L
Journal of Experimental Marine Biology and Ecology 247 (2000) 223–232
www.elsevier.nl / locate / jembe
A note on a possible influence of traps on assessment of the
diet of Jasus lalandii (H. Milne-Edwards)
*
Morgan K.P. Griffiths, Stephen Mayfield , George M. Branch
Coastal Ecology Unit, Zoology Department, University of Cape Town, Private Bag,
Rondebosch7701 Cape Town, South Africa
Received 22 April 1999; received in revised form 14 December 1999; accepted 18 December 1999
Abstract
Dietary analyses are important components of ecological studies. However, some methods of collecting organisms may expose them to exceptionally high densities of prey items, leading to inaccurate dietary assessments. These methods include the use of baited traps. We hypothesised that such a ‘‘trap effect’’ occurred during work on the diet of the rock lobster Jasus lalandii, because baited traps attracted isopods, which were then eaten opportunistically by trapped J. lalandii. To test this hypothesis, rock lobsters were collected at two sites using both baited-traps and Scuba diving. Results showed that large numbers of isopods were attracted to, and consumed, the trap-bait. Analyses of the stomach contents of trap-caught J. lalandii from both localities reflected a larger occurrence and significantly greater abundance of isopods in stomach samples from trap- rather than Scuba-caught rock lobsters. For probably similar reasons, small fish were significantly over-represented in the gut contents of trap-caught rock lobsters, although the evidence is less clear-cut and there may be other explanations for the high proportions of fish in the diet. Although isopods and fish may be naturally consumed at a low rate, predation on them is greatly inflated in traps, and the dietary analyses of trap-caught J. lalandii. All assessments of the diets of animals traditionally caught with traps should consider and account for such ‘‘trap effects’’, by calibrating the data for trap-caught animals against an alternative sampling method.
2000 Elsevier Science B.V. All rights reserved.
Keywords: Diet assessment; Isopods; Jasus lalandii; Lobsters; Trap effects; Trapping
1. Introduction
Dietary studies based upon analysis of stomach contents are a standard practice in fish
*Corresponding author. Tel.:127-21-6503-620; fax:127-21-6503-301.
E-mail address: [email protected] (S. Mayfield)
ecology (Hyslop, 1980), and the diet of the West Coast rock lobster (Jasus lalandii ) is similarly assessed (Heydorn, 1969; Newman and Pollock, 1969, 1974; Griffiths and Seiderer, 1980; Pollock, 1979, 1986; Pollock et al., 1982; Barkai and Branch, 1988a; Barkai et al., 1996; Mayfield, 1998). Natural diet is an essential component of studies on an animal’s nutrition and interactions with other organisms, as well as its potential for culture (Hyslop, 1980; Williams, 1981). Analyses of diet traditionally involve visual assessment of stomach contents (Berg, 1979; Hyslop, 1980), though direct observation of feeding (Robles et al., 1990), prey ‘fingerprinting’ using protein electrophoresis (Giller, 1986) and gas chromatography (Knutsen and Vogt, 1985a,b) have proved successful alternative techniques.
Circumstances sometimes favour or compel the use of baited traps to collect specimens for dietary analyses. Catches obtained from such traps are influenced by the interactions between various organisms attracted to the bait, environmental factors and the trap itself (Bennett, 1974; Morgan, 1974; Williams and Hill, 1982; Robertson, 1989; Miller and Addison, 1995). Useful reviews can be found in Bennett and Brown (1979) and Caddy (1979). Conducting trap experiments may interfere with natural conditions (Peterson and Black, 1994) and runs the risk of introducing biases (Dayton, 1985; Underwood, 1986; Spanier et al., 1994). In addition, controls do not always account for such ‘trap-effects’ (Peterson and Black, 1994).
