the nature of adsorptive rather then the distribution of pore size and also the reason not to take this curve region for the calculation of mesopore size distribution[29]. In these cases adsorption branch corresponds better to equilibrium and should be used for calcula-tions of pore size distribution[30]. The distribution based on the desorption data is indicative of the pore opening/mouth while the adsorption data provides information of the actual (interior) pore size. To enable the comparison of samples with different M(III) content and taking into account that the samples with ex-tended M(III) substitution have this type of hysteresis loop, the adsorption branch was chosen for the calculation of pore size dis-tribution using BJH method (Figs. 3 and 4).
The BET surface areas of synthesized layer double hydroxides are between 70 and 100 m2g1. The BET surface area values of cal-cined samples are given inTable 3. The calcination results in in-crease of BET surface area caused by the formation of small venting holes (crater) at the crystal surface built during the
ex-Fig. 1.XRD patterns of the Mg–Fe as-synthesized samples.
T.J. Vulic et al. / Chemical Engineering Journal xxx (2012) xxx–xxx
haust of water and CO2[31]. The increase in M(III) ion content in-creases in general the BET surface area evidenced also in pore size distribution.
With the increase of M(III) ion content, the bimodal size distri-bution changes simultaneously (Figs. 3 and 4), the fraction of small mesopores (2–3 nm) increases and the fraction of bigger
mesop-ores shifts from 30 nm to 6 nm. The changes in the pore size distri-bution could be related to the initial ordering of the LDH structure prior to calcination. The XRD analysis showed that with the increasing amount of aluminum in the LDH samples, the intensity of characteristic XRD reflections decreases and diffraction lines broaden, suggesting a decrease in the ordering of the LDH
struc-Table 1
Precipitation products phase composition and lattice parameters, initial metal amounts and the metal amounts measured by AAS and EDS of Mg–Fe samples.
Sample XRD analysis Initial amounts (mol%) AAS amounts (mol%) EDS amounts (mol%)
Phase d0(nm) a0(nm) c0(nm) Mg Fe Mg Fe Mg Fe
HS-Mg70Fe30 LDH 0.775 0.311 2.324 70 30 72.2 27.8 66.7 33.3
LS-Mg70Fe30 LDH 0.785 0.311 2.355 70 30 72.6 27.4 80.3 19.7
Table 2
Data from TG/DTA analysis: mass loss of the firstm1, secondm2and third transition stagem3,total mass lossmtot, temperatures of endothermic peaks corresponding to the first T1, the secondT2and to the third transitionT3.
m1(%) m2(%) m3(%) mtot(%) T1(°C) T2andT02(°C) T3(°C)
HS-Mg70Fe30 17 17 5 39 212 336, 379 >1000
HS-Mg70Al25Fe5 19 20 4 43 236 418 827
LS-Mg70Fe30 15 17 5 37 179 324, 373 >1000
LS-Mg70Al25Fe5 18 21 4 43 213 356, 405 691
Fig. 2.Adsorption isotherms of Mg–Al–Fe samples after calcination.
Fig. 3.Pore size distribution of Mg–Al–Fe samples after calcinations.
ture. The formation of additional Bayerite phase causes a decrease in the amount of carbonate bounded in LDH interlayer and conse-quently a decrease in surface area of the calcined samples. Lower surface area of HS samples with extended M(III)ion substitution (xP0.5) when compared to corresponding LS samples, which have
no Bayerite phase of smaller amount of it, also supports this state-ment. The increase of surface area with increasing amount of alu-minum in the LDH samples eventually leads to increased amounts of alumina after calcination, which contributes to the higher sur-face area compared to the Mg/M(III) mixed oxides.
The pore size distribution of Mg–Fe samples is similar to the pore size distribution of the Mg–Al–Fe samples with the same amount of M(III) ions samples (Fig. 4). The Mg–Fe samples have smaller fraction of small mesopores (ca. 2.5 nm) and consequently lower surface area, which could be explained with the smaller amount of physisorbed and interlayer water in these samples de-tected by TG analysis (Table 2).
3.4. Acidic properties
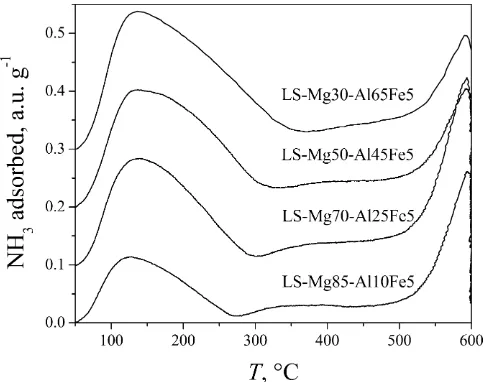
Ammonia TPD profile of all LS-Mg–Al–Fe samples are presented in Fig. 5. All other samples have similar TPD profiles. Simulta-neously with TPD measurement the evolved gases were analyzed by mass spectrometry, MS. The MS analysis showed that the first peak with temperatures between 120 and 140°C corresponds to the amount of ammonia desorbed and that the second one at
tem-peratures around 590°C corresponds to the amount of water
desorbed.
