Ethylene feedback mechanisms in tomato and strawberry
fruit tissues in relation to fruit ripening and climacteric
patterns
Mordy A. Atta-Aly *, Jeffrey K. Brecht, Donald J. Huber
Horticultural Sciences Department,Uni6ersity of Florida,Gaines6ille,FL32611-0690,USA
Received 16 August 1999; accepted 18 May 2000
Abstract
Exposing pericarp tissue excised from immature tomato fruit to 4.5 mmol l−1 C2H4 revealed a negative C2H4
feedback mechanism in relation to its biosynthesis since ACC concentration and C2H4production by the tissue were
reduced. An opposite trend (positive C2H4feedback mechanism) was observed in pericarp tissue excised from fruit at
the pink stage. At the mature-green stage however, tissue showed a transition from negative to positive C2H4feedback
mechanism with the onset of tissue ripening. In strawberry tissues excised from green, white and half-coloured fruits however, C2H4application caused a short-term increase in C2H4 production followed by a sharp reduction to the
control level along with a marked reduction in ACC levels. In both tomato and strawberry fruit tissues, C2H4
application significantly induced ACC oxidase (ACO) activity at all ripening stages, as measured by in vivo ACC conversion to C2H4. This strongly suggests that ACC synthesis is the limiting step in C2H4autocatalysis and the only
limiting step in C2H4autoinhibition. In tomato pericarp tissues, C2H4 autoinhibition and autocatalysis caused by
C2H4application in immature and pink fruits, respectively, were eliminated when tissues were transferred to air and
re-occurred when tissues were returned back to C2H4. These responses did not occur in all strawberry tissues due to
the sharp reduction in C2H4production with the time course of C2H4application. Inhibiting C2H4action with STS
pretreatment inhibited both negative and positive C2H4feedback mechanisms in both tomato and strawberry tissues
indicating that C2H4feedback mechanism is one sort of C2H4action. In addition, only tomato fruit tissue showed
significant increases in CO2 production with C2H4 application. In contrast to the nonclimacteric behaviour of
strawberry fruit which exhibits only a negative C2H4 feedback mechanism, these data strongly suggest that the
transition of the C2H4feedback mechanism from negative to positive, which occurs in tomato fruit only with ripening
initiation and progress, may be the reason behind the climacteric behaviour of tomato fruit. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Ethylene feedback mechanism; Climacteric behaviour; Tomato fruit; Strawberry fruit
www.elsevier.com/locate/postharvbio
* Corresponding author. Present and permanent address: Department of Horticulture, Faculty of Agriculture, Ain Shams University, P.O. Box 68, Hadayek Shoubra 11241, Cairo, Egypt. Tel.: +20-2-4447317; fax: +20-2-4444460.
1. Introduction
The rate of C2H4 production varies with the
type of plant tissue and its stage of development. In climacteric fruits, C2H4is produced at different
rates based on fruit stage of growth. Such fruit is characterized by a low rate of C2H4 production
during the preclimacteric or unripe stage (basal C2H4), followed by the climacteric, a sudden
in-crease in C2H4production during fruit ripening, a
phenomenon referred to as autocatalytic C2H4
(Abeles, 1973). After the climacteric rise, C2H4
production significantly declines during the post-climacteric phase (Hoffman and Yang, 1980). Nonclimacteric fruits, on the other hand, exhibit no increase in C2H4 production during
matura-tion and ripening (Knee et al., 1977).
Autocatalytic C2H4 production is a common
feature of ripening in climacteric fruit, in which increased synthesis of C2H4is triggered by
exoge-nous C2H4 application (Burg and Burg, 1965;
Abeles, 1973). Several reports however, have demonstrated autoinhibition of C2H4 production.
McMurchie et al. (1972) reported that C2H4
treat-ment inhibited C2H4 production of banana pulp
slices. Similarly, propylene treatment, which ini-tiated ripening, suppressed C2H4 production in
intact green bananas. In the non-ripening stages of sycamore fig, C2H4 acts as an autoinhibitor of
its own production, but this does not occur in the ripening stages (Zeroni et al., 1976). C2H4
autoin-hibition was also noticed in avocado fruit (Za-uberman and Fuchs, 1973), immature tomato locule gel tissue (Atta-Aly et al., 2000) and pea segments (Saltveit and Dilley, 1978).