Mayfield (1998) found a high incidence by numbers (85%) and a large proportion by volume (70%) of isopods (Ciralana spp.) in the stomachs of trap-caught Jasus lalandii. Previous studies on the diet of this species, which were based on diver-caught specimens, failed to record isopods in the gut contents (Heydorn, 1969; Newman and Pollock, 1969, 1974; Griffiths and Seiderer, 1980; Pollock, 1979, 1986; Pollock et al., 1982; Barkai and Branch, 1988a; Barkai et al., 1996).
We hypothesised that isopods are attracted to the bait in the rock-lobster traps and become available to the rock lobsters, either as accidental prey items consumed as the rock lobsters attempted to feed on the bait, or as opportunistic prey selected by the rock lobsters while within the traps. If this is true, isopods recorded in the stomachs of trap-caught rock lobsters are an artefact of the trap sampling technique and must be excluded from any diet analysis on trap-caught individuals. Small fish may also be attracted to traps and preyed upon, similarly biasing diet, but the primary focus of our study was on the isopods.
This note tests the hypothesis that the diet of trap- and dive-caught J. lalandii will be dissimilar unless trap effects are accounted for. We predict that (1) the diet of trap-caught rock lobsters will have a higher proportion of isopods in their gut contents than Scuba-caught individuals, and (2) that following removal of the isopods from the diet analysis, the diets of Scuba- and trap-caught individuals will be the same. The results impact on all dietary studies conducted on organisms traditionally sampled using baited traps or pots, and researchers will need to calibrate diets of trap-caught animals against an alternate sampling method.
2. Materials and methods
communities on the West Coast of South Africa. These sites were (1) Olifantsbos (348049 S, 188209 E), a commercial rock lobster fishing ground, where the benthic
community was dominated by barnacles (Notomegabalanus algicola), sponges, encrust-ing corallines and foliar algae, with few mussels (Field et al., 1980; Mayfield, 1998) and (2) the Knol (348169 S, 188229 E), a rock-lobster sanctuary, in which the benthic
community was dominated by mussels (Aulacomya ater), N. algicola, foliar red and coralline algae (pers. observation).
2.1. Experiment 1: rates of arrival of isopods and rock lobsters at the traps
At both sites, 80 traps divided between four treatments were set in a randomised pattern along four trap-lines. Standard commercial rock-lobster traps (ca. 130.6 30.7
m; mesh size 60390 mm) were deployed at an average depth of 25 m. Twenty traps
had their entrance funnels closed to prevent rock lobsters from entering the traps. These traps and an additional 20 open traps were baited with whole fish (the Maasbanker, Trachurus trachurus), which was secured in thick shadecloth bait-bags (mesh size 4 mm), so as to prevent rock lobsters having access to the bait which could otherwise have biased their stomach contents. Isopods, however, had unrestricted access to the bait by virtue of their small size. The bait in a further 20 open traps was not protected so that the rock lobsters were able to freely consume it. The remaining 20 traps served as controls and were left open and unbaited. All traps had terylene funnel nets (mesh size 1.5 mm) secured around their bases to collect the isopods and any other organisms vacating the traps as they were pulled to the surface.
Five traps per treatment were set for each of four soak-times viz.: 2, 6, 12 and 22 h. The traps were pulled up 1 h after dawn so as to coincide with the end of the rock lobsters’ crepuscular feeding session (Paterson, 1969; Zoutendyk, 1988). All rock lobsters caught in the traps were counted, measured and released. Isopods caught in the funnel nets and bait bags were weighed and an estimate of their numbers was calculated (by counting the number of individuals in a weighed subsample).