The conclusions about strength and the amount of acid sites, presented inTable 3, were taken considering the temperature of
the first peak maxima and its area. The amount of iron in samples decreases acidity compared to samples with aluminum. The in-crease of M(III) content inin-creases also the acidity. All samples have acid sites of similar strength, although the strength of acid sites in LS-Mg–Al–Fe samples is slightly higher than in HS-Mg–Al–Fe sam-ples. According to reported findings[32,33]LDHs have Lewis acid sites with medium–high acidic strength.
Fig. 4.Pore size distribution of Mg–Al–Fe and Mg–Fe samples after calcinations havingx= 0.3.
Table 3
The initial M(III) molar ratio,x, BET surface area of calcined samples,S, the temperatures of the first,T1-TPRand second TPR peak maxima,T2-TPR, the temperature of the first TPD peak maximaT1-TPDand area of the first TPD peakA1-TPD.
Sample x, – S(m2g1) T
1-TPR(°C) T2-TPR(°C) T1-TPD(°C) A1-TPD(a.u. g1)
HS-Mg70Fe30 0.3 126 385 625 124 130
HS-Mg85Al10-Fe5 0.15 167 450 789 129 122
HS-Mg70Al25Fe5 0.3 264 473 737 136 229
HS-Mg50Al45Fe5 0.5 281 409 764 130 243
HS-Mg30Al65Fe5 0.7 303 442 762 129 292
LS-Mg70Fe30 0.3 142 385 736 122 146
LS-Mg85Al10-Fe5 0.15 163 448 816 125 101
LS-Mg70Al25Fe5 0.3 266 466 745 136 187
LS-Mg50Al45Fe5 0.5 362 409 760 139 239
LS-Mg30Al65Fe5 0.7 344 454 746 139 300
Fig. 5.TPD profiles of LS-Mg–Al–Fe samples. T.J. Vulic et al. / Chemical Engineering Journal xxx (2012) xxx–xxx
3.5. Mössbauer spectroscopy analysis
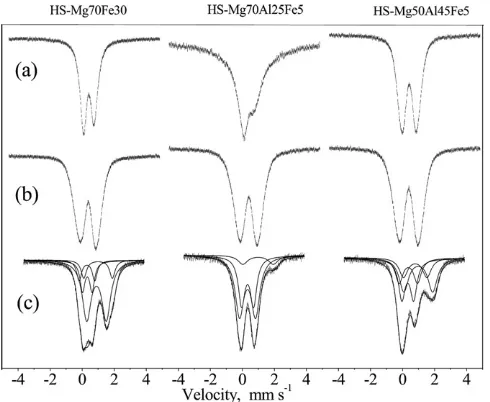
The Mössbauer spectra of the HS and LS samples are shown in the as synthesized, calcined and CO treated sequence in Figs. 6 and 7, the data obtained from the corresponding evaluations are presented inTables 4–6.
The as synthesized samples exhibit asymmetric Fe3+doublet. This asymmetry in the doublet of the as synthesized materials has been reported for other LDHs as well. The asymmetry can be correlated either with relaxation effects emerging between distant and separated Fe3+ions at lower iron content[34], or by distribu-tion of quadrupole splitting at higher iron contents, as suggested in
[26,35]. The presence of asymmetric doublet is prevalent in the x =0.3 samples, thus fitting with regard to relaxation phenomena was applied for them. For samples of larger extent of substitution (x =0.5) existence and separation of two different iron environ-ments are assumed, in correspondence with results of previous studies where Fe3+components with a smaller and a larger qudru-pole splittings are distinguished[35–37]. These two Fe3+ compo-nents are shown as Fe3+ (1) and Fe3+ (2) in the corresponding tables. An additional parameter,I/BLis also displayed. This param-eter is the relative intensity of the total spectrum related to the base line (spectral area in count numbers related to that of the base line), it carries information on the average value of the probability of the Mössbauer effect and is strongly correlated to the bonding strength of the iron species present in the sample[38].