It has been suggested that C2H4 autocatalysis
involves increased synthesis of ACC synthase and the enzyme responsible for the conversion of ACC to C2H4 (Riov and Yang 1982; Atta-Aly et al.,
2000), whereas autoinhibition involves suppres-sion of the activity of either both enzymes (Riov and Yang, 1982) or only ACC synthase (Atta-Aly et al., 2000). Ethylene, therefore, seems to play a role in regulating its own production (Yang and Hoffman, 1984). Studies involving treatment with exogenous ethylene or propylene have indicated that fruit response to C2H4 may also serve to
distinguish between climacteric and nonclimac-teric fruits (McMurchie et al., 1972). The response of harvested fruit to applied C2H4 depends on
various factors, including tissue sensitivity and stage of maturation, as well as whether or not the fruit is climacteric (Biale and Young, 1981).
The objectives of this work, therefore, were to study C2H4 feedback mechanisms (autocatalysis
and autoinhibition) in tomato and strawberry fruits at different developmental stages; to deter-mine the step(s) in C2H4 biosynthesis which
con-trol C2H4 feedback mechanism; to examine the
relation between C2H4 feedback mechanism and
the behaviour of both tomato and strawberry, climacteric and nonclimacteric fruit, respectively; to determine the most suitable stage to induce fruit ripening with exogenous C2H4 application.
2. Material and methods
2.1. Plant material
Full size tomato (Lycopersicon esculentumMill cv. Sunny.) fruits were harvested from a commer-cial field in south Florida at immature (IM), mature-green (MG) and pink (P) stages. Blossom-end dark and light-green colours were used to distinguish between IM and MG stages, respec-tively, since the former has no jelly-like locular materials in any fruit locules while only one or two locules of the latter developed jelly-like mate-rials. Strawberry (Fragaria X ananassa) fruits cv. Chandler, were picked from Gainesville area, FL, at full size green (G), white (W) and half-coloured (HC) stages. Tomato and strawberry fruits were transferred to the laboratory on the same day. Fruits were washed with chlorinated water (3.4 mM NaOCl), sorted, regarding to size and devel-opmental stage, and kept at 15°C and 95% RH overnight for treatment preparation. Experiments were repeated three times using tomato and straw-berry fruits from the same sources.
2.2. Tissue sampling
the equator of the fruit using a stainless steel cork borer at IM, MG and P stages (one disk/fruit). Disks were trimmed to remove excess jelly-like locular materials which had developed only in MG and P fruits. The presence of jelly-like mate-rials was used to distinguish between MG and IM tomato fruit. Directly after excision, disks were placed epidermal surface down, inside glass tubes (17 ml vol.; 2 cm diam.; one disk/tube).
Strawberry flesh cylinders were longitudinally excised from fruit central flesh using the cork borer after removing 0.5 cm from blossom and stem ends to obtain achene-free fleshy cylinders. This was done to exclude the effect of auxins on C2H4 biosynthesis since it is known that the
ach-enes are the main source of auxins in strawberry fruit (Archbold and Dennis, 1985). Excised tissue cylinders were then placed vertically inside the tubes, which contained 3-mm glass beads at the bottom of each tube to protect the tissue base from anaerobic conditions. Both fruit tissues were then distributed among chemical solution treat-ments and exposed thereafter to C2H4 using a
gas-flow system as described below.
2.3. Chemical treatments and tissue analysis
For each treatment, 100ml of each solution was
applied to the locular surface of tomato disks or to the vascular tissue of the strawberry flesh cylin-ders. With the exception of ACC, which was applied 3 days after continuous C2H4exposure to
eliminate wound C2H4 interaction, all solutions
were applied immediately after excision. Chemical solutions were applied to both tomato and straw-berry tissues as described below.
2.3.1. Control treatments
These tissues were divided into three groups. The first group was used to measure initial C2H4
and CO2 production, immediately after excision,
with an incubation period of 30 min. This incuba-tion period was enough for measuring basal C2H4
levels and less than that required for wound C2H4
to be initiated (Atta-Aly, 1992). The second and the third groups, however, were continuously ex-posed to an air flow94.5 mmol l−1 C2H4 for 5
days, either for monitoring C2H4 and CO2
pro-duction 3, 4 and 5 days after excision or for ACC analysis 4 days after excision. Plant tissue pro-duces a large amount of wound C2H4 which
di-minishes within 72 h of excision (Atta-Aly et al., 1987). The gas flow system removed wound C2H4
produced during the duration of the experiment and the first C2H4analysis, therefore, was carried
out after 3 days of excision.
