Summary Adaptive physiological changes were investi-gated in seven populations of Sitka (Picea sitchensis (Bong.) Carr.) × interior spruce (P. glauca (Moench) Voss ×P. engel-mannii Parry ex Engelm.) spanning the Nass--Skeena transition zone in British Columbia, Canada. Each population was repre-sented by an Si rDNA index that was calculated from the relative optical densities on a gel autoradiogram of five ribo-somal DNA bands characteristic of Sitka spruce and interior spruce. This index estimates the proportion of the genome contributed by interior spruce. Physiological adaptations were assessed by gas exchange parameters measured under both well-watered and drought conditions. Under well-watered con-ditions, Sitka spruce populations had higher maximal photo-synthesis at saturating light and ambient CO2, higher quantum
yield at the light compensation point, and higher dark respira-tion than interior spruce popularespira-tions. Sitka spruce popularespira-tions also reached maximal photosynthesis at lower photosyntheti-cally active radiation and higher CO2 concentrations, and had
higher stomatal densities that resulted in lower stomatal limi-tations to photosynthesis than interior spruce populations. In contrast, interior spruce populations exhibited greater drought tolerance than Sitka spruce populations. Their gas exchange rates declined at a slower rate in response to drought. They maintained higher gas exchange rates in response to moderate to severe drought (predawn plant water potentials = −1.5 MPa), and their photosynthetic rates recovered faster when they were rewatered after exposure to drought. Comparison of the seven populations indicated that physiological parameters were sig-nificantly related to the Si rDNA index. An increase in Si rDNA index was associated with proportional changes in physiologi-cal measurements, suggesting that genetic interchange among species with contrasting ecological adaptations can enhance the environmental adaptation of natural populations.
Keywords: DNA analysis, drought, Picea engelmannii, Picea glauca, Picea sitchensis.
Introduction
Introgressive hybridization occurs between Sitka spruce (Picea sitchensis (Bong.) Carr.) and interior spruce (P. glauca (Moench) Voss × P. engelmannii Parry ex. Engelm.) in the Nass--Skeena transition zone in northwestern British
Colum-bia, Canada (Roche 1969, Daubenmire 1972). Sitka spruce naturally occurs in the maritime climate on the Pacific coast of North America (Burns and Honkala 1990). The parental spe-cies of interior spruce, Engelmann spruce (P. engelmannii) and white spruce (P. glauca), have contrasting ecological adapta-tions. Engelmann spruce grows in cool, humid environments at high elevations, whereas white spruce is found in dry, cool, continental conditions across North America (Burns and Honkala 1990). Reproductive difficulties sometimes occur when two distinctive spruce species are crossed, but Sitka, Engelmann, and white spruce are phylogenetically close and hybridize easily (Wright 1955). It is important to understand the consequences of introgression between these spruce spe-cies, which may help develop breeding and dispersal strate-gies.
Populations taken from the Sitka × interior spruce introgres-sion zone are highly heterogeneous in both their nuclear and plastid DNA (Sutton et al. 1991a, 1991b, 1994). Sitka spruce has different ecological adaptations than interior spruce, there-fore, the DNA exchange between these species has caused corresponding alterations in physiological attributes. Changes in drought and freezing tolerance of Sitka × interior spruce populations correspond with their nuclear ribosomal DNA (rDNA) patterns (Grossnickle et al. 1996). These results are consistent with evolutionary studies, which suggest that intro-gression may produce new ecotypes both by transferring exist-ing adaptive characteristics from one population to another and by giving rise to new adaptations (Rieseberg and Wendel 1993).
Because of the scarcity of experimental evidence for adap-tive variabilities resulting from DNA exchange among species with different ecological adaptations (Grossnickle et al.1996), little is known about the significance of DNA changes on morphological, physiological and phenological attributes of trees (Mitton 1995). Individual forest tree species are usually distributed over a large geographic range and their genotype and phenotype vary with environmental variation across the range (Abrams 1994). It is important, therefore, to establish a relationship between genotypic (DNA) and phenotypic (i.e., physiological) attributes that is independent of environmental variability.
Following the study of Grossnickle et al. (1996), we exam-ined the adaptive significances of DNA exchange in seven
Relationships between gas exchange adaptation of Sitka
××
interior
spruce genotypes and ribosomal DNA markers
SHIHE FAN, STEVEN C. GROSSNICKLE and BEN C. S. SUTTON
Forest Biotechnology Centre, B.C. Research, Inc., 3650 Wesbrook Mall, Vancouver, B.C. V3S 2L2, Canada
Received February 10, 1996
Sitka × interior spruce introgressive hybrid populations from the Nass--Skeena transition zone. Specifically, gas exchange characteristics were measured under both well-watered and drought conditions, and their relationships with the rDNA values of the populations examined. Results suggested that DNA exchange between Sitka spruce and interior spruce has broadened the ecological adaptations of spruce populations in the Nass--Skeena transition zone.
