G Protein–Mediated cAMP Signaling in
Bipolar I Disorder
Masoumeh Emamghoreishi, Peter P. Li, Lyanne Schlichter, Sagar V. Parikh,
Robert Cooke, and Jerry J. Warsh
Background: Evidence of extensive cross-talk between
calcium (Ca21)- and cAMP-mediated signaling systems suggests that previously reported abnormalities in Ca21 homeostasis in bipolar I (BP-I) patients may be linked to disturbances in the function of G proteins that mediate cAMP signaling.
Methods: To test this hypothesis, theb-adrenergic ago-nist, isoproterenol, and the G protein activator, sodium fluoride (NaF), were used to stimulate cAMP production in B lymphoblasts from healthy and BP-I subjects pheno-typed on basal intracellular calcium concentration ([Ca21]
B). cAMP was measured by radioimmunoassay
and [Ca21]
Bby ratiometric fluorometry with fura-2. Results: Isoproterenol- (10mM) stimulated cAMP forma-tion was lower in intact B lymphoblasts from BP-I patients with high [Ca21]
B($2 SD above the mean concentration
of healthy subjects) compared with patients having normal B lymphoblast [Ca21]B and with healthy subjects.
Al-though basal and NaF-stimulated cAMP production was greater in B lymphoblast membranes from male BP-I patients with high versus normal [Ca21]B, there were no
differences in the percent stimulation. This suggests the differences in NaF response resulted from higher basal adenylyl cyclase activity.
Conclusions: These findings suggest that trait-dependent
disturbances in processes regulatingb-adrenergic recep-tor sensitivity and G protein–mediated cAMP signaling occur in conjunction with altered Ca21 homeostasis in those BP-I patients with high B lymphoblast [Ca21]
B.
Biol Psychiatry 2000;48:665– 673 © 2000 Society of Bi-ological Psychiatry
Key Words: Bipolar disorder, G proteins, signal
transduc-tion, cAMP, calcium homeostasis, B lymphoblasts
Introduction
E
vidence from studies of postmortem brain and periph-eral blood cells implicate disturbances in G protein– mediated cAMP signaling and Ca21 homeostasis in the pathophysiology of bipolar affective disorder (BD; re-viewed in Li et al 2000; Manji 1992; Warsh and Li 1996). For instance, increased stimulatory G protein a subunit (Gas) levels (Manji et al 1995; Young et al 1994), blunted receptor- and G protein–stimulated cAMP accumulation (Ebstein et al 1988; Mann et al 1985; Pandey et al 1979) and increased basal intracellular calcium levels ([Ca21]B;
Dubovsky et al 1992b; Emamghoreishi et al 1997) have all been observed in mononuclear leukocytes from patients with BD. Moreover, some of these signaling disturbances appear to be trait dependent, possibly reflecting underlying molecular “vulnerability” factors in BD (Emamghoreishi et al 1997; Warsh et al 1999). Although it has been speculated that these signal transduction abnormalities are linked through cross-talk mechanisms (Dubovsky et al 1992a; Warsh and Li 1996), to our knowledge there is no empirical evidence for such a link.
Extensive cell-type specific cross-talk occurs between G protein– coupled cAMP- and phospholipase C (PLC)– linked second-messenger systems (Hill and Kendall 1989; Liu and Simon 1996). Multiple adenylyl cyclase (AC) subtypes provide the potential for Ca21either to synergise or attenuate cAMP signaling (Sunahara et al 1996). Thus, Ca21/CaM directly activates AC isotypes I and VIII, whereas Ca21inhibits isotypes V and VI (Sunahara et al 1996). Ca21-activated protein kinase C (PKC) may also increase or decrease cAMP levels by phosphorylating b-adrenergic receptors (b-ARs; Bell and Brunton 1987), Gsa, Gia (Katada et al 1985; Sugden and Klein 1988; Yoshimasa et al 1987) or some AC subtypes (Cooper et al 1995). Recently, it has been shown that Ca21-activated
From the Section of Biochemical Psychiatry (ME, PPL, JJW) and Mood Disorder Program (SVP, RC, JJW), Centre for Addiction and Mental Health, Clarke Site; Playfair Neuroscience Unit, The Toronto Hospital (LS); and the Departments of Psychiatry (PPL, SVP, RC, JJW), Pharmacology (PPL, JJW), and Physiology (LS) and the Institute for Medical Sciences (JJW), University of Toronto, Toronto, Canada.
Address reprint requests to J.J. Warsh, M.D., Ph.D., University of Toronto, Centre for Addiction and Mental Health, Clarke Division, 250 College Street, Toronto Ontario M5T 1R8, Canada.