After calcination at 500°C, profound changes in structure are observed: iron ions became more strongly bonded, as the signifi-cant increase of theI/BL parameter attests. The ca. 50% increase is significant and it clearly demonstrates that upon calcination the open, layered structure transforms to a close, three dimen-sional matrix. LDHs with similar compositions may be transformed to fluorite-type periclase or spinel oxides, with corresponding Mössbauer parameters different from those of LDHs[20,34]. Our spectra were decomposed to three components Fe3+(3), Fe3+(4) and Fe3+(5). The first of them can probably be assigned to the com-ponent of periclase structure [20], the second and third can be attributed to the different sites in the spinel matrix[20,24,26]. It should be emphasized here that the method of Mössbauer spec-troscopy provides local information from the very vicinity of iron.
Thus, e.g. spinel structure may already appear in the local environ-ment of iron at the 500°C treatment, however the transformation resulting in the formation of separate extended spinel phase pro-ceeds only at 800°C as TG and XRD studies revealed.
The third stage, the in situ treatment with CO at 340°C, was
intended originally to demonstrate the existence of Fe3+–
O–Fe3+–O–Fe3+chains, with the assumption that reduction of iron with CO may proceed via extraction of oxygen from the chain with simultaneous reduction of two neighboring Fe3+ to Fe2+. This assumption seems to be proven in different extents. This kind of reduction is less expressed in the samples of low M(III) substitu-tion (x= 0.3), it reaches only 10% for the HS-Mg70–Al25–Fe5 and 23% for the LS-Mg70–Al25–Fe5 sample. The increase in aluminum amount increases also the extent of reduction, as the results from
x= 0.5 samples measurements show. The Fe–O–Fe–O chains are
probably originated from the presence of a minor ferrihydrite phase formed already at the synthesis of LDHs. This ferrihydrite may probably be incorporated into the bayerite phase detected by XRD. Its assignment by the Mössbauer method is less successful, since its parameters are similar to those of Fe3+(1). Further, the brownish color of the samples is a convincing indication for the presence of an amorphous ferric oxihydroxide. The same experi-ences were collected and the assumptions proven by low temper-ature (268.8°C) Mössbauer measurements[24,36]. The effect of the CO treatment is not restricted only to the assumed ferrihy-drite-bayerite phase, the spinel oxide is also modified. For instance, the Fe3+(5) component fully disappears (is reduced to Fe2+), and the quadrupole splittings of the Fe3+(3) and Fe3+(4) components are noticeably reduced.
3.6. Redox properties
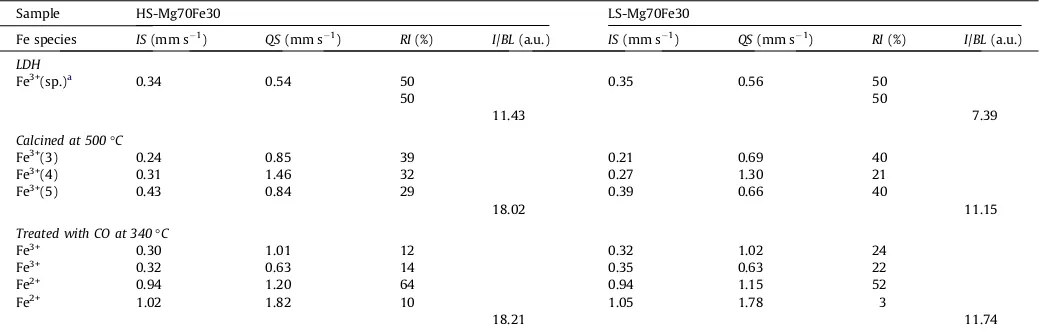
The TPR measurements of all iron containing materials have
two characteristic peaks at temperatures lower than 900°C
(Fig. 8, e.g. HS samples). For all of the samples, TPR signal did not reach the base line until 1000°C and presumably the complete reduction of iron was not achieved. The hydrogen consumption was represented per mol iron to enable the comparison of samples with different iron content. All HS and LS samples with the same initial chemical composition have almost the same TPR profiles
Fig. 6.Mössbauer spectra of samples HS-Mg70Fe30 (right), HS-Mg70Al25Fe5 (middle) and HS-Mg50Al45Fe5 (left) recorded: (a) as synthesized; (b) calcined at 500°C and (c) treated with CO at 340°C. Decomposition of spectra containing Fe2+
components is also displayed in row (c) for illustration.
Fig. 7.Mössbauer spectra of samples LS-Mg70Fe30 (right), LS-Mg70Al25Fe5 (middle) and LS-Mg50Al45Fe5 (left) recorded: (a) as synthesized; (b) calcined at 500°C and (c) treated with CO at 340°C Decomposition of spectra containing Fe2+
components is also displayed in row (c) for illustration.
Table 4
Mössbauer parameters and assignments of iron species recorded for samples HS-Mg70Al25Fe5 and LS-Mg70Al25Fe5 as synthesized; calcined at 500°C and after treatment with CO at 340°C.