2.3.2. In 6i6o estimation of ACC oxidase (ACO) acti6ity
Tissues were treated with water or 0.5 mM AVG directly after excision and then exposed to C2H4for 3 days, when water or 100mM ACC was
added to the tissues 2 h before measuring C2H4
production as an indicator of ACO activity.
2.3.3. C2H4 action
This was achieved in two different ways as follows:
1. Tissues were treated with water and then di-vided into two groups. The first group was exposed to air for 3 days, then transferred to the 4.5mmol l−1 C2H4 atmosphere for 1 day,
then returned to air for another day, while the second group was exposed to the above atmo-spheres in the opposite order. C2H4 and CO2
production were analyzed at the time of each atmosphere transfer.
2. STS (silver thiosulfate; 0.5 mM) was applied to the tissues while water was the control treatment. After 3 days of gas treatments, C2H4 and CO2 produced by the tissues were
analyzed.
2.4. Ethylene treatments
Based on the highest respiratory levels of ex-cised tomato and strawberry fruit tissues, mea-sured 1 day ahead, an air flow system was calculated and adjusted to a rate of 3.5 l h−1
for supplying normal O2levels around the tissue. CO2
levels in the air flow were checked twice per day and its concentration was always below 0.5% throughout the experiment. The air flow94.5
mmol l−1 (100 ml l−l) C2H4 was passed through
Excised tissues were placed inside the 17-ml volume glass tubes, chemically treated and then divided into two groups for either air or C2H4
treatment. Each group was placed inside 10-l gas-flow containers. The containers were kept at 20°C and 95% RH throughout the experiment. Time between tissue excision and gas exposure for each treatment was less than 30 min.
Since applied C2H4 may emanate during tissue
incubation and interfere with the measurement of endogenous levels, 100 g of tomato and straw-berry fruit tissues, excised at each developmental stage, were exposed to the air flow94.5mmol l−1
C2H4 for 3 days, thoroughly flushed with C2H4
-free air for 60 s and exposed to the vacuum procedure described by Saltveit (1982) for measur-ing internal C2H4 concentrations. No significant
differences were found between air and C2H4
-treated tissues in internal C2H4 levels at each
developmental stage of both fruits. All tissues, therefore, were thoroughly flushed for 60 s with C2H4-free air prior to each C2H4 analysis.
In a separate experiment, tissue was exposed to 580 mmol l−1 propylene gas instead of 4.5 mmol
l−1
ethylene. C2H4 production by both tomato
and strawberry fruit tissues was similar to that obtained with C2H4 application when the tissue
was flushed with C2H4-free air for 60 s before
incubation.
2.5. C2H4, CO2 and ACC analysis
At each sampling time, the tubes were removed, thoroughly flushed with C2H4-free air for 60 s,
and then sealed with rubber stoppers. After 30 min of incubation at 20°C, 1-ml gas samples were withdrawn and used for C2H4 and CO2
measure-ments. A Hewlett Packard gas chromatograph Model 5080A with FID was used for C2H4
analy-sis, while a Gow Mac Model 60, with TCD (Gow Mac Instrument Co., NJ) was used for CO2
mea-surements. After withdrawing the gas samples the tubes holding tissues were unsealed and returned to the gas flow containers.
For ACC analysis, fruit tissues were removed after 4 days of continuous exposure, frozen in liquid nitrogen and kept at −20°C. Two grams of the frozen tissues were homogenized in 10 ml
0.2 mM trichloroacetic acid (TCA) (Atta-Aly et al., 1987). The mixture was centrifuged at 1000×
g for 10 min and the supernatant decanted. Aliquots were assayed for ACC with a modified version of the procedure used by Lizada and Yang (1979).
2.6. Experimental design and statistical analysis
Experiments were designed as factorial arrange-ments in completely randomized designs with five replicates each consisting of 15 samples. Experi-ments were repeated three times and data were subjected to combined analysis. Means were ana-lyzed for statistically significant differences using the LSD test at the 5% level (Little and Hills, 1978).