Received June 29, 1999; revised March 16, 2000; accepted March 20, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
PKC suppresses protein kinase A (PKA)-mediated signal-ing in fibroblast cell lines (Dobbelsignal-ing and Berchtold 1996), providing another site of interaction between these signaling pathways.
cAMP-mediated processes also affect Ca21 signaling by inhibiting Ca21influx (Rasmussen 1986), desensitizing inositol trisphosphate receptors (Supattapone et al 1988), stimulating Ca21 removal from the cytosol by Ca21 ATPase (Helman et al 1986), and inhibiting PLCb2(Liu and Simon 1996). In other instances, G protein–mediated cAMP signaling enhances that of Ca21, including PKA-mediated phosphorylation of voltage-dependent Ca21 channels in excitable cells (Chad et al 1987), or the ion gate that closes K1 channels in cells in general, thus prolonging depolarization and enhancing Ca21 influx (Kandel et al 1987). There is also evidence that in non-excitable cells, receptor-activated Ca21 channels are opened by an increase in the concentration of cytoplasmic cAMP (Barritt 1999). Such mechanisms as noted above would account for the types of interaction (i.e., stimula-tion/suppression) that occur between cAMP and phospho-inositide signaling pathways.
As disturbances in both G protein–mediated cAMP and Ca21 signaling have been previously reported in BD, cross-talk regulatory mechanisms are potentially impor-tant candidates to be considered in explaining abnormali-ties in these two signal transduction pathways. Thus, the increased lymphocyte and platelet intracellular Ca21 ([Ca21]
i) reported in BD might result from the altered G
protein function previously identified in these patients, or vice versa. In effect, defects in one signaling system may decompensate or, alternatively, induce adaptive changes in other signaling systems in an attempt to maintain homeostasis.
To test this hypothesis, we used Epstein–Barr virus (EBV)–immortalized B lymphoblasts derived from BD patients, a cellular model that demonstrates trait-depen-dent abnormalities in Ca21 homeostasis (Emamghoreishi et al 1997) and inb-AR sensitivity (Kay et al 1993) in this disorder. These cell lines can be grown in continuous culture removed from in vivo neurohormonal influences
and medications, which circumvents the potential of such variables to hamper detection of signal transduction ab-normalities and interactions between signaling cascades. To screen for possible relationships between disturbed G protein function and altered basal intracellular [Ca21] ([Ca21]
B), isoproterenol and sodium fluoride
(NaF)-stim-ulated cAMP formation were determined in B lympho-blasts from bipolar I (BP-I) patients stratified based on [Ca21]
Bcompared with healthy subjects. We report here
the coexistence of altered receptor- and G protein–medi-ated cAMP signaling and calcium homeostasis, supporting the view that these abnormalities are both interrelated and trait-dependent.
Methods and Materials
Subjects
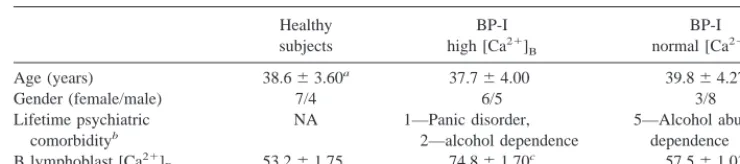
Cell lines were selected from a subset of BP-I patients and healthy subjects recruited and assessed as previously described (Emamghoreishi et al 1997). Briefly, subjects were recruited from the Mood Disorders inpatient and outpatient units of the Clarke Institute of Psychiatry. After a clinical diagnosis of BP-I disorder was made by a consulting psychiatrist, the diagnosis was confirmed for research purposes using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I/P, version 2.0; First et al 1995b) administered by a research psychiatrist or trained psychiatric research assistant, complemented by review of medical records. All interview and historical material was further reviewed by a research psychiatrist to ensure the reliabil-ity of the diagnoses. Patients had no history of recent (.3 months) substance abuse. Healthy comparison subjects were recruited through posted advertisements in the University of Toronto and were assessed by a trained research assistant and a research psychiatrist using the SCID-I/NP, version 2.0 (First et al 1995a). Healthy subjects had no past or family (first-degree relatives) history of psychiatric disorder. All subjects were physically healthy, had no past history of serious medical illness, and had no personal or family history of diabetes or hyperten-sion. The study was approved by the Human Subjects Review Committee of the University of Toronto and, after providing a complete description of the study, all subjects provided written informed consent. Table 1 shows the demographics and [Ca21
]B
Gender (female/male) 7/4 6/5 3/8
Lifetime psychiatric
bBP patients with a lifetime history of alcohol abuse or dependence had no recent history (.3 months) of substance abuse at
the time of study. One BP-I subject with normal [Ca21]
Balso had a past, but not recent, history of polysubstance abuse. cTukey p,.001, compared with healthy subjects and BP-I patients with normal [Ca21]
of the BP-I patients and healthy subjects from whom cell lines were selected for study.
B Lymphoblast Transformation and Intracellular Calcium Measurements
Mononuclear leukocyte preparations were isolated by density gradient separation through Ficoll-Hypaque and B lymphocytes in these isolates were transformed by infection with EBV as previously described (Emamghoreishi et al 1997). The trans-formed, immortalized B lymphoblasts were grown in RPMI-1640 medium containing L-glutamine (2 mmol/L), pyruvate (1 mmol/L), 20% fetal bovine serum (FBS), 100mg/mL streptomy-cin, 100 units/mL penicillin in an incubator (95% air/5% CO2at
37°C) with passages every 3– 4 days. At 12–15 passages, aliquots of cells were removed and [Ca21
]B determined as described
below. The remaining cells were frozen in aliquots (107
cells/ mL) and stored over the vapour phase of liquid nitrogen until they were regrown and assayed for receptor- and G protein– stimulated cAMP formation.