Materials and methods
Plant materials
Seven Sitka (Picea sitchensis) × interior (P. glauca × P. engel-mannii) spruce populations were sampled from the Nass--Skeena transition zone in northwestern B.C., Canada. Four were wild seed collections, one was an operational seedlot pooled from the Bulkley Valley seed orchard, and two were seedlots from open pollinated families within the Bulkley Valley seed orchard (each consisting of five families based on the localities of the original parental trees). A brief summary of the seed collection is given in Table 1 and a detailed descrip-tion has been given by Sutton et al. (1994).
The history of seedling culture has been detailed by Gross-nickle et al. (1996). In brief, seedlings were 1-year-old con-tainer-grown plants raised at the B.C. Ministry of Forests Saanich nursery (Sidney, B.C., Canada; 48°28′ N and 124°10′ W). Seedlings were transplanted in the second year to a farm field site at the University of B.C. (Vancouver, B.C., 49°15′ N and 123°15′ W). Seedlings used in the experiment had been planted in 11-liter pots to facilitate rapid and nondisruptive removal for physiological testing. The pots were randomly placed within the field site design and submerged in the soil so that seedlings were exposed to normal site edaphic conditions. Just before the third growing season, potted seedlings were relocated to an outdoor compound at B.C. Research, Inc. (2 km away from the farm field site). During the third year, seedlings were watered every three days and fertilized every other week with 20,8,20 (N,P,K) Plant Prod forestry seedling fertilizer (Plant Products Co., Ltd., Brampton, ON, Canada) (equivalent to 200 ppm N). Experiments were started in late June immedi-ately after seedlings had set terminal buds.
DNA Analysis
Each value of Si rDNA index listed in Table 1 was calculated from rDNA data taken on eight experimental seedlings within a population. Details of the DNA analysis procedures have been described previously (Sutton et al. 1994, Grossnickle et al. 1996). In brief, rDNA was isolated from three buds from each seedling. After purification, rDNA was digested with the restriction enzyme, HindIII, followed by electrophoresis in a 0.8% agarose gel. Ribosomal DNA was transferred to Hybond filters (Amersham, Oakville, ON, Canada) by Southern blot-ting, and hybridized with a α32P-dATP labeled probe, Eco 2.0 (yeast 18S RNA gene). Ribosomal DNA bands were visual-ized by autoradiography, and an Si rDNA index was then calculated, as described previously, for each seedling accord-ing to the optical density of the five characteristic bands (Sut-ton et al. 1994, Grossnickle et al. 1996). The Si rDNA index gives an overall estimation of interior spruce DNA contribu-tion to the genome of each seedling.
Photosynthetic characterization of well-watered and drought-treated seedlings
Photosynthetically active radiation (PAR) versus net photo-synthesis (Pn) curves and intercellular CO2 (Ci) versus Pn
curves were constructed from the seven test populations. Measurements were taken on newly developed, unshaded, lateral branches in the upper crown with an LI-6200 Portable (closed-loop) Photosynthesis System and a 250-ml leaf cuvette (Li-Cor, Inc., Lincoln, NE). Curves were constructed from six seedlings for each population.
The PAR--Pn response curves were measured twice on the
same branch of each seedling under both well-watered (pre-dawn plant water potentials (Ψpd) of about −0.2 MPa) and
moderate drought (Ψpd of −1.0 to −1.1 MPa) conditions.
Meas-urements were taken in an environmentally controlled growth room providing the following conditions: 22 °C air tempera-ture, 40 ± 5% relative humidity, and 400 ± 10 µl l−1 ambient
CO2. Light was provided by two Sylvania 1000 W Metalarc®
metal halide lamps (Osram Sylvania Inc., Manchester, NH). To obtain the PAR--Pn response curve, PAR was varied by
chang-ing the thickness of plastic sheets between the seedlchang-ing and the light. Air temperature in the leaf cuvette was held virtually constant during measurements with a 360-mm external electri-cal fan. Measurements were started with dark-adapted
seed-Table 1. Summary of seed source information and the interior spruce rDNA index (mean ± SE; n = 8) for each test population.
Source Seedlot Seed zone1 Location Elevation (m) Si rDNA Index
1 1004 NST Kitwanga, 55°20′ N; 128°05′ W 305 0.51 ± 0.06
lings. After a change in PAR, further measurements were not made until after an interval of 15--20 min, which in a prelimi-nary trial was found sufficient to allow photosynthesis to reach a steady state.