2.2. Experiment 2: diets of trap-caught versus diver-caught rock lobsters
At Olifantsbos, rock lobsters were collected from the five open, bait-protected traps after these had been deployed for 2 h. At the Knol, a 6-h soak-time was required to capture sufficient individuals. Traps were again pulled 1 h after dawn. From each of four of these traps, up to 70 rock lobsters (carapace length (CL) 65–85 mm) were collected and anaesthetised in a water / ice slush. Their stomachs were removed immediately thereafter to prevent further digestion of the stomach contents and to minimise the time
´
elapsed since the last meal (Sarda and Valladares, 1990). The first 35 ‘full’ stomachs obtained from lobsters in each trap were frozen (2208C) for later analysis. Full stomachs
3
were defined as those with a bolus$1 cm . Simultaneously with the sampling of the
trap-caught J. lalandii, 150 rock lobsters were caught by Scuba divers in the vicinity of the traps, at both locations. They were processed in the same way to obtain a sample of 50 full stomachs.
as a measure of gut fullness (Berg, 1979). The stomach contents were flushed into a Petri dish, examined under a dissecting microscope, and prey fragments identified to the lowest possible taxon and assigned into one of eight food categories (viz.: mussels, isopods, fish, barnacles, rock lobster, sponges, crustaceans and other). The occurrence of each food item was calculated following the method of Williams (1981): for each of the traps and the sample sites as a whole, the number of stomachs containing each prey item was recorded and expressed as a percentage of the total number of stomachs. This method provided a qualitative picture of the prey spectrum. The relative abundance of each prey item in the diet was calculated by applying Shepherd’s (1973) equation:
% abundance for prey item I5S P /SS P * 100
i i
where the proportion of each prey item (P) was estimated according to the contribution it made to the total volume of each stomach. This value was then multiplied by the gut mass of the food bolus (F, expressed as the wet weight in grams). PF was summed for all the stomachs, and divided by the total relative value of all the prey items from all the stomach contents of the sample.
Following the methods of Field (1971), PRIMER v6.0 (Clarke et al., 1994) was used to analyse relationships in the occurrence and abundance data between the trap and dive replicates. For this paper, the data were arcsine transformed into frequencies.
3. Results
3.1. Rates of arrival of isopods and rock lobsters at the traps
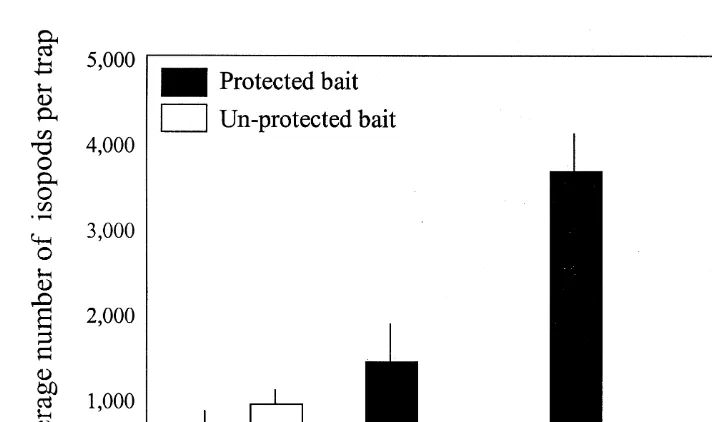
At both sites, large numbers of cirolanid isopods and amphipods were caught in traps that contained bait (up to 3500 per trap). Three species of cirolanid: Cirolana hirtipes, C. cranchii and C. imposita, and one species of amphipod, Amaryllis macrophthalma, were identified. The isopods contributed 75% by numbers to these catches. For periods longer than 2 h, larger numbers of isopods were caught in the traps with protected bait when compared to the traps with unprotected bait (Fig. 1). None were caught in the baitless trap controls.
3.2. Diets of trap-caught and diver-caught rock lobsters
Fig. 1. Number of isopods caught per trap after different soak times for traps with protected (solid bars) and unprotected bait (clear bars). Error bars show standard error.