[Ca21
]B was measured in B lymphoblasts using ratiometric
fluorometry and the calcium sensitive dye, fura 2-acetoxymeth-ylester, as described previously (Emamghoreishi et al 1997). BP-I subjects were stratified based on [Ca21
]B greater than
(high) or less than (normal) two standard deviations above the mean [Ca21
]B determined in cells from healthy subjects, to
classify patients into these two phenotypes.
Regeneration of B lymphoblast Cell Lines
B lymphoblasts were removed from the liquid nitrogen freezer and immediately placed on dry ice. Cells were then thawed quickly (,5 min) at 37°C and transferred gently into 25-cm2
culture flasks containing 5 mL pre-warmed (37°C) B lympho-blast medium and regrown as previously described (Emamghore-ishi et al 1997). After nine passages from the time of regeneration (total passages 21–24), cells were transferred to B lymphoblast medium containing 10% FBS and incubated for three additional passages. Finally, cells were transferred to B lymphoblast me-dium without FBS and incubated for 24 hours, then harvested and divided in two aliquots. One aliquot (8 –103107
cells) was used immediately to assay isoproterenol-stimulated cAMP for-mation, and the other was stored at270°C until used to assay G protein-stimulated AC activity.
Determination of Basal and Isoproterenol-Stimulated cAMP Production in Intact B Lymphoblasts
Isoproterenol was used to assess b-adrenergic receptor-stimu-lated cAMP formation in intact B lymphoblasts using a method modified from Kay et al (1993). Cells were washed once in buffer containing 130 mmol/L NaCl, 0.54 mmol/L KCl, 0.005 mmol/L CaCl2, 0.098 mmol/L MgCl2, 0.1% glucose and 15
mmol/L Tris.HCl pH 7.6, resuspended at 33106cells/mL, and incubated in 0.9 mL aliquots (30 min, 37°C, 95% air/5% CO2).
The reactions were begun by adding 50mL isobutyl-methylxan-thine (IBMX, final concentration 0.5 mmol/L), 50ml ascorbic
acid (final concentration 0.1 mmol/L) with or without isoproter-enol, and incubating at 37°C for 10 min. Reactions were stopped by boiling for 10 min, then centrifuging at 12,000 g for 10 min at 4°C. The protein concentration in each pellet was determined by the Lowry method (Lowry et al 1951). The supernatants, which contained the cAMP, were evaporated to dryness under vacuum, stored at270°C until needed, then reconstituted with 100mL assay buffer containing 50 mmol/L sodium acetate at pH 6.2, and 0.01% sodium azide. The cAMP content was measured using an 125
I-radioimmunoassay kit following the supplier’s instructions (Mandel, Guelph, Canada). Maximally stimulating and saturating concentrations of isoproterenol (1 and 10mmol/L, respectively) were determined in preliminary dose-response studies, and subsequently used to assess B lymphoblast receptor-stimulated cAMP production in the comparison groups.
Determination of Basal and NaF-Stimulated AC Activity in B Lymphoblast Membranes
MEMBRANE PREPARATION. B lymphoblasts were thawed
on ice, resuspended in 20 volumes ice-cold buffer (10 mmol/L Tris, pH 7.4, 1 mmol/L EGTA) and homogenized by hand using a glass-teflon homogenizer (15 strokes). The homogenates were centrifuged at 20,000 g (20 min, 4°C), then the pellets were re-suspended and centrifuged twice more. Each pellet was resuspended in 500mL ice-cold TEMS buffer (50 mmol/L Tris, pH 7.4, 1 mmol/L EGTA, 5 mmol/L MgCl2, 12% sucrose), and
protein concentrations were determined by the method of Brad-ford (BradBrad-ford 1976), using bovine serum albumin as a standard.
ASSAY OF NAF-STIMULATED AC ACTIVITY. Membrane
homogenates were diluted with TEMS buffer to give 1 mg membrane protein/ml and the samples kept on ice until use. Reaction mixtures (200mL) contained 50 mmol/L Tris, pH 7.4, 1 mmol/L EGTA, 3.5 mmol/L MgCl2, 0.1 mmol/L IBMX, 1.5
mg/mL BSA, 0.5 mmol/L adenosine triphosphate (ATP), 25 mmol/L phosphocreatine, and 250 units/mL creatine phosphoki-nase. Membrane homogenates (50 mL) were incubated at 37°C for 15 min in 100mL of reaction mixture. The reactions were initiated by adding 50mL ATP (2 mmol/L) in the absence or presence of NaF (2.5, 5, and 10 mmol/L), followed by gently vortexing and incubation at 37°C for 10 min with gentle shaking. The reaction was terminated by boiling the tubes for 10 min, followed by cooling on ice and centrifuging at 12,000 g for 10 min at 4°C. The supernatants were then removed and stored at
270°C until used for cAMP measurements as described above.
Statistical Analysis
1979; Pandey et al 1979] and increased NaF-stimulated cAMP formation [Young et al 1993] in B lymphoblasts from BP-I patients). Thus, one-tailed probability values of p ,.05 were used to distinguish statistically significant differences. Statistical analyses were performed using the SPSS (release 7.0) statistical software package (SPSS Inc., Chicago).