The Ci--Pn curves were measured on well-watered seedlings
under the conditions described above except that PAR was 750 µmol m−2 s−1 and CO2 in the leaf cuvette was progressively
reduced from an initially high concentration (1100--1200 µl l−1) by seedling photosynthesis (McDermitt et al. 1989). Before each Ci--Pn measurement, the initial CO2 concentration was
sustained for 10 min by slowly pumping CO2 (compressed
CO2 mixed with air) into the LI-6200 system through an
external flow switch using a Li-Cor 6000-01 gas calibration cylinder. This treatment acclimated stomata and photosynthe-sis to the high CO2 environment and stabilized stomatal
con-ductance without significant variation during the 30- to 50-min measuring period. After this acclimation treatment, CO2 in the
cuvette was drawn down by the seedling (McDermitt et al. 1989), and data logging was started when CO2 in the cuvette
was 900 to 1000 µl l−1. When CO
2 in the cuvette reached the
CO2 compensation point for Pn, the remaining CO2 in the
system was drawn down further by both the online and an external soda lime tube attached to the external flow switch. This allowed the Ci--Pn curves to be extended to a CO2
concen-tration approaching zero. Both Pn and Ci were corrected for
system leakage following the manufacturer’s recommenda-tions (Application No. 103, Li-Cor, Inc. 1991).
Gas exchange response to drought
After the measurements of PAR--Pn and Ci--Pn under
well-wa-tered conditions, seedlings were subjected to soil drought by withholding water for approximately 35 to 40 days. The drought was simulated under an outdoor rain shelter. The evening before each measuring day, seedlings were moved to the growth room and placed on a darkened growth table for 10 h. The next morning, Ψpd was measured with a pressure
chamber (Model 3005, Soil Moisture Equipment Corp. Santa Barbara, CA) on a lateral branch from the upper crown of each seedling before the lights were turned on. Two hours later, gas exchange parameters (net photosynthesis (Pn), stomatal
con-ductance to water vapor (gwv) and transpiration flux density
(TFD)) were measured on an upper crown lateral branch with the LI-6200 system under conditions similar to those for Ci--Pn
measurements, except that CO2 concentration in the leaf
cu-vette was 390 ± 10 µl l−1. The same branch was measured
throughout the drought and rewatering period. When Pn
de-creased to just less than 0, the soil was fully rehydrated. Gas exchange was measured again exactly 24 h after rehydration. To compare the relative rates of gas exchange recovery after rewatering, rates relative to the drought values are pre-sented. Total needle surface area of each lateral branch sample enclosed in the leaf chamber for gas exchange measurements was determined from a relationship with oven-dried (70 °C) needle dry weight. Gas exchange measurements were then recalculated accordingly.
Stomatal morphology
Stomatal sizes were measured on current-year needles of five selected populations (Nos. 1--4, and 7 in Table 1, Nos. 5 and 6 were excluded to reduce sample sizes). Three needles from each of the top, middle, and bottom positions on an upper-crown branch were randomly excised, cut in half, and the mesophyll removed. Stomatal sizes (nine per needle) on the needle epidermis were then determined under a light micro-scope. Stomatal density assessment (nine views per impres-sion) was made on needle surface impressions made from fingernail polish. Three impressions were made for each seed-ling.
model parameters. The last negative Pn data point of each
seedling was excluded from the data set used for modeling. To compare the dehydration tolerance of populations, the values of Ψpd at which Pn, gwv, or TFD were half their pre-drought
maxima were determined by means of Equation 1. The higher the Ψpd value, the lower the dehydration tolerance. Equation 1
was also used to normalize Pn, gwv, and TFD to −1.5 MPa for
urements (because real Rd is generally inhibited at PAR above
500 µmol m−2 s−1 (Villar et al. 1994)); b is the potential
maximum Pn under saturating PAR or ambient CO2; and c is
the light utilization or CO2 affinity constant when Pn was half
maximal (Leopold and Kriedemann 1975). Equation 2 gener-ally fitted the data well (r2 from0.97 to 1.00 for Ci--Pn curves
and 0.94 to 0.98 for PAR--Pn curves, respectively). Quantum
yield (φ), the initial slope at the light compensation point for Pn, and carboxylation efficiency of ribulose-1,5-bisphosphate
carboxylase/oxygenase (Rubisco) (VRuBP), the initial slope at
the CO2 compensation point for Pn, were estimated from the
PAR--Pn and Ci--Pn curves, respectively (Farquhar et al. 1980).
Stomatal limitations (I) to Pn were estimated from Ci--Pn
curves by the differential method (Jones 1985):
I = 1/gwv
1/gwv + 1/gm
, (3)
where gwv was the slope of the ‘‘supply function,’’ i.e.,
curve, at the operating point. The operating point was deter-mined according to the ‘‘demand’’ and ‘‘supply’’ functions fitted or measured for each individual seedling.
The relationship of the Si rDNA index to physiological measurements in the seven populations was examined by the Pearson product-moment correlation analysis, because the Si rDNA index was designed to describe the genotype of a population in a specific subzone in the Nass--Skeena transition zone, and not individual experimental seedlings. This analysis acknowledged the means and standard errors of both Si rDNA indices and physiological measurements of each population. This established a relationship between the Si rDNA index and physiological parameters under both well-watered and drought conditions. Population differences for specific physiological parameters were evaluated by analysis of variance (with Systat software, SYSTAT, Inc., Evanston, IL), except when effects of DNA exchange were examined.