Sample HS-Mg70Al25Fe5 LS-Mg70Al25Fe5
Fe species IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.) IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.)
LDH
Fe3+(sp)a 0.34 0.58 50 0.34 0.67 50
50 50
7.36 8.17
Calcined at 500°C
Fe3+(3) 0.24 0.81 44 0.23 0.79 28
Fe3+(4) 0.30 1.47 25 0.29 1.45 45
Fe3+(5) 0.44 0.80 31 0.43 0.76 27
13.66 13.86
Treated with CO at 340°C
Fe3+ 0.32 1.12 55 0.31 1.10 53
Fe3+ 0.33 0.69 35 0.33 0.64 24
Fe2+ 1.00 1.60 18
Fe2+ 1.02 1.92 10 1.00 2.25 5
14.07 14.00
a Special fitting: the doublet is fitted assuming the same intensity but the same line width is not imposed (as otherwise is the common case at all the other fits).
Table 5
Mössbauer parameters and assignments of iron species recorded for samples HS-Mg50Al45Fe5 and LS-Mg50Al45Fe5 as synthesized; calcined at 500°C and after treatment with CO at 340°C.
Sample HS-Mg50Al45Fe5 LS-Mg50Al45Fe5
Fe species IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.) IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.)
LDH
Fe3+(1) 0.27 0.68 46 0.34 0.61 58
Fe3+(2) 0.36 1.19 16 0.35 1.00 42
Fe3+(5) 0.43 0.68 37
10.89 8.23
Calcined at 500°C
Fe3+(3) 0.24 0.85 39 0.25 0.81 42
Fe3+(4) 0.31 1.47 32 0.31 1.42 35
Fe3+(5) 0.43 0.84 29 0.44 0.80 23
14.69 11.40
Treated with CO at 340°C
Fe3+ 0.36 1.19 21 0.35 1.22 30
Fe3+ 0.32 0.78 29 0.36 0.72 41
Fe2+ 0.82 1.46 18 0.91 1.61 20
Fe2+ 1.05 1.81 32 0.96 2.23 9
14.82 12.21
Table 6
Mössbauer parameters and assignments of iron species recorded for samples HS- Mg70Fe30 and LS- Mg70Fe30 as synthesized; calcined at 500°C and after treatment with CO at 340oC.
Sample HS-Mg70Fe30 LS-Mg70Fe30
Fe species IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.) IS(mm s1) QS(mm s1) RI(%) I/BL(a.u.)
LDH
Fe3+(sp.)a 0.34 0.54 50 0.35 0.56 50
50 50
11.43 7.39
Calcined at 500°C
Fe3+(3) 0.24 0.85 39 0.21 0.69 40
Fe3+(4) 0.31 1.46 32 0.27 1.30 21
Fe3+(5) 0.43 0.84 29 0.39 0.66 40
18.02 11.15
Treated with CO at 340°C
Fe3+ 0.30 1.01 12 0.32 1.02 24
Fe3+ 0.32 0.63 14 0.35 0.63 22
Fe2+ 0.94 1.20 64 0.94 1.15 52
Fe2+ 1.02 1.82 10 1.05 1.78 3
18.21 11.74
a Special fitting: the doublet is fitted assuming the same intensity but the same line width is not imposed (as otherwise is the common case at all the other fits). T.J. Vulic et al. / Chemical Engineering Journal xxx (2012) xxx–xxx
with small differences in temperatures of both peak maxima listed inTable 3.
The Mg–Fe samples with 30% of iron have two separated peaks, first sharp and symmetrical and second broad and nonsymmetri-cal. This type of reduction behavior was already reported for Mg– Fe–LDH derived mixed oxides with different iron amount between 10 and 50%[19]and with 50% of iron[39]concluding that the pres-ence of Mg2+cations retards the reduction of iron and suggesting that the two reduction peaks in TPR profiles correspond to the sequentional reduction of iron species from Fe3+ to Fe2+ in first stage and from Fe2+ to Fe0 in second, presented with following equations:
MgFe2IIIO4þH2¼Mg1xFexIIOþH2O ð1Þ
Mg1xFexIIOþH2¼
a
FeþMgOþH2O ð2ÞOn the contrary, the TPR profiles of the Mg–Al–Fe–LDH derived mixed oxides with 5% of iron have broad and overlapping peaks shifted to higher temperatures showing that the second reduction stage begins before the first stage is completed. This indicates stron-ger interaction among different Mg–Al–Fe–O components of mixed oxide. Similar reduction behavior was reported for Mg–Al–Fe–LDH derived mixed oxides (with 50% of Mg and Fe content varied be-tween 12.5% and 50%)[39]explaining the first stage as the partial reduction of iron species from Fe3+to Fe2+:
MgFeIIIAlO
4þH2¼Mg1xFe
II
xOþH2OþMgðAlÞO ð3Þ
and the second stage as the reduction from the rest of Fe3+species to Fe2+, proceeding according to Eq.(3), and the reduction of Fe2+ species to Fe0, proceeding according to Eq.(2).