3. Results and discussion
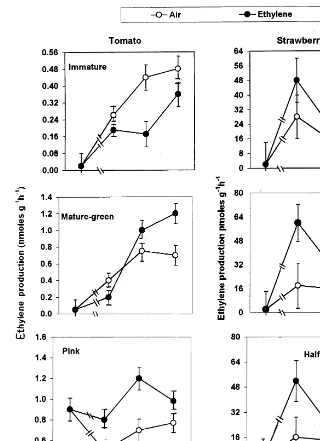
Immature tomato fruit tissue showed a pattern of C2H4 autoinhibition (negative C2H4 feedback
mechanism) since C2H4production by such tissue
was strongly inhibited upon exposure to exoge-nously applied C2H4 (Fig. l). An opposite trend
(C2H4 autocatalysis or positive feedback
mecha-nism) however, was observed when tomato fruit tissue at the pink stage was used (Fig. l). With the first visual sign of red colour which occurred in MG tissue 4 days after exogenous C2H4exposure,
a transition phase from C2H4 autoinhibition to
autocatalysis was detected (Fig. 1). All develop-mental stages of strawberry fruit tissues, on the other hand, showed a short-term increase in C2H4
production upon exogenous C2H4application
fol-lowed by a dramatic reduction to that of control levels after 4 days of continuous C2H4application
(Fig. 1). Since 5.8 mmol l−1 propylene has the
same impact on fruit ripening as 0.045 mmol l−1
C2H4(McMurchie et al., 1972), separate tests with
tomato and strawberry tissues were also carried out using propylene rather than C2H4. Results
showed similar levels of C2H4 production in both
treatments.
Since ACC formation and its conversion to C2H4 are the two main limiting steps in C2H4
Fig. 1. Effect of exogenous ethylene treatment on ethylene production by tomato and strawberry fruit tissues at different developmental stages. Vertical bars superimposed on datapoints at each sampling date represent the L.S.D. values at the 5% level.
1984) and also in C2H4 feedback mechanism
(Nakatsuka et al., 1998; Atta-Aly et al., 2000), both ACC concentration and in vivo ACO activ-ity were determined in both fruit tissues at all developmental stages. While ACO activity
signifi-cantly increased in both fruit tissues at all devel-opmental stages with exogenous C2H4 treatment
de-Table 1
ACC oxidase activity (C2H4nmol g−1h−1) in tomato pericarp and strawberry flesh tissues exposed to exogenous C2H4treatmenta
C2H4exposure
Treatment Fruit developmental stages
Tomato Strawberry
Immature Mature green Pink Green White Half coloured
0.04b 0.02b
AVG Air 0.04a 0.18a 0.31a 0.01a 0.01a
0.01a
C2H4 0.03a 0.13a 0.39a 0.01a 0.01a
0.38b 0.14b
ACC Air 2.15b 1.88b 2.07b 0.20b
0.90a
AVG+ACC 0.36b 0.10b 0.10b
0.21a
aFruit tissues were treated with either H2O or 0.5 mM AVG immediately after excision and then exposed to either air or 4.5
mmol
l−1C
2H4for 3 days with H2O or 100mM ACC application 2 h before C2H4measurements. Values within each treatment for each fruit developmental stage followed by the same letter are not statistically different at the 5% level.
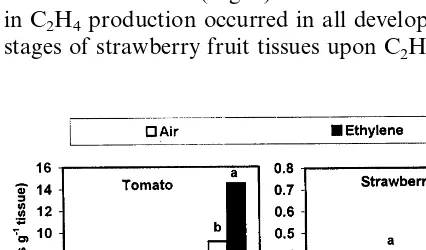
veloped, but decreased in immature tomato fruit tissue and in all developmental stages of straw-berry fruit (Fig. 2). Regardless of C2H4
applica-tion, ACC concentration increased the maturation of both fruit tissues (Fig. 2). A short-term increase in C2H4production occurred in all developmental
stages of strawberry fruit tissues upon C2H4
treat-ment, due mainly to induced ACO activity. This increase was diminished thereafter when ACC concentration became limiting. The reduction in ACC concentration which occurred in strawberry fruit tissues (Fig. 2) may be due not only to the high level of its consumption during the first 3 days as a result of induced ACO activity (Table 1), but also to the reduction in tissue ACC synthe-sis, since after the first 3 days, C2H4-treated tissue
contained lower ACC concentrations but pro-duced C2H4 levels at a rate equal to that of
air-treated ones (Figs. 1 and 2). These data sug-gest that both ACC and its conversion to C2H4
are limiting steps in C2H4 autocatalysis, while
only ACC is the controlling step in C2H4
auto-inhibition.