Results
Photosynthetic characterization of well-watered and drought-treated seedlings
Sitka spruce populations had higher maximum Pn in saturating
light and under well-watered conditions (open symbols, Ψpd
approximately −0.2 MPa) and lower maximum Pn in moderate
drought (filled symbols, Ψpd of −1.0 to −1.1 MPa) (Figure 1a)
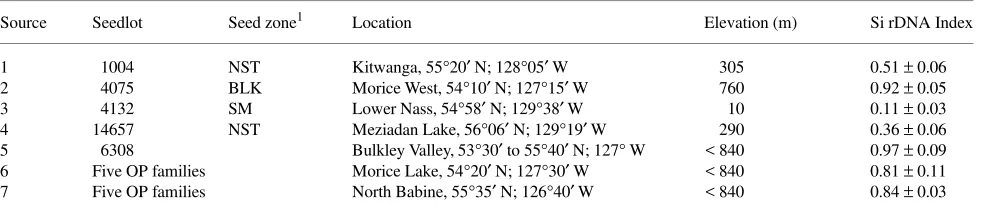
than interior spruce populations. Under well-watered and moderate drought conditions, Sitka spruce populations (Si rDNA index = 0.11) reached half maximal Pn at lower PAR
than interior spruce populations (Si rDNA index = 0.97), with hybrids in between (Figure 1b). The light compensation point for Pn was similar in both well-watered (52 to 54 µmol m−2 s−1)
and moderate drought (31 to 41 µmol m−2 s−1) conditions in all
Sitka, interior, and hybrid spruce populations.
Quantum yield (φ) of photosynthesis was higher (P > 0.04) in Sitka spruce populations than in interior spruce populations, and tended to decline as the Si rDNA index increased (Fig-ure 1c). This trend of φ remained the same in both well-wa-tered and drought-treated seedlings.
Under well-watered conditions, Sitka spruce populations had higher potential maximal Pn at saturating CO2
concentra-tions than interior spruce populaconcentra-tions (Figure 2a). Carbon dioxide saturation of Pn also occurred at higher concentrations
in Sitka spruce populations than in interior spruce populations. As the Si rDNA index increased, Ci at half maximal Pn
oc-curred at lower CO2 concentrations (Figure 2b). However,
there was no difference in carboxylation efficiency of Rubisco between all measured Sitka and interior spruce populations (Figure 2c).
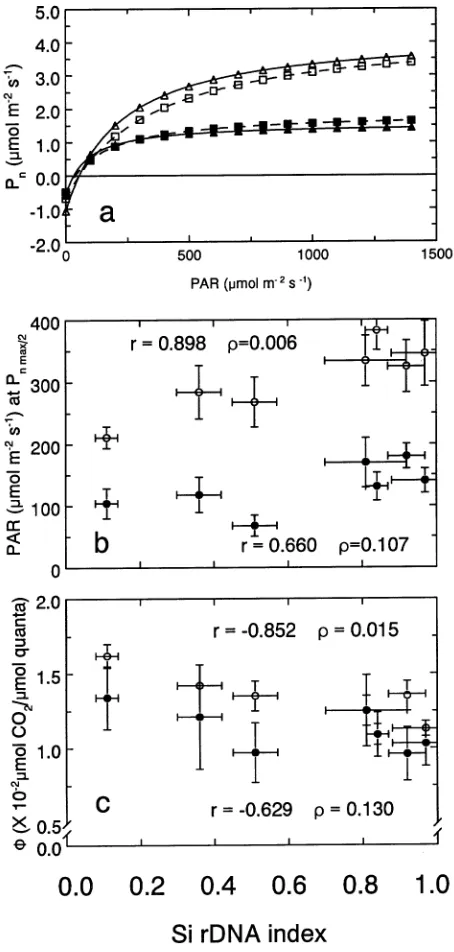
Under well-watered conditions, stomatal limitations to Pn
were slightly lower in Sitka spruce than in interior spruce populations (Figure 3a). Population differences in stomatal limitations were primarily a result of differences in stomatal densities, which were higher in Sitka spruce populations than in interior spruce populations (Figure 3b). Three to six rows of stomata were common on the adaxial side of Sika spruce needles, whereas two to three (two in most cases) rows of
stomata were typically observed on the adaxial side of interior spruce needles. In contrast, stomatal size tended to be larger in populations having higher Si rDNA indices (29.0 ± 0.9 µm for interior spruce (Si rDNA index = 0.97)) than in populations
Figure 1. (a) Net photosynthesis (Pn) versus photosynthetically active
radiation (PAR) in Sitka (Si rDNA index = 0.11, triangles) and interior (Si rDNA index = 0.97, squares) spruce seedlings. (b) Photosyntheti-cally active radiation at half maximal Pn in relation to Si rDNA index
in Sitka × interior spruce populations. (c) Photosynthetic quantum yield at the light compensation point (φ) versus Si rDNA index in Sitka
× interior spruce populations. Open symbols indicate well-watered conditions, filled symbols signify moderate (Ψpd at −1.0 to −1.1 MPa)
having lower Si rDNA indices (26.3 ± 0.8 µm for Sitka spruce (Si rDNA index = 0.11)), although they were not statistically different.