Results
Basal and Isoproterenol-Stimulated cAMP Production in Intact B Lymphoblasts
Repeated-measures analysis of variance (ANOVA) per-formed on the absolute values for cAMP formation showed a significant effect of isoproterenol [F(2,60) 5 26.1, p ,.001], but no significant interaction between diagnosis and isoproterenol [F(4,60) 5 1.0, p5 .2] or main effect of diagnosis [F(2,30) 5 0.71, p 5 .25]. When the isoproterenol-stimulated AC activity was ex-pressed as the percent stimulation above basal, there was a significant interaction between diagnosis and the percent stimulation [F(4,60) 5 2.04, p 5 .05] and a trend toward a significant main effect of diagnosis [F(2,30)5 1.92, p 5 .08]. Post hoc comparison of cell means by contrasts showed a significant reduction in the percent stimulation above basal at 10 mmol/L isoproterenol in BP-I patients with high [Ca21]
B compared with normal
[Ca21]
B, and compared with healthy subjects (59%, t 5
1.95, p 5 .03 and 58%, t 5 1.85, p 5 .035, respectively; Table 2). The increase in absolute cAMP levels was 17% lower than that achieved with 1 mmol/L isoproterenol stimulation in B lymphoblasts from BP-I patients with high [Ca21]
B, but was not statistically
significant compared with the cAMP levels reached fol-lowing stimulation of B lymphoblasts from healthy sub-jects with 10 mmol/L isoproterenol. Reduced percent stimulation above basal was also observed with 1mmol/L isoproterenol in BP-I patients with high [Ca21]
Bcompared
with healthy subjects, although this difference did not reach statistical significance (37%, t51.58, p5 .065).
Additionally, the reduced isoproterenol-stimulated cAMP production in BP-I subjects with high [Ca21]
Bwas more
marked at 10mmol/L and was significantly different from that determined at 1mmol/L isoproterenol (t 5 2.0, p5 .025). In preliminary experiments using membrane prep-arations, isoproterenol-stimulation was very low and vari-able, as we have also observed for other tissues (Young et al 1993). Thus, there was no further attempt to measure isoproterenol-stimulated cAMP formation in membranes from the subject samples studied.
Basal and NaF-Stimulated AC Activity in B Lymphoblast Membranes
NaF increased cAMP production in a concentration-de-pendent manner, with maximal stimulation at 5 mmol/L and a reduced response at 10 mmol/L NaF (Figure 1A). A repeated-measures MANOVA test showed significant main effects of NaF concentration [F(3,72) 5 23.1, p ,.001] and gender [F(1,24) 5 4.5, p5 .02], but not of diagnosis [F(2,24) 5 1.5, p 5 .12]. Moreover, statistically significant interactions were found between sex and NaF concentration [F(3,72)5 6.5, p5 .001], and between diagnosis and NaF [F(6,72) 5 1.95, p 5 .04]. Simple effects were performed to examine the interactions. Contrasts of cell means revealed that male subjects were significantly different from female subjects in the magnitude of the response to 2.5 mmol/L NaF [F(1,24) 5 21, p,.001] and in the reduced response to 10 mmol/L versus 5 mmol/L NaF [F(1,24) 5 4.96, p 5 .02) (Figure 1, C and D).
When cAMP levels were expressed as percent change above the basal level (Figure 1B), repeated-measures MANOVA tests also showed a significant effect of the NaF concentration [F(2,46) 5 33.9, p ,.001], an interaction between gender and NaF concentration [F(2,46) 5 4.0, p 5 .01], and a significant interaction between NaF concentration and diagnosis [F(4,46) 5 2.1, p 5 .046]. Male subjects differed significantly in
Table 2. Basal and Isoproterenol-Stimulated cAMP Production in B Lymphoblasts from Healthy Subjects and Bipolar I Patients with High (.69.4 nmol/L) and Normal (,69.4 nmol/L) [Ca21
]B
Healthy
Bipolar I disorder
High [Ca21]
B Normal [Ca
21]
B
Basal 9.2461.28 10.761.71 12.061.62
Isoproterenol (1mmol/L) 16.962.55 16.864.24 20.563.08
% stimulation 82.9612.5 52.3615.7 69.0612.6
Isoproterenol (10mmol/L) 16.663.00 14.763.83 21.663.42
% stimulation 79.4619.4 33.5615.2a,b 81.8617.6
cAMP production (see Methods) is expressed as pmol/mg protein/10 min (mean6SEM, 11 subjects in each group). The % stimulation over the basal level is shown.
aSignificantly different (t52.0, p5.025) from stimulation by 1mmol/L isoproterenol in the same patient group.
bSignificantly different from BP-I subjects with normal [Ca21]
the extent of reduction in response to 10 mmol/L com-pared to 5 mmol/L NaF [F(1,23) 5 6.2, p 5.01] (Figure 1 E and F).