The presence of aluminum intensifies interactions among dif-ferent Mg–Al–Fe–O species, since it retards the reduction of iron. This also agrees with the assumption derived from the thermal analysis that the presence of aluminum stabilizes layered structure of LDHs. It has also been reported that the presence of aluminum cations inside LDH framework retards the reduction of iron[39], but the reduction of complex mixed oxides is affected by the
for-mation of solid solutions after calcination and in case of samples with additional Bayerite phase, evidenced by both Mössbauer and TPR analysis, extra LDH framework aluminum cations support the reduction of iron. An increase in temperature of the first TPR peak is followed by a decrease of the second reduction peak tem-perature (corresponding to the reduction of Fe2+ Fe0and the rest of Fe3+ Fe2+). This behavior favors the reduction of the first stage (Fe3+ Fe2+). Since the limit for the incorporation of M(III) ions into the LDH matrix is aroundx= 0.5, the samples with this M(III) ion content have the weakest interaction among different Mg– Al–Fe–O components and therefore the lowest temperature of the first TPR maxima, compared to other Mg–Al–Fe containing samples. On the contrary, the samples withx= 0.3 present the samples with single LDH phase, the most intensive XRD peaks, the strongest interaction among different Mg–Al–Fe–O compo-nents and in return the highest temperature of the first TPR maxima.
3.7. Catalytic tests
The nitrous oxide molecule is quite stable at room temperature and its simplest form of catalytic decomposition can be described as an adsorption of N2O at the active center followed by a decom-position giving N2 and surface oxygen O(Eq.(4)). This surface oxygen desorbs in combination with another oxygen atom or in di-rect reaction with another N2O (Eqs.(5)and (6))[15]:
N2Oþ !N2þO ð4Þ
N2OþO !N2þO2þ ð5Þ
2O
$O2þ2 ð6Þ
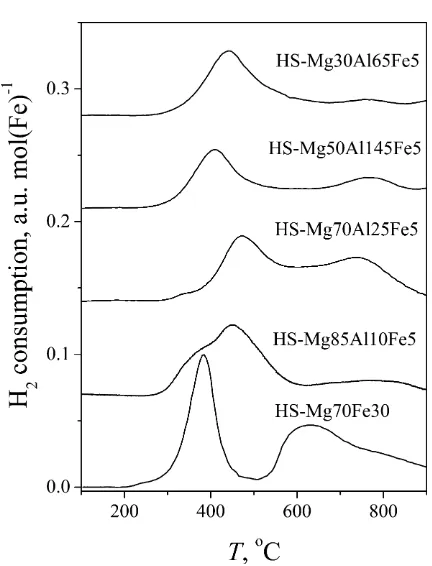
The experimental results of catalytic N2O decomposition are presented inFig. 9. Low iron containing samples (5%), independent on Mg/Al–ratio, have very low N2O conversion. The increase of iron content (30%) improves conversion and confirms the crucial role of iron in the catalytic act, proved also with a very small conversion in case of iron free sample (at 500°C <0.04). This is in correspondence with reduction behavior, since the decomposition of N2O is based on the redox cycle Fe3+ Fe2+[15]. The influence of Mg/Al ratio on N2O decomposition for the samples with 5% iron cannot be estab-lished because no significant difference in the range of measure-ment accuracy was observed. These samples all have very low conversion probably because, according to TPR results, their reduc-tion cycle starts at temperatures above 450°C. Better performance of LS-Mg70Fe30 sample compared to HS-Mg70Fe30 sample can be explained with their reduction behavior. Both samples have the same temperature of the peak maxima in the first reduction stage (Fe3+ Fe2+) but the temperature of the second reduction stage is for the sample LS-Mg70Fe30 about 110°C higher enabling better com-pletion of the first reduction stage responsible for the catalytic reaction. The Mössbauer analysis confirmed it also showing that after calcination sample LS-Mg70Fe30 has higher amount (40%) of the most easily reduced Fe3+species
Fe3+(5) component, than HS-Mg70Fe30 sample (29%).
The experimental results of the catalytic N2O reduction with NH3are presented inFig. 10. The other reaction path with reducing agent has overall higher activity, since the presence of reducing agent e.g. ammonia, boosts the removal of surface oxygen O⁄ [16](Eq.(7)), which is the reaction rate determining step in cata-lytic decomposition of N2O[15].