Gas exchange response to drought
Under well-watered conditions, Sitka spruce populations had higher Pn, gwv, TFD, and Rd, than interior spruce populations,
with gas exchange parameters decreasing as the Si rDNA index increased (Figure 4). However, when seedlings were subjected to drought, Pn, gwv and TFD (normalized at Ψpd of −1.5 MPa)
were all lower in Sitka spruce populations having a lower Si rDNA index (Figures 4a--4c). The exception, under drought conditions, was Rd (measured at Ψpd of −1.0 −1.1 MPa), which
was similar for all Sitka × interior spruce populations (Fig-ure 4d).
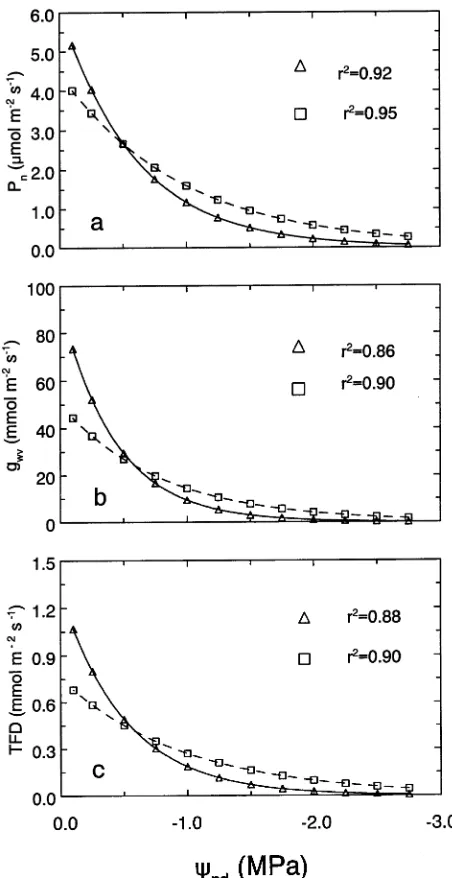
Lower gas exchange rates in drought-treated Sitka spruce populations than in interior spruce populations could be as-cribed to an intolerance to dehydration (Figure 5). Although initially higher under well-watered conditions, Pn, gwv, and
TFD all dropped sharply in Sitka spruce populations as seed-ling Ψpd decreased. In contrast, these parameters declined at a
slower rate in drought-treated interior spruce populations. Be-cause of these differences in sensitivity to dehydration, Pn, gwv,
and TFD fell to half of their predrought maximum at lower Ψpd
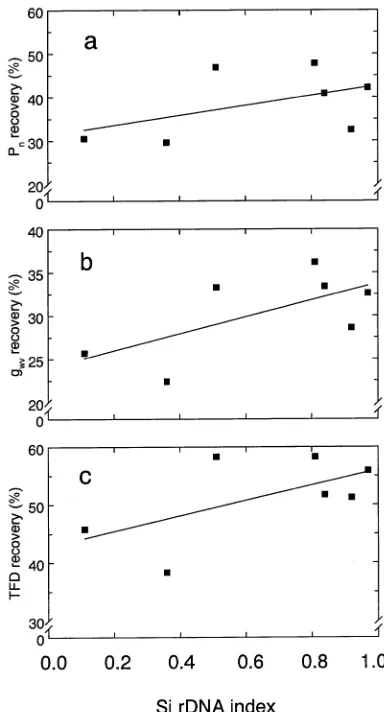
in populations with a higher Si rDNA index (Figure 6). Twenty-four hours after drought-treated seedlings had been watered, Pn, gwv, and TFD recovered partially in all
popula-tions, but the degree of recovery varied (Figure 7). Populations with a higher Si rDNA index generally had a more rapid recovery of Pn, gwv, and TFD after drought, measured by
recovery to predrought values, than populations with a lower
Figure 2. (a) Net photosynthesis (Pn) versus intercellular CO2
concen-tration (Ci) in Sitka (Si rDNA index = 0.11, triangles) and interior
(Si rDNA index = 0.97, squares) spruce seedlings. (b) Intercellular CO2 concentrations (Ci) at half maximal Pn, and (c) Rubisco
carboxy-lation efficiency at the light compensation point (VRuBP) under
well-watered conditions versus Si rDNA index in Sitka × interior spruce populations.
Figure 3. (a) Stomatal limitations to Pn, and (b) stomatal densities on
Si rDNA index. Of the gas exchange processes, recovery from drought was highest for TFD (45 to 65%), followed by Pn (25
to 45%), and gwv (18 to 35%).