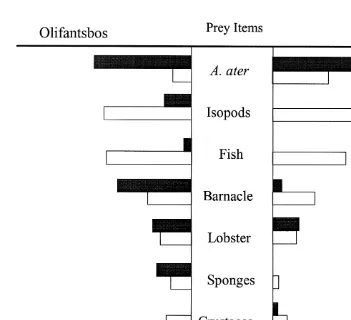
Frequency of occurrences of gut content categories, for the pooled dive and trap samples at Olifantsbos and at the Knol are shown in Fig. 2. There were large differences in the percentage occurrences of A. ater, isopods and fish between the dives and traps at both locations. There were significant differences in the abundance of isopods and fish between the dive and trap samples at both sites (Olifantsbos isopods: Mann–Whitney U51195; Olifantsbos fish: U51063; Knol isopods: U5286; Knol fish U51254; P all ,0.001). Overall, there were significant differences between the diets of rock lobsters
caught using the two methods for both prey abundance (ANOSIM: Global R50.691)
and occurrence (Global R50.839).
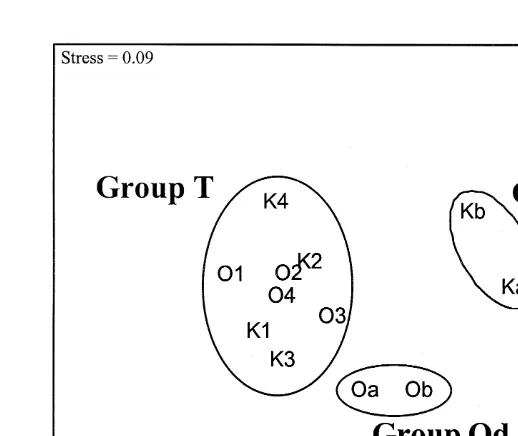
Based on abundance data, the cluster analysis (Fig. 3) clearly separated the data into 3 groups viz.: the Olifantsbos dives (Od), the Knol dives (Kd) and the traps from both sites (T). The stress on the MDS plot was small (0.09) which suggests the hierarchical relationships between the replicates are shown without substantial distortion.
The within-group similarities based on abundance were all large (SIMPER analyses: 75.1–78.6%), but between group similarities were smaller (47–63%). Isopods and fish were apparently responsible for the observed differences between the trap and dive groups (SIMPER, see above). To confirm this, both were excluded from the data, which were then reanalysed. The diets obtained from traps and by diving was then similar for both sites. ANOSIM showed no significant differences between the methods, at either site, for occurrence (P.0.05) or abundance (P.0.5) data. An MDS plot (based on
Fig. 2. Percentage occurrence of prey items in the gut contents of trap- (clear bars) and diver-caught (solid bars) rock lobsters at Olifantsbos and the Knol.
4. Discussion
Isopods were attracted in large numbers into the traps: up to 3500 per trap (Fig. 1). None of the unbaited control traps caught isopods, which indicates isopods are being lured to the traps by the bait, perhaps in quantities large enough for the isopods to be easily available for opportunistic consumption by the rock lobsters.
Data from both sites showed a significant difference (P,0.001) in the abundance of
both isopods and fish in rock-lobster stomachs between the dive and trap samples. At Olifantsbos, fish and isopods contributed about half the gut contents for the trap samples – but only 15% in the dive samples. The contrast was much stronger for the Knol; isopods comprised 90% of the stomach contents of trap-caught rock lobsters but no isopods were recorded in the dive samples. The occurrence data reflected similar results (Fig. 2).
Fig. 3. Multidimensional scaling plot (based on prey species abundance) to compare the groupings of the diet of rock lobsters caught at two sites (first letters: K5Knol, O5Olifantsbos) using the different collection methods (a and b5dive replicates, 1–45trap replicates).
dive groups were respectively 64% and 47% similar to the combined trap group. Isopods and fish contributed .46% to the cumulative differences observed between the two dive
groups and the traps. Similar analyses on the abundance data showed the same result – isopods and fish were again responsible for most of the differences in diet observed between the dives and the combined trap group. Reanalysis of the data following removal of the fish and isopods from the diet information showed, as expected, no separation of dive- and trap-caught samples. The similarity between dive and trap samples increased to about 80% (compared to about 60%). Thus, we conclude that isopods and fish found in the stomach contents of Jasus lalandii were only eaten because they were encountered in the traps, where they had been attracted by the bait. They are therefore artefacts of the trap sampling technique and need to be excluded from any dietary analyses of trap-caught J. lalandii.