Figure 1 (C–F) shows NaF-stimulated cAMP produc-tion expressed in absolute values and as a percent above basal cAMP production, in the comparison groups strati-fied by gender. Because the number of female BP-I subjects with normal [Ca21]
Bwas small, further statistical
analysis of data was performed only on male bipolar subjects. Repeated-measures MANOVA showed signifi-cant effects of NaF concentration [F(3,42) 5 48.8, p ,.001], an interaction between NaF concentration and
diagnosis [F(6,42) 5 2.4, p 5 .02], and a significant main effect of diagnosis [F(2,14) 5 3.5, p 5 .028; Figure 1C]. Male BP-I patients with high [Ca21]
B had
significantly higher absolute values of basal cAMP (142% greater, p , .05) and NaF-stimulated cAMP production (86% greater at 2.5 mmol/L NaF, p 5 .05; 94% at 5 mmol/L, p,.05; 81% at 10 mmol/L, p ,.05) compared with male BP-I patients with normal [Ca21]
B. Their cAMP
values were also higher than healthy male subjects, but the differences were not statistically significant (84% at 2.5 mmol/L, p 5 .09; 73% at 5 mmol/L, p 5 .08). In contrast, analysis of the NaF-stimulated cAMP levels in B
Figure 1. Sodium fluoride (NaF)–stimulated adenylyl cyclase (AC) activity production: dose–response curves for B lymphoblast membranes from bipolar I (BP-I) patients with high [Ca21
]B(.69.4 nmol/L,E), normal [Ca 21
]B(,69.4 nmol/L,‚), and their age-and gender-matched healthy controls (h). (A, B) Males and female subjects combined (n510). (C–F) Results stratified by gender (n 5 4 – 8 male and 2– 6 female subjects per subject comparison group). Repeated-measures multivariate analysis of variance (MANOVA) tests revealed no significant differences in basal or NaF-stimulated AC activity among comparison groups (A and B). For male subjects (C), repeated-measures MANOVA tests showed a significant main effect of diagnosis [F(2,14)53.5, p5 .028]. *Significant differences (p #.05) compared with corresponding NaF-stimulated response in the male BP-I group with normal [Ca21
lymphoblast membranes from male subjects expressed as a percent increase above their respective basal activities (Figure 1E) showed only a significant effect of NaF concentration [F(2,28) 5 35.4, p ,.001].
Discussion
We have used B lymphoblasts from BP-I patients that display high [Ca21]
Bto test whether alterations in calcium
homeostasis are related to disturbances in receptor-G protein coupled cAMP formation in BP-I patients mani-festing this cellular endophenotype. The principal findings are reducedb-AR-stimulated cAMP formation in intact B lymphoblasts, and elevated basal G protein/effector-de-pendent cAMP production in membranes of B lympho-blasts from BP-I patients with high [Ca21]
B compared
with healthy subjects. Specifically, theb-AR response to 10mmol/L isoproterenol was significantly lower in BP-I patients with high [Ca21]
Bthan in healthy subjects, and
although the response to 1mmol/L isoproterenol was also lower, it was not statistically significant. Male BP-I patients with elevated B lymphoblast [Ca21]
B also had
significantly higher basal and NaF-stimulated AC activity compared with male BP-I subjects with normal [Ca21]
B
and a trend towards higher NaF-stimulated AC activity compared with healthy subjects. These findings suggest that the alterations in intracellular calcium homeostasis in B lymphoblasts from BP-I patients are indeed associated with disturbances in receptor- and G protein– coupled AC activity. Moreover, the co-expression of these distur-bances in cells from BP-I subjects implies that this spectrum of signal transduction abnormalities is trait dependent.
Maximally stimulating and saturating concentrations of isoproterenol were used to assessb-AR-stimulated cAMP production in B lymphoblasts because of the limited signal-to-noise ratio of the response and to ensure a maximal level of cAMP stimulation was achieved in all cases in the absence of full dose response studies on each cell line. b-ARs in transformed B lymphoblasts show qualitatively similar ligand binding characteristics and AC activation to those in B lymphocytes, but lower density and maximal enzyme activity (Ebstein et al 1985; Elliot 1987). Furthermore, because the functional interaction between receptors and G proteins is strongly influenced by their relative expression levels (Kenakin 1996), the small responses observed here likely reflect the low density of b-ARs in these cell lines. Dose-response studies were not performed, and inferences about the efficacy of receptor-G protein coupling (reflected in the EC50) and exact nature of
the altered responsivity will require future concentration-response characterization.
The small magnitude and inter-sample variability in the
isoproterenol-stimulated cAMP production at 1 mmol/L may have contributed to the lack of statistically significant differences in B lymphoblast b-AR responses, between BP-I patients with high [Ca21]
Band either patients with
normal [Ca21]
B or healthy subjects. Nevertheless, our
results highlight the possibility that factors regulating the responsivity of the b-AR-G protein-AC complex are differentially altered in BP-I patients who manifest the endophenotype of high B lymphoblast [Ca21]B. Although
b-AR density was not determined in this study, previous studies have shown reducedb-AR-stimulated AC activity in lymphocytes (Mann et al 1985) and B lymphoblasts (Kay et al 1993) from BD patients in the absence of changes inb-AR density or affinity. Furthermore, we have not found differences in Gas and Gai levels in B lympho-blasts from BP-I patients compared with their matched healthy subjects (unpublished data). These observations argue against the possibility that altered levels of b-AR and of these G proteinasubunits account for the reduced b-AR-stimulated response in B lymphoblasts from BP-I patients observed here. Other downstream processes that regulate the response of the receptor-G protein-AC trans-duction apparatus should be considered, and it is notable that Kay et al (1993) found lessb-AR down-regulation in response to isoproterenol in B lymphoblasts from BD patients than in cells from control subjects. These obser-vations suggested the process(es) regulatingb-AR desen-sitization and/or down-regulation may be compromised in BD.b-AR down-regulation and/or desensitization is me-diated by receptor phosphorylation through PKA and/or b-AR kinase (Sibley et al 1987). Therefore, the lower b-AR responsiveness in BP-I patients with high [Ca21]
B,
compared with healthy subjects and BP-I patients with normal [Ca21]B, could similarly be related to alterations in
b-AR kinase or PKA activity.