Discussion
Populations with a higher Si rDNA index exhibited lower maximum Pn under both saturating PAR and CO2 and
well-wa-tered conditions than those with a lower Si rDNA index. When other environmental factors are not limiting, rates of CO2
diffusion to, and CO2 fixation at, the photosynthetic sites in
chloroplasts can limit photosynthesis. Carbon dioxide diffu-sion rates can be affected by stomatal and mesophyll conduc-tance. Fixation of CO2 can be limited by the carboxylation
efficiency of Rubisco at low Ci, the regeneration of ribulose
1,5-bisphosphate at intermediate Ci, and the recycling of
inor-Figure 4. Net photosynthesis (Pn) (a), stomatal conductance to water
vapor (gwv) (b), transpiration flux density (TFD) (c), and dark
respira-tion rates (Rd) (d) versus Si rDNA index in Sitka × interior spruce
populations maintained under well-watered conditions (open symbols) or subjected to moderate drought (Ψpd at −1.5 MPa) (filled symbols),
except dark respiration which was measured at −1.0 to −1.1 MPa.
Figure 5. Changes in net photosynthesis (Pn) (a), stomatal
conduc-tance to water vapor (gwv) (b), and transpiration flux density (TFD) (c)
in relation to decreases in predawn water potentials (Ψpd) for Sitka
(Si rDNA index of 0.11, n) and interior (Si rDNA index of 0.97, u)
ganic phosphate at high Ci concentrations (Farquhar et al.
1980, Sharkey 1985). Rubisco carboxylation efficiency, CO2
and light compensation points for Pn were all similar among
the seven populations, and corresponded to reported values for Sitka spruce (Ludlow and Jarvis 1971, Leverenz and Jarvis 1979) and Engelmann spruce (DeLucia 1986). Carboxylation efficiency could not, therefore, explain the differences in maxi-mum Pn between Sitka and interior spruce populations.
Unlike carboxylation efficiency, gwv differed among the
populations as a result of differences in stomatal densities on needle surfaces. Higher stomatal densities resulted in lower stomatal limitations to Pn, and could partially explain higher
Pn in Sitka spruce populations than in populations having a
higher Si rDNA index. The importance of CO2 diffusion rate
to Pn was further suggested by the higher maximum Pn in
CO2-saturated than in light-saturated conditions in the Sitka ×
interior populations. Similar findings were found when com-paring a coastal with a coastal × interior ponderosa pine (Pinus ponderosa Dougl. ex Laws) population (Monson and Grant 1989). The estimated stomatal limitations for all Sitka × inte-rior populations were comparable to reports for black spruce (Picea mariana (Mill.) B.S.P.) (Stewart et al. 1995) and lob-lolly pine (Pinus taeda L.) (Teskey et al. 1986). Stomatal anatomy seemed to be more important than carboxylation efficiency for describing maximum Pn in the Sitka × interior
populations.
In contrast to well-watered conditions, interior spruce popu-lations had greater dehydration tolerance of gas exchange
Figure 6. Predawn plant water potentials (Ψpd) at which net
photosyn-thesis (Pn) (a), stomatal conductance to water vapor (gwv) (b), and
transpiration flux density (TFD) (c) were at half of maximal values versus Si rDNA index in Sitka × interior spruce populations. The higher the Ψpd values (less negative) the lower a population’s
dehydra-tion tolerance.
Figure 7. The recovery of net photosynthesis (Pn) (a), stomatal
con-ductance to water vapor (gwv) (b), and transpiration flux density (TFD)
(c) of drought-treated seedlings in relation to Si rDNA index in Sitka
processes than Sitka spruce populations, measured by the Ψpd
at which Pn, gwv and TFD are at half their maximal values, and
percent recovery of gas exchange processes after drought. Because stomatal limitations to Pn increase under drought
conditions in spruce species (cf Stewart et al. 1995), higher gwv
in interior than in Sitka spruce populations would explain their higher Pn. Black spruce populations that have greater
dehydra-tion tolerance are able to maintain higher gwv and Pn under
drought, and grow faster on dry sites than populations with more limited dehydration tolerance (Tan et al. 1992, Tan and Blake 1993). Trees occurring naturally on dry sites are gener-ally more dehydration tolerant (Kuhns et al. 1993) and better able to maintain high gas exchange rates under drought condi-tions than trees of the same species occupying more mesic sites (Larsen 1981, Seiler and Johnson 1988). Our data are consis-tent with previous findings of greater drought tolerance in interior spruce than in Sitka spruce (Grossnickle et al. 1996).
Because of its unique geographic location, the Nass--Skeena transition zone has a heterogeneous, transitional climate with both maritime and continental characteristics (Roche and Had-dock 1987, Sutton et al. 1994). Environmental heterogeneity tends to sustain genetic polymorphism (Hedrick 1986), and trees with high degrees of heterozygosity are thought to be more tolerant to environmental stresses (Müller-Starck 1985, Stutz and Mitton 1988, Mitton et al. 1989). Gene transfer between ecologically differing species as the result of intro-gressive hybridization can result in improved environmental adaptation (Oliver et al. 1995) as appears to be true in the case of Sitka and interior spruces. Incorporation of interior spruce genes into the Sitka spruce genome has produced hybrids that have both retained the high photosynthetic capability of Sitka spruce under nondrought conditions, and acquired the greater drought and freezing tolerance of interior spruce (Grossnickle et al. 1996). These diverse physiological features allow Sitka × interior spruce hybrids to occupy environments over a range of transitional habitats, and compete for sites that are not well utilized by either pure Sitka or interior spruce. Thus, environ-mental variability and selection pressure during evolution may explain the existence of hybrid populations between Sitka and interior spruce in the Nass--Skeena transition zone.