The dietary composition of the dive-caught rock lobsters was assumed to represent the natural diet of Jasus lalandii. The 15% occurrence, but only 2% of the abundance, of cirolanids in the Olifantsbos dive samples was unexpected, and was thought to be from accidental ingestion by rock lobsters scavenging on other prey. Many ribbed mussels (Aulacomya ater) find refuge within the holdfasts of kelp. Rock lobsters preying on those mussels may inadvertently consume isopods, which occur in high densities within
22
but are unlikely to even approach the proportions or quantities recorded for trap-caught animals.
Of considerable interest was the apparent predation on fish by trap-caught J. lalandii. Mayfield (1998) also noted fish in the diet of rock lobsters, especially in rock lobsters larger than 80 mm (carapace length) and in all animals caught in deep water (50 m). Rock lobsters are known to be opportunistic feeders (Barkai and Branch, 1988b), and probably eat the most readily available protein they can find. Indeed, the commercial rock-lobster fishery is based on the principle of attracting rock lobsters into the traps using fish-bait. However, the presence of fish has not been recorded in any other previous studies on J. lalandii diet (other than those of Mayfield, 1998), so these results warrant careful consideration.
Pieces of fish found in the stomachs were typically skull bones and vertebrae of small fish (vertebrae: 2–3 mm in diameter), and were identified as coming from species other than that used for bait (Trachurus trachurus). Rock lobsters scavenging on dead or wounded fish could account for some, but not all of the high occurrence (48%) and abundance (35%) of fish in the guts. Wassenberg and Hill (1989) and Lawton and Lavalli (1995) reported fish in the diets of lobsters, but suspected it was from discarded bait from commercial trawlers. Equally possible is that rock lobsters are actively preying on small fish. Lawton and Lavalli (1995) and Mayfield (1998) noted lobsters preying on fish in the field. The lobsters could be attacking sleeping fish which were experiencing reduced awareness. Night-time Scuba divers have anecdotal evidence to suggest that this could be possible, in that sleeping fish are sluggish and slow to respond to touch. The rock lobsters may also be ambushing small cryptic fish species (Clinus spp.), that become trapped within rock crevices by Jasus lalandii. It is also possible that clinids may be caught by J. lalandii while the rock lobsters are feeding on other foods such as mussels. Clinids are inquisitive fish that will readily dart in to snatch scraps when the opportunity presents itself (personal observation) and may be captured by rock lobsters in the process.
Fish were more frequently found in guts of trap-caught than diver-caught J. lalandii. Clinid fish were often caught in the traps, which suggests that, like cirolanids, small fish are attracted into traps by the bait and therefore could have become opportunistic prey for the trapped J. lalandii. Thus, it remains plausible that fish are actually captured by lobsters. However, the significantly higher proportion of fish in the diet of trap-caught versus diver-caught lobsters suggests very strongly that their contribution to the diet is inflated by a ‘‘trap effect’’, i.e. an artefact of the sampling technique. Researchers using traps to collect animals for dietary studies should therefore make allowances for possible trap effects on their results.
Acknowledgements
particular Dr A. Cockcroft, D. Van Zyl and M. Noffke, and research crew of the SFRV ‘‘Sardinops’’. Financial support was provided through a Foundation for Research Development grant to Prof. G.M. Branch, Coastal Ecology Unit, University of Cape Town. [AU]
References
Barkai, A., Branch, G.M., 1988a. Energy requirements for a dense population of rock lobsters Jasus lalandii: novel importance of unorthodox food sources. Mar. Ecol. Prog. Ser. 50, 83–96.
Barkai, A., Branch, G.M., 1988b. Contrasts between the benthic communities of subtidal hard substrata at Marcus and Malgas Islands: a case of alternate stable states. S. Afr. J. Mar. Sci. 7, 117–137.