Disturbances in cross-talk mechanisms could also be involved. For example, PKC inducesb-AR sequestration and desensitization (Kassis et al 1985, Pitcher et al 1992; Sibley et al 1987) and decreases b-AR affinity and coupling to G protein in S49 lymphoma cells (Bell and Brunton 1987). Ca21may also affect PKA activity, either directly or indirectly (Blumenthal et al 1986; Dobbeling and Berchtold 1996). Thus, disturbances in Ca21 ho-meostasis in BD could readily affect b-AR-mediated cAMP signaling at the receptor level through the intricate framework of cross talk that exists between these signaling systems.
et al 1986). Our NaF stimulation studies, although not definitive, show some potentially important trends. Al-though there was no main effect of diagnosis in the NaF-induced responses, there was a significant but unex-pected interaction with gender, as well as an interaction between increasing concentrations of NaF and the diagno-sis. Male subjects differed from female subjects in their concentration-response curves for NaF-stimulated AC ac-tivity. For this reason, and because of the small number of female BP-I patients with normal [Ca21]B(n5 3), only
male subjects were considered in further statistical analy-ses. Male BP-I patients with high [Ca21]
Bshowed higher
basal (142%) and NaF-stimulated AC activity (81%–94%) compared with BP-I patients with normal [Ca21]B.
NaF-stimulated AC activity was also higher (73%– 84%) in male BP-I patients with high [Ca21]B compared with
healthy subjects, although these differences did not reach statistical significance, possibly because of high variability and small sample size; however, the greater NaF-stimu-lated AC activity in BP-I patients with high [Ca21]Blikely
reflects higher basal AC activity in this group, as there were no significant differences in percent stimulation over basal levels among the comparison groups.
In contrast to the significantly higher basal cAMP formation in the B lymphoblast membrane preparations from male BP-I patients with high [Ca21]Bcompared to
other comparison groups, no difference was observed in basal cAMP formation in intact B lymphoblasts. This discrepancy is most readily explained by the different cellular matrices and assay conditions used: the NaF-stimulation employed a membrane preparation, whereas intact cells were used in the isoproterenol studies. Basal and stimulated responses in intact cells were assayed in the presence of Ca21(i.e., at physiological extracellular Ca21 concentrations), whereas the membrane assay used EGTA-washed membranes, and was therefore Ca21free. The differences in basal cAMP formation in membranes unmasked by removing Ca21suggest altered regulation of one or more Ca21-dependent processes that influence cAMP formation. In effect, basal cAMP formation appears to be “locked” at a higher baseline in membranes from BD compared with healthy male subjects and is not further upregulated in the presence of Ca21, as may occur in B lymphoblast membranes from healthy subjects. Because cAMP formation was measured following phosphodiester-ase inhibition with IBMX, it is most likely that the posited dysregulation of cAMP formation in B lymphoblast mem-branes from BP-I patients with high [Ca21]Binvolves the
AC subtypes expressed in these cells and/or factors mod-ulating their activity. Multiple AC isotypes exist that differ in their activation and inhibition by G proteina andbg subunits, Ca21, PKC, and PKA (Sunahara et al 1996). Therefore, higher basal AC activity in B lymphoblasts
from BP-I patients with high [Ca21]B than in the other
comparison groups might reflect differences in catalytic activity or levels of AC isotype (Pieroni et al 1995), particularly because G protein a subunit levels did not differ; however, the specific AC isotypes expressed in human B lymphoblasts have not been characterized.
The finding of higher basal AC activity in male BP-I patients with high [Ca21]Bmay be important
pathophysi-ologically. In addition to the factors discussed above, the levels and activity of phosphodiesterase isozymes (Hous-lay and Milligan 1997) and the extent and type of cross talk (Cooper et al 1995) also contribute to determining basal cAMP levels. If basal cAMP is near the threshold for activating PKA, a small increase in cAMP either from increased AC activity or reduced phosphodiesterase activ-ity will have a large and rapid effect (Houslay and Milligan 1997). In contrast, when basal AC activity is low, increased AC activity may be the predominant means of triggering PKA responses. Thus, the predicted physiolog-ical consequences of modulating PDE and/or AC activity can differ.