In this and previous work (Grossnickle et al. 1996), seed-lings of all seven populations were treated under identical environmental conditions. Differences in physiological attrib-utes (phenotypic variation) between populations were related to their corresponding Si rDNA index (genotypic variation) values. This is consistent with Cheverud’s (1988) argument that phenotypic analysis can sometimes substitute for genetic analysis. However, genetic estimates provide a more direct method of defining population differences (Willis et al. 1991). Nevertheless, caution is required in using physiological (phenotypic) measurements for screening populations with unknown genetic background. Apart from the genetic depend-ency, physiology of tree species is strongly influenced by environmental conditions. Different physiological processes may respond in various ways to environmental conditions. For instance, Pn, gwv, and TFD responses in Sitka and interior
spruce populations changed under different watering regimes,
whereas these populations had similar PAR for maximum Pn,
φ and Rd as soil water availability varied. Elucidation of the
interactions among physiological processes and environ-mental conditions will lead to an understanding of the adaptive significances of interspecific DNA exchange and the use of physiological tests. Testing under both limiting and non-limiting environmental conditions may provide more reliable information for the understanding of the evolutionary conse-quences of species introgression.
In conclusion, introgressive hybridization between Sitka spruce and interior spruce in British Columbia’s Nass--Skeena transition zone has resulted in a wide array of genotypes. Gradual mixing of interior and Sitka spruce populations has caused a proportional reduction in high gas exchange capabil-ity under well-watered conditions that is associated with Sitka spruce populations. On the other hand, greater gas exchange capability under drought conditions is related to greater dehy-dration tolerance in interior spruce than in Sitka spruce popu-lations. The wide range of physiological responses found in Sitka × interior spruce populations could explain the persist-ence of a spruce hybrid swarm in the heterogeneous habitats of the Nass--Skeena transition zone.
Acknowledgments
Funding for this research was provided by the Science Council of British Columbia (Contract No. 175 (T-5)), the British Columbia Ministry of Forests (Contract No. 18605-20/SXSS) grants to Steven C. Grossnickle and Ben C.S. Sutton, and a Natural Science and Engineering Research Council of Canada industrial postdoctoral fel-lowship to Shihe Fan.
References
Abrams, M.D. 1994. Genotypic and phenotypic variation as stress adaptations in temperate tree species: a review of several case studies. Tree Physiol. 14:833--442.
Burns, R.M. and B.H. Honkala. 1990. Silvics of North America. Volume 1, Conifers. USDA For. Serv., Agric. Handbook No. 654. Washington, DC, pp 187--226, 260--267.
Cheverud, J.M. 1988. A comparison of genetic and phenotypic corre-lations. Evolution 42:958--968.
Daubenmire, R. 1972. Taxonomic and ecological relationships be-tween Picea glauca and Picea sitchensis and their ecological inter-pretation. Can. J. Bot. 46:787--798.
DeLucia, E.H. 1986. Effect of low temperature on net photosynthesis, stomatal conductance and carbohydrate concentration in Engel-mann spruce (Picea engelmannii Parry ex Engelm.) seedlings. Tree Physiol. 2:143--154.
Farquhar, G.D., S. von Caemmerer and J.A. Berry. 1980. A biochemi-cal model of photosynthetic (CO2) assimilation in leaves of C3
species. Planta 149:78--90.
Grossnickle, S.C., B.C.S. Sutton, R.S. Folk and J.R. Gawley. 1996. Relationship between nuclear DNA markers and physiological pa-rameters for Sitka × interior spruce populations. Tree Physiol. 16:547--556.
Hedrick, P.W. 1986. Genetic polymorphism in heterogeneous environ-ments: a decade later. Annu. Rev. Ecol. System. 17:535--566. Jones, H.G. 1985. Partitioning stomatal and nonstomatal limitations to
Kuhns, M.R., W.W. Stroup and G.M. Gebre. 1993. Dehydration toler-ance of five bur oak (Quercus macrocarpa) seed sources from Texas, Nebraska, Minnesota, and New York. Can. J. For. Res.
23:387--393.
Larsen, J.B. 1981. Geographic variation in winter drought resistance of Douglas-fir (Pseudotsuga menziesii Mirb. Franco). Silvae Genet.
30:4--5.
Leopold, A.C. and P.E. Kriedemann. 1975. Plant growth and develop-ment. McGraw-Hill Book Co., Toronto, pp 102--103.