Barkai, A., Davis, C.L., Tugwell, S., 1996. Prey selection by the South African Cape Rock Lobster, ecological and physiological approaches. Bull. Mar. Sci. 58 (1), 1–8.
Bennett, D.B., 1974. The effects of pot immersion times on catches of crabs, Cancer pagurus (L.) and lobsters,
Homarus grammarus (L.). Journal du Conseil International pour l’Exploration de La Mer 35 (3), 332–336.
Bennett, D.B., Brown, C.G., 1979. The problem of pot immersion time in recording and analysing catch-effort data from a trap fishery. Rapportes et Proces-Verbaux des Reunions, Conseil International pour l’Explora-tion de la Mer 175, 186–189.
Berg, J., 1979. Discussion of the methods of investigating the food of fishes, with reference to a preliminary study of the prey of Gobiusculus flavescens (Gobiidae). Mar. Biol. 50, 263–273.
Caddy, J.F., 1979. Some considerations underlying definitions of catchability and fishing effort in shellfish industries, and their relevance for stock assessment purposes. Manuscr. Rep. Fish. Mar. Serv. Can. 1489, 1–24.
Clarke, K.R., Warwick, R.M., Carr, M., 1994. PRIMER.v4.2.Plymouth Marine Laboratory, Plymouth, U.K. Dayton, P.K., 1985. Ecology: a science and a religion. In: Livingston, R.J. (Ed.), Ecological Processes in
Coastal and Marine Systems, Plenum Press, New York, pp. 3–18.
Field, J.G., 1971. A numerical analysis of changes in the soft-bottom fauna along a transect across False Bay, South Africa. J. Exp. Mar. Biol. Ecol. 7, 215–253.
Field, J.G., Griffiths, C.L., Griffiths, R., Jarman, J., Zoutendyk, P., Velimerov, B., Bowes, A., 1980. Variations in the structure and biomass of kelp communities along the West Coast of South Africa. Trans. Roy. Soc. S. Afr. 56, 24–36.
Giller, P.S., 1986. The natural diet of the Notonectidae: field trials using electrophoresis. Ecol. Entomol. 11, 163–172.
Griffiths, C.L., Seiderer, J.L., 1980. Rock lobsters and mussels – limitations and preferences in a predator– prey interaction. J. Exp. Mar. Biol. Ecol. 44, 95–109.
Heydorn, A.E.F., 1969. The rock lobster of the South African West Coast Jasus lalandii (H. Milne-Edwards) 2: population study, behaviour, reproduction, moulting, growth and migration. Investl. Rep. Div. Sea Fish. S. Afr. 71, 1–52.
Hyslop, E.J., 1980. Stomach content analysis – a review of methods and application. J. Fish. Biol. 17, 411–429.
Knutsen, H., Vogt, N.B., 1985a. An approach to identifying the feeding patterns of lobsters using chemical analysis and pattern recognition by the method of SIMCA 1: identification of prey organism, Artemia salina (L.) in the stomachs of juvenile lobsters, Homarus grammarus (L.). J. Exp. Mar. Biol. Ecol. 89, 109–119. Knutsen, H., Vogt, N.B., 1985b. An approach to identifying the feeding patterns of lobsters using chemical analysis and pattern recognition by the method of SIMCA 2: attempts at assigning stomach contents of lobsters, Homarus grammarus (L.), to infauna and detritus. J. Exp. Mar. Biol. Ecol. 89, 121–134. Lawton, P., Lavalli, K.L., 1995. Postlarval, juvenile, adolescent, and adult ecology. In: Factor, J.R. (Ed.),
Biology of the Lobster (homarus Americanus), Academic Press, New York, pp. 47–88.
Miller, R.J., Addison, J.D., 1995. Trapping interactions of crabs and American lobsters in laboratory tanks. Can J. Fish. Aquat. Sci. 52, 315–324.