2NH3þ3O$N2þ3H2Oþ3 ð7Þ
In this reaction path, the crucial role of iron is also confirmed, but the activity of samples with lower iron content is generally increased being dependent on the Mg/Al-ratio among the series.
Fig. 8.TPR profiles of HS samples with different Mg:Al:Fe content.
Differentiation of lower iron content samples could be made in
cor-respondence with reduction behavior supplemented with
Mössbauer analysis. Other sample properties such as BET surface, acid properties and thermal behavior, do not influence the catalytic behavior in both reactions significantly. The order of the activity within the Mg–Al–Fe series follows the order obtained with TPR analysis of the first reduction stage.
Better performance between the samples with the same chem-ical composition, synthesized with different methods, having the same temperature of the first TRP maxima was already mentioned
for LS-Mg70Fe30 and HS-Mg70Fe30 samples in N2O
decomposi-tion reacdecomposi-tion and explained with the strength of Mg–Fe–O interac-tions. This can also be applied within the Mg–Al–Fe–LDH derived mixed oxide series taking into account the strength of Mg– Al–Fe–O interactions. In case of HS-Mg50Al45Fe5 and LS-Mg50Al45Fe5 samples having the same temperature of the first
TRP maxima, catalytically better performing sample,
HS-Mg50Al45Fe5, has higher temperature of the second reduction stage and, as Mössbauer analysis showed, higher amount of
Fe3+(5) component (29% compared to 23% in LS-Mg50Al45Fe5)
responsible for the Fe3+ Fe2+redox cycle.
4. Conclusions
Mg–Al–Fe and Mg–Fe layered double hydroxides were success-fully synthesized by two different coprecipitation methods and in a wide range of M(III) ions. The extended M(III) substitution influ-ences the structure and surface properties of Mg–Al–Fe–LDHs and their derived mixed oxides, weakens Mg-Al-Fe-O interactions and improves catalytic behavior. Thermally activated iron-containing LDHs exhibited catalytic activity in N2O decomposition and reduction with NH3. This feature is mainly influenced by reduction behavior and can be correlated with the presence of Fe–O–Fe–O–Fe entities providing possibility for facilitated extrac-tion of oxygen with simultaneous redox Fe3+ Fe2+ conversion. The best results are obtained when the amount M(III) substitution is near the limit for the incorporation into the LDH matrix (x= 0.5) and the methastabile mixed oxides are formed with the weakest interactions between constituent metals and oxygen. The presence of small amount of additional Bayerite phase, e.g. extra-LDH-framework aluminum (and iron), in x= 0.5 samples, positively influences catalytic behavior, but higher concentrations of extra-LDH-framework aluminum (x= 0.7) lead to the significant
Fig. 9.N2O decomposition atGHSVmod¼2:17 cm
3
NTPgcat1s1with all Mg–Al–Fe and Mg–Fe catalysts.
Fig. 10.N2O reduction with NH3atGHSVmod¼2:17 cm
3
NTPgcat1 s1with all Mg–Al–Fe and Mg–Fe catalysts. T.J. Vulic et al. / Chemical Engineering Journal xxx (2012) xxx–xxx
formation of Bayerite phase and decrease catalytic activity. The high supersaturation synthesis method, HS, provides LDHs with more structural defects, especially in stacking of layers which facilitates metastability of derived mixed oxides. This feature is favorable for the catalytic activity in N2O decomposition and reduction with NH3.
Acknowledgements
The financial support received from DAAD, 38th International Seminar for Research and Teaching in Chemical Engineering and Physical Chemistry, Universitaet Karlsruhe, Germany and from Ser-bian Ministry of Education and Science (Contract No. II145008) is gratefully acknowledged.
References
[1] P.S. Braterman, Z.P. Xu, F. Yarberry, Layered double hydroxides (LDHs), in: S.M. Auerbach, K.A. Carrado, P.K. Dutta (Eds.), Handbook of layered materials, Marcel Dekker, Inc., New York, 2004, pp. 420–449.
[2] F. Li, X. Duan, Applications of layered double hydroxides, Struct. Bond. 119 (2006) 193–223.
[3] Z.P. Xu, J. Zhang, M.O. Adebajo, H. Zhang, C. Zhou, Catalytic applications of layered double hydroxides and derivatives, Appl. Clay Sci. 53 (2011) 139–150. [4] Kostas S. Triantafyllidis, Efrosyni N. Peleka, Vasilis G. Komvokis, Paul P. Mavros, Iron-modified hydrotalcite-like materials as highly efficient phosphate sorbents, J. Colloid Interf. Sci. 342 (2010) 427–436.