In summary, the reduced isoproterenol stimulation and elevated basal AC activity in B lymphoblasts from BP-I patients with high [Ca21]Bsuggests a link between
distur-bances in calcium homeostasis and processes regulating b-AR sensitivity and G protein-coupled AC functionality in a subgroup of patients with BD. Further replications are warranted, however, to ensure the replicability of these preliminary findings given the small sample size. Which signaling system (Ca21or cAMP) is first affected, and to what extent the changes in the linked transduction cascade are secondary or adaptive responses, remain to be eluci-dated. Regardless, these disturbances in signal transduc-tion systems, which are expressed in transformed B lymphoblasts from BP-I patients, appear to represent trait-dependent abnormalities that distinguish a patho-physiologically distinct subgroup of BP-I disorder.
Supported by Medical Council of Canada Grant No. MT12500 (JJW). The contributions of the following individuals are greatly appreciated: Dr. Jane Mitchell for her helpful advice about the G protein stimulation assays; Arvind Kamble for assistance with B lymphoblast transforma-tion, growth of cell lines and cAMP assays; Liliana Mihic and Veronica Hoang for assistance in subject recruitment and interview; and Kathy Spegg for advice regarding the statistical analyses.
References
Barritt GJ (1999): Receptor-activated Ca21
inflow in animal cells: A variety of pathways tailored to meet different intracellular Ca21
signaling requirements. Biochem J 337: 153–169.
hormone-sensitive adenylate cyclase activity in S49 lym-phoma cells. Am J Physiology 252:E783–E789.
Blumenthal DK, Takio K, Hansen RS, Krebs EG (1986): Dephosphorylation of cAMP-dependent protein kinase regu-latory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J Biol
Chem 261:8140 – 8145.
Bradford M (1976): A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248 –252. Chad T, Kalman D, Armstrong D (1987): The role of cAMP-dependent phosphorylation in the maintenance and modula-tion of voltage-dependent calcium channels. Soc Gen Physiol
Ser 42:167–186.
Cooper DMF, Mons N, Karpen JW (1995): Adenylyl cyclases and the interaction between calcium and cAMP signaling.
Nature 347:421– 424.
Dobbeling U, Berchtold MW (1996) Down-regulation of the protein kinase A pathway by activators of protein kinase C and intracellular Ca21
in fibroblasts cells. FEBS Lett 391: 131–133.
Dubovsky SL, Murphy J, Christiano J, Lee C (1992a): The calcium second messenger system in bipolar disorders: Data supporting new research directions. J Neuropsychiatry 4:3–14.
Dubovsky SL, Murphy J, Thomas M, Rademacher BD (1992b): Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 149: 118 –120.
Ebstein RP, Lerer B, Shapira B, Shamesh Z, Moscovich D, Kindler S (1988): cAMP second messenger signal amplifica-tion in depression. Br J Psychiatry 152:665– 669.
Ebstein RP, Steinitz M, Mintzer J, Lipshitz I, Stessman J (1985):
b-Adrenergic-stimulated adenylate cyclase activity in normal and EBV-transformed lymphocytes. Experientia 41:1552–1554. Elliott JM (1987): Characterization of [125
I]-iodocyanopindolol binding to cultured human lymphoblast cells. Br J Pharmacol 92:734P.
Emamghoreishi M, Schlichter L, Li PP, Parikh S, Sen J, Kamble A, Warsh JJ (1997): High intracellular calcium concentrations in transformed lymphoblasts from subjects with bipolar I disorder. Am J Psychiatry 154:976 –982.
Extein I, Tallman J, Smith CC, Goodwin FK (1979): Changes in lymphocyteb-adrenergic receptors in depression and mania.
Psychiatry Res 1:191–197.
First MB, Spitzer RL, Robert L, Gibbon M, Williams JBW (1995a): Structured Clinical Interview for DSM-IV Axis I
Disorders—Non-patient Edition (SCID-I/NP, Version 2.0).
New York: New York State Psychiatric Institute, Biometric Research Department.
First MB, Spitzer RL, Robert L, Gibbon M, Williams JBW (1995b): Structured Clinical Interview for DSM-IV Axis I
Disorders—Patient Edition (SCID-I/P, Version 2.0). New
York: New York State Psychiatric Institute, Biometric Re-search Department.
Helman J, Kuyatt BL, Takuma T, Seligmann B, Baum BJ (1986): ATP-dependent calcium transport in rat parotid basolateral membrane vesicles. Modulation by agents which elevate cyclic AMP. J Biol Chem 261:8919 – 8923.
Hill SJ, Kendall DA (1989): Cross-talk between different
recep-tor-effector systems in the mammalian CNS. Cell Signal 1:135–141.
Houslay MD, Milligan G (1997): Tailoring cAMP-signaling responses through isoform multiplicity. Trends Biochem Sci 22:217–224.
Kandel ER, Castellucci VF, Goelet P, Schacher S (1987): Cell-biological interrelationship between short-term and long-term memory. In: Kandel ER, editor. Molecular
Neuro-biology in Neurology and Psychiatry. New York: Raven,
111–132.
Kassis S, Zaremba T, Patel J, Fishman PH (1985): Phorbol esters andb-adrenergic agonists mediate desensitization of adenyl-ate cyclase in rat glioma C6 cells by distinct mechanisms.
J Biol Chem 260:8911–9817.