Morgan, G.R., 1974. Aspects of the population dynamics of the Western rock lobster, Panulirus cygnus George, 2 Seasonal changes in the catchability coefficient. Aust. J. Mar. Freshwater Res. 25, 249–259. Newman, G.G., Pollock, D.E., 1969. The efficiency of rock lobster fishing gear. S. Afr. Shipp. News Fish. Ind.
Rev. 24, 79–81.
Newman, G.G., Pollock, D.E., 1974. Growth of the rock lobster Jasus lalandii and its relationship to benthos. Mar. Biol. 24, 339–346.
Paterson, N.E., 1969. The behaviour of captive Cape rock lobsters, Jasus lalandii (H. Milne-Edwards). Ann. S. Afr. Mus. 52 (10), 225–266.
Peterson, C.H., Black, R., 1994. An experimentalist’s challenge: when artefacts of intervention interact with treatments. Mar. Ecol. Prog. Ser. 111, 289–297.
Pollock, D.E., 1979. Predator–prey relationships between the rock lobster Jasus lalandii and the mussel
Aulacomya ater at Robben Island on the Cape West Coast of Africa. Mar. Biol. 52, 347–356.
Pollock, D.E., 1982. The fishery for and the population dynamics of West Coast rock lobster related to the environment in the Lamberts Bay and Port Nolloth areas. Investl. Rep. Sea Fish. Inst. S. Afr. 124, 1–57. Pollock, D.E., 1986. Review of the fishery for and the biology of the Cape rock lobster Jasus lalandii with
notes on larval recruitment. Can. J. Fish. Aquat. Sci. 43, 2107–2117.
Pollock, D.E., Augustyn, C.J., Goosen, P.C., 1982. The rock lobster Jasus lalandii and its environmental biology on the Saldanha Columbine off the Cape West Coast, 1978–1981. Investl. Rep. Sea Fish. Inst. S. Afr. 125, 1–30.
Robertson, W.D., 1989. Factors affecting catches of the crab, Scylla serrata (Forskal) (Decapoda:Portunidae) in baited traps: soaktime of day and accessibility of the bait. Estuarine, Coast. and Shelf Sci. 10, 161–170. Robles, C., Sweetnam, D., Eminike, J., 1990. Lobster predation on mussels: shore level differences in prey
vulnerability and predator preference. Ecology 71 (4), 1564–1577. `
Sarda, F., Valladares, F.J., 1990. Gastric evacuation of different foods by Nephrops norvegicus (Crustacea: Decapoda) and estimation of soft tissue ingested, maximum food intake and cannibalism in captivity. Mar. Biol. 104, 25–30.
Shafir, A., Field, J.G., 1980. Importance of a small carnivorous isopod in energy transfer. Mar. Ecol. Prog. Ser. 3, 203–215.
Shepherd, S.A., 1973. Studies on Southern Australian abalone (genus Haliotis) 1: Ecology of five sympatric species. Aust. J. Mar. Freshwat. Res. 24, 217–257.
Spanier, E., Stanley Cobb, J., Clancy, M., 1994. Impacts of remotely operated vehicles (ROVs) on the behaviour of marine animals: an example using American lobsters. Mar. Ecol. Prog. Ser. 104, 257–266. Underwood, A.J., 1986. The analysis of competition by field experiments. In: Kikkawa J Anderson, D.J. (Ed.),
Community Ecology: Pattern and Process, Blackwell Scientific Press, London, pp. 240–268.
Wassenberg, T.J., Hill, B.J., 1989. Diets of four decapod crustaceans (Linuparus trigonus, Metanephrops
andamanicus, M. Australienis and M. boschmani ) from the continental shelf around Australia. Mar. Biol. 103, 161–167.
Williams, M.J., 1981. Methods for analysis of natural diet in portunid crabs (Crustacea: Decapoda: Portunidae). J. Exp. Mar. Biol. Ecol. 52, 103–113.
Williams, M.J., Hill, B.J., 1982. Factors influencing pot catches and population estimates of the portunid crab
Scylla serrata. Mar. Biol. 71, 187–192.