[5] A. Vaccari, Preparation and catalytic properties of cationic and anionic clays, Catal. Today 41 (1998) 53–71.
[6] E.M. Serwicka, K. Bahranowski, Environmental catalysis by tailored materials derived from layered minerals, Catal. Today 90 (2004) 85–92.
[7] S.P. Newman, W. Jones, in: W. Jones, C.N.R. Rao (Eds.), Supramolecular Organisation and Material Design, Cambridge University Press, Cambridge, UK, 2002, pp. 295–331.
[8] H. Wang, H. Yi, P. Ning, X. Tang, L. Yu, D. He, S. Zhao, Calcined hydrotalcite-like compounds as catalysts for hydrolysis carbonyl sulfide at low temperature, Chem. Eng. J. 166 (2011) 99–104.
[9] J. Oi, A. Obushi, A. Ogata, G.R. Bamwenda, R. Tanak, T. Hibino, S. Kushiyama, Zn, Al, Rh-mixed oxides derived from hydrotalcite-like compound and their catalytic properties for N2O decomposition, Appl. Catal. B: Environ. 13 (1997)
197–203.
[10] J. Perez-Ramirez, J. Overeijnder, F. Kaptejn, J.A. Moulijn, Structural promotion and stabilizing effect of Mg in the catalytic decomposition of nitrous oxide over calcined hydrotalcite-like compounds, Appl. Catal. B: Environ. 23 (1999) 59–72.
[11] K.S. Chang, H. Song, Y.-S. Park, J. -W. Woo, Analysis of N2O decomposition over
fixed bed mixed metal oxide catalysts made from hydrotalcite-type precursors, Appl.Catal.A: Gen. 273 (2004) 223–231.
[12] L. Obalova, K. Pacultova, J. Balabanova, K. Jiratova, Z. Bastlc, M. Valaskova, Z. Lacny, F. Kovanda, Effect of Mn/Al ratio in Co–Mn–Al mixed oxide catalysts prepared from hydrotalcite-like precursors on catalytic decomposition of N2O,
Catal. Today 119 (2007) 233–238.
[13] J.J. Yu, J. Cheng, C.Y. Ma, H.L. Wang, L.D. Li, Z.P. Hao, Z.P. Xu, NOx
decomposition, storage and reduction over novel mixed oxide catalysts derived from hydrotalcite-like compounds, J. Colloid. Interf. Sci. 333 (2009) 423–430.
[14] K. Karásková, L. Obalová, K. Jirátová, F. Kovanda, Effect of promoters in Co–Mn– Al mixed oxide catalyst on N2O decomposition, Chem. Eng. J. 160 (2010) 480– 487.
[15] F. Kapteijn, J. Rodriguez-Mirasol, J.A. Moulijn, Heterogeneous catalytic decomposition of nitrous oxide, Appl. Catal. B: Environ. 9 (1996) 25–64. [16] B. Coq, M. Mauvezin, G. Dalay, S. Kieger, Kinetics and mechanism of the N2O
reduction by NH3on a Fe-zeolite-Beta catalyst, J. Catal. 195 (2000) 298–303.
[17] G. Delahay, M. Mouvezin, A. Guzman-Vargas, B. Coq, Effect of the reductant nature on the catalytic removal of N2O on Fe-zeolite-b catalysts, Catal.
Commun. 3 (2002) 385–389.
[18] T. Nobukawa, M. Yoshida, S. Kameoka, S.-I. Ito, K. Tomishige, K. Kunompri, In-Situ observation of reaction intermediate in the selective catalytic reduction of N2O with CH4 over Fe ion-exchanged BEA zeolite catalyst for the
elucidation of its reaction mechanism using FTIR, J.Phys.Chem.B 108 (2004) 4071–4079.
[19] J. Shen, B. Guang, M. Tu, Y. Chen, Preparationa and characterization of Fe/MgO catalysts obtained from hydrotalcite-like compounds, Catal. Today 30 (1996) 77–82.
[20] Y. Ohishi, T. Kawabata, T. Shishido, K. Takaki, Q. Zhang, Y. Wang, K. Nomura, K. Takehira, Mg–Fe–Al mixed oxides with mesoporous properties prepared from hydrotalcite as precursors: catalytic behavior in ethylbenzene dehydrogenation, Appl. Catal.A: Gen. 288 (2005) 220–231.
[21] P. Kustowsi, A. Rafalska-Lasocha, D. Majda, D. Tomaszewska, R. Dzimbaj, Preparation and characterization of new Mg–Al–Fe oxide catalyst precursors for dehydrogenation of ethylbenzene in the presence of carbon dioxide, Solid State Ionics 141–142 (2001) 237–242.