Katada T, Gilman AG, Watanabe Y, Bauer S, Jakobs KH (1985): Protein kinase C phosphorylates the inhibitory guanine-nucleotide binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem 151:431– 437.
Kay G, Sargeant M, McGuffin P, Whatley S, Marchbanks R, Baldwin D, et al (1993): The lymphoblast b-adrenergic receptor in bipolar depressed patients: Characterization and down-regulation. J Affec Disord 27:163–172.
Kenakin T (1996): The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev 48:413– 463.
Li PP, Andreopoulos A, Warsh JJ (2000): Signal transduction abnormalities in bipolar affective disorder. In: Reith MEA, editor. Cerebral Signal Transduction: From First to Fourth
Messengers. Totowa, NJ: Humana, 283–309.
Liu M, Simon MI (1996): Regulation by cAMP-dependent protein kinase of a G protein-mediated phospholipase C.
Nature 382:83– 87.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275.
Manji HK (1992): G-proteins—implications for psychiatry. Am J
Psychiatry 149:746 –760.
Manji HK, Chen G, Shimon H, Hsiao JK, Potter WZ, Belmaker RH (1995): Guanine nucleotide binding proteins in bipolar affective disorder: Effects of long-term lithium treatment.
Arch Gen Psychiatry 52:135–144.
Mann JJ, Brown RP, Halper JP, Sweeney JA, Kocsis JH, Stokes PE, Bilezikian JP (1985): Reduced sensitivity of lymphocyte
b-adrenergic receptors in patients with endogenous depres-sion and psychomotor agitation. N Engl J Med 313:715–720. Pandey GN, Dysken MW, Garver DL, Davis J (1979): Beta-adrenergic receptor function in affective illness. Am J
Psy-chiatry 136:675– 678.
Pieroni JP, Harry A, Chen J, Jacobowitz O, Magnusson RP, Iyengar R (1995): Distinct characteristics of the basal activ-ities of adenylyl cyclases 2 and 6. J Biol Chem 270:21368 – 21373.
Pitcher J, Lohse MJ, Codina J, Caron MG, Lefkowitz RJ (1992): Desensitization of the isolated b2-adrenergic receptor by
b-adrenergic receptor kinase, cAMP-dependent protein ki-nase and protein kiki-nase C occurs via distinct molecular mechanisms. Biochemistry 31:3193–3197.
Rasmussen H (1986): The calcium messenger system. N Engl
Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ (1987): Molecular mechanisms ofb-adrenergic receptor desensitiza-tion. Adv Exp Med Biol 221:253–273.
Sugden D, Klein DC (1988): Activators of protein kinase C act at a postreceptor site to amplify cAMP production in rat pinealocytes. J Neurochem 50:149 –155.
Sunahara RK, Dessauer CW, Gilman AG (1996): Complexity and diversity of mammalian adenylyl cyclases. Annu Rev
Pharmacol Toxicol 36:461– 480.
Supattapone S, Danoff SK, Theibert A, Joseph SK, Steiner J, Snyder SH (1988): Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A 85:8747– 8750. Warsh JJ, Emamghoreishi E, Li PP (1999): Elevated B lymphoblast
resting intracellular calcium is a trait-dependent abnormality specific to bipolar-I disorder. Bipolar Disord 1(suppl 1):57.
Warsh JJ, Li PP (1996): Second messenger systems and mood disorders. Curr Opin Psychiatry 9:23–29.
Yamanaka G, Eckstein F, Stryer L (1986): Interaction of retinal transducin with guanine triphosphate analogues: Specificity of the g-phosphate binding region. Biochemistry 25:6149 – 6153.
Yoshimasa T, Sibley DR, Bouvier M, Lefkowitz RJ, Caron MG (1987): Cross-talk between cellular signalling pathways sug-gested by phorbol ester induced adenylate cyclase phosphor-ylation. Nature 327:67–70.
Young LT, Li PP, Kamble A, Siu KP, Warsh JJ (1994): Increased mononuclear leukocyte G proteins in bipolar but not unipolar depression. Am J Psychiatry 151:594 –596. Young LT, Li PP, Kish SJ, Siu KP, Kamble A, Hornykiewicz O,
Warsh JJ (1993): Cerebral cortex Gas protein levels and
![Table 2. Basal and Isoproterenol-Stimulated cAMP Production in B Lymphoblasts from HealthySubjects and Bipolar I Patients with High (�69.4 nmol/L) and Normal (�69.4 nmol/L) [Ca2�]B](https://thumb-ap.123doks.com/thumbv2/123dok/3143537.1383557/4.612.121.487.106.192/isoproterenol-stimulated-production-lymphoblasts-healthysubjects-bipolar-patients-normal.webp)
![Figure 1. Sodium fluoride (NaF)–stimulated adenylyl cyclase (AC) activity production: dose–response curves for B lymphoblastmembranes from bipolar I (BP-I) patients with high [Ca*Significant differences (2�]B (�69.4 nmol/L, E), normal [Ca2�]B (�69.4 nmol/L](https://thumb-ap.123doks.com/thumbv2/123dok/3143537.1383557/5.612.122.486.80.474/fluoride-stimulated-activity-production-response-lymphoblastmembranes-significant-differences.webp)