Leverenz, J.W. and P.G. Jarvis. 1979. Photosynthesis in Sitka spruce. VIII. The effects of light flux density and direction on the rate of net photosynthesis and the stomatal conductance of needles. J. Appl. Ecol. 16:919--932.
Ludlow, M.M. and P.G. Jarvis. 1971. Photosynthesis in Sitka spruce (Picea sitchensis (Bong.) Carr.). J. Appl. Ecol. 8:925--953. Mitton, J.B. 1995. Genetics and the physiological ecology of conifers.
In Ecophysiology of Coniferous Forests. Eds. W.K. Smith and T.M. Hinckley. Academic Press, New York, pp 1--36.
Mitton, J.B., H.P. Stutz, W.S. Schuster and K.L. Shea. 1989. Geno-typic differentiation at PGM in Engelmann spruce from wet and dry sites. Silvae Genet. 38:217--221.
Mcdermitt, D.K., J.M. Norman, J.T. Davis, T.M. Ball, T.J. Arkebauer, J.M. Welles and S.R. Roemer. 1989. CO2 response curves can be
measured with a field-portable closed-loop photosynthesis system. Ann. Sci. For. 46 (suppl.):416s--420s.
Monson, R.K. and M.C. Grant. 1989. Experimental studies of ponder-osa pine. III. Differences in photosynthesis, stomatal conductance, and water-use efficiency between two genetic lines. Am. J. Bot.
76:1041--1047.
Müller-Starck, G. 1985. Genetic differences between ‘tolerant’ and ‘sensitive’ beeches (Fagus sylvatica L.) in an environmentally stressed adult forest stand. Silvae Genet. 34:241--246.
Oliver, M.J., D.L. Ferguson and J.J. Burke. 1995. Interspecific gene transfer: implications for broadening temperature characteristics of plant metabolic processes. Plant Physiol. 107:429--434.
Rieseberg, L.H. and J.F. Wendel. 1993. Introgression and its conse-quences in plants. In Hybrid Zones and Evolutionary Processes. Ed. R.G. Harrison. Oxford University Press, New York, pp 70--109. Roche, L. 1969. A genecological study of the genus Picea in British
Columbia. New Phytol. 68:505--554.
Roche, L. and P.G. Haddock. 1987. Sitka spruce (Picea sitchensis) in North America with special reference to its role in British forestry. Proc. Royal Soc., Edinburgh, 93B:1--12.
Seiler, J.R. and J.D. Johnson. 1988. Physiological and morphological responses of three half-sib families of loblolly pine to water-stress conditioning. For. Sci. 34:487--495.
Sharkey, T.D. 1985. Photosynthesis in intact leaves of C3 plants:
physics, physiology and rate limitations. Bot. Rev. 51:53--105. Stewart, J.D., A. Zine El Abidine and P.Y. Bernier. 1995. Stomatal and
mesophyll limitations of photosynthesis in black spruce seedlings during multiple cycles of drought. Tree Physiol. 15:57--64. Stutz, H.P. and J.B. Mitton. 1988. Variation in Engelmann spruce
associated with variation in soil moisture. Arct. Alp. Res. 20:461--465.
Sutton, B.C.S., D.J. Flanagan and Y.A. El-Kassaby. 1991a. A simple and rapid method for estimating representation of species in spruce seedlots using chloroplast DNA restriction fragment. Silvae Genet. 38:119--123.
Sutton, B.C.S., D.J. Flanagan, J.R. Gawley, C.H. Newton, D.T. Lester and Y.A. El-Kassaby. 1991b. Inheritance of chloroplast and mito-chondrial DNA in Picea and composition of hybrids from introgres-sion zones. Theor. Appl. Genet. 82:242--248.
Sutton, B.C.S., S.C. Pritchard, J.R. Gawley, C.H. Newton and G.K. Kiss. 1994. Analysis of Sitka spruce-interior spruce introgression in British Columbia using cytoplasmic and nuclear DNA probes. Can. J. For. Res. 24:278--285.
Tan, W. and T.J. Blake. 1993. Drought tolerance, abscisic acid and electrolyte leakage in faster- and slower-growing black spruce (Picea mariana) progenies. Physiol. Plant. 89:817--823.
Tan, W., T.J. Blake and T.J.B. Boyle. 1992. Drought tolerance in faster-and slower-growing black spruce (Picea mariana) progenies: I. Stomatal and gas exchange responses to osmotic stress. Physiol. Plant. 85:639--644.
Teskey, R.O., J.A. Fites, L.J. Samuelson and B.C. Bongarten. 1986. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol. 2:131--142.
Villar, R., A.A. Hel and J. Merino. 1994. Comparison of methods to estimate dark respiration in the light in leaves of two woody species. Plant Physiol. 105:167--72.
Willis, J.H., J.A. Coyne and M. Kirkpatrick. 1991. Can one predict the evolution of quantitative characters without genetics? Evolution 45:441--444.