[22] Y. Ohishi, T. Kawabata, T. Shishido, K. Takaki, Q. Zhang, Y. Wang, K. Nomura, K. Takehira, Mg–Fe–Al mixed oxides with mesoporous properties prepared from hydrotalcite as precursors: catalytic behaviour in ethylbenzene dehydrogenation, Appl. Catal. A: Gen. 288 (2005) 220–231.
[23] S.M. Auer, J.-D. Grunwaldt, R.A. Koeppel, A. Baiker, Reduction of 4-nitrotoluene over Fe–Mg–Al lamellar double hydroxides, J. Mol. Chem. A: Chem. 139 (1999) 305–313.
[24] P.S. Kumbhar, J. Sanchez-Valente, J.M.M. Millet, F. Figueras, Mg–Fe hydrotalcite as a catalyst for the reduction of aromatic nitro compounds with hydrazine hydrate, J. Catal. 191 (2000) 467–473.
[25] T. Vulic, A. Reitzmann, J. Ranogajec, R. Marinkovic-Neducin, The influence of synthesis method and Mg-Al-Fe content on the thermal stability of layered double hydroxides, J. Therm. Anal. Calorim. (2012),http://dx.doi.org/10.1007/ s10973-012-2230-9.
[26] O.P. Ferreira, O.L. Alves, D.X. Gouveia, A.G. Souza Filho, J.A.C. de Paiva, J. Mendes Filho, Thermal decomposition and structural reconstruction effect on Mg–Febased hydrotalcite compounds, J. Solid State Chem. 177 (2004) 3058– 3069.
[27] J.M. Fernandez, M.A. Ulibarri, F.M. Labajos, V. Rives, The effect of iron on the crystalline phases formed upon thermal decomposition of Mg–Al–Fe hydrotalcites, J. Mater. Chem. 8 (1998) 2507–2514.
[28] N. Benselka-Hadj Abdelkader, A. Bentouamib, Z. Derriche, N. Bettahar, L.-C. de Ménorval, Synthesis and characterization of Mg–Fe layer double hydroxides and its application on adsorption of orange G from aqueous solution, Chem. Eng. J. 169 (2011) 231–238.
[29] K.S.W. Sing, J. Rouquerol, in: G. Ertl, H. Knoezinger, J. Wetkamp (Eds.), Handbook of Heterogeneous Catalysis, vol. 2, VCH Verlagsgesellschaft mbH, Weinheim, 1997, pp. 427–432. ISBN: 3-527-29212-8.
[30] S.J. Gregg, K.S.W. Sing, Adsorption, Surface Area and Porosity, Academic Press Inc. Ltd. LCCCN: 66-29432, London, UK, 1967.
[31] W.T. Reichle, S.Y. Kang, D.S. Everhardt, The nature of the thermal decomposition of a catalytically active anionic clay mineral, J. Catal. 101 (1986) 352–359.
[32] F. Prinetto, G. Ghiotti, R. Durand, D. Tichit, Investigation of acidbase properties of catalysts obtained from layered double hydroxides, J. Phys. Chem. B 104 (2000) 11117–11126.
[33] S. Casanave, H. Martinez, C. Guimon, A. Auroux, V. Hulea, E. Dimitriu, Acid-Base properties of MgCuAl mixed oxides, J. Therm. Anal. Cal. 72 (2003) 191– 198.
[34] M. Blume, Magnetic relaxation and asymmetric quadrupole doublets in the Mössbauer effect, Phys. Rev. Lett. 14 (1965) 96–98.
[35] C.B. Koch, Structures and properties of anionic clay minerals, Hyp. Interact. 117 (1998) 131–157.
[36] J. Sanchez-Valente, J.M.M. Millet, F. Figueras, L. Fournes, Mössbauer spectroscopic study of iron containing hydrotalcite catalysts for the reduction of aromatic nitro compounds, Hyp. Interact. 131 (2000) 43–50. [37] P. Kustrowski, A. Wegrzyn, A. Rafalska-Lasocha, A. Pattek-Janczyk, R. Dziembaj,
Substitution of Fe3+for Al3+cations in layered double hydroxide [LiAl 2(OH)6]2
CO3nH2O, Clays Clay Min. 53 (2005) 18–27.
[38] J.W. Niemantsverdriet, A.M. van der Kraan, W.N. Delgass, Characterization of surface phases in bimetallic FeRhSiO2 catalysts by in situ Mössbauer spectroscopy at cryogenic temperatures, J. Catal. 89 (1984) 138–147. [39] X. Ge, M. Li, J. Shen, The reduction of Mg–Fe–O and Mg–Fe–Al–O complex
oxides studied by temperature-programmed reduction combined with in situ Mössbauer spectroscopy, J. Solid State Chem. 161 (2001) 38–44.