Riov and Yang (1982) suggested that autoinhi-bition of C2H4production in wounded citrus peel
tissue is attributable to the suppression of ACC formation due to the inhibition of ACC synthase formation and activity. Since the in vivo activity of the ACO enzyme relies on the available level of ACC in the tissue (Yang, 1980), it could be sug-gested that in the long term, ACC synthesis is the limiting step in C2H4 feedback mechanism.
Thus the climacteric behaviour of tomato fruit Fig. 2. Effect of exogenous ethylene treatment for 4 days on
during ripening initiation and development may be due mainly to the presence of C2H4 catalysis,
while the absence of this mechanism and the
presence of C2H4 autoinhibition are the reasons
for the nonclimacteric behaviour occurring in strawberry fruit and during the nonripening
stages of tomato fruit. Zeroni et al. (1976) reported that C2H4 acts as an autoinhibitor of its own
production in the immature stages of sycamore fig but not during ripening. Studies involving treat-ment of fruit with exogenous ethylene or propylene indicated that fruit response to C2H4 may also
serve to distinguish between climacteric and non-climacteric fruit (McMurchie et al., 1972). The response of harvested fruit to applied C2H4
de-pends on various features, including tissue sensitiv-ity and stage of maturation, as well as whether or not the fruit is climacteric (Biale and Young, 1981). These data suggest that exogenous C2H4
applica-tion to climacteric fruit should not be applied until fruit become mature to obtain acceptable ripening uniformity and quality, since positive C2H4
feed-back mechanism does not occur at earlier stages. To test the impact of C2H4feedback mechanism
on C2H4 production in relation to fruit
develop-mental stage and its climacteric pattern, both tomato and strawberry fruit tissues at three specific developmental stages were transferred between air and C2H4atmospheres. This was carried out in two
opposite sequences: air – C2H4– air, or C2H4– air –
C2H4, starting with 3 days in the first atmosphere
to eliminate wound C2H4 followed by one
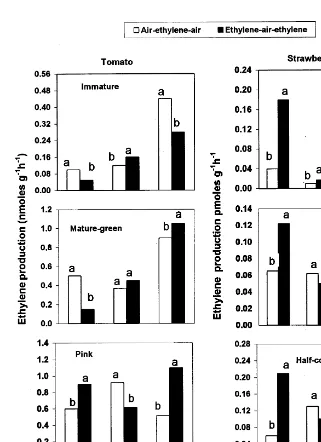
addi-tional day for each subsequent atmosphere change. During the immature stage of tomato fruit devel-opment, C2H4 production was significantly
re-duced when tissue was exposed to C2H4 or
transferred from air to C2H4 in comparison with
that exposed to air or transferred from C2H4to air
(Fig. 3). As maturation progressed, exposure to exogenous C2H4 significantly induced C2H4
pro-duction. This induction was eliminated upon trans-ferring the tissue to air and re-occurred when tissue was returned back to C2H4 (Fig. 3). The same
pattern of response was also obtained using tomato locule gel tissue (Atta-Aly et al., 2000).
In strawberry fruit tissues however, C2H4
pro-duction strongly increased during the 3rd day of exposure to exogenous C2H4compared with those
exposed to air. After those first 3 days however, the impact on C2H4 production of transferring tissues
between air and C2H4atmospheres was diminished
(Fig. 3). Zauberman and Fuchs (1973) reported that C2H4 feedback mechanisms may last after
removing the tissue from an C2H4 atmosphere.
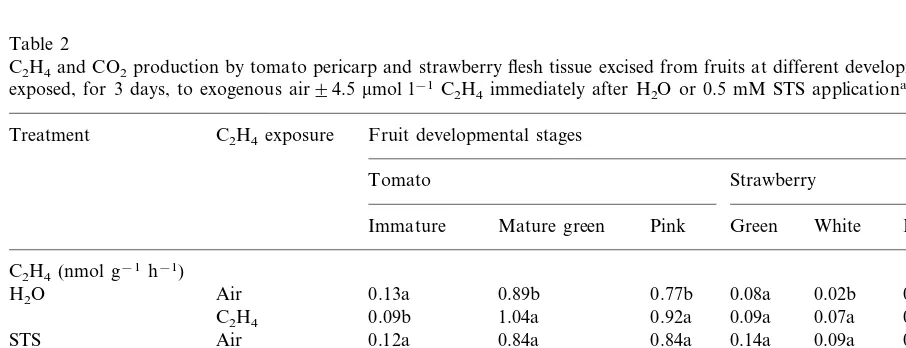
Table 2
C2H4and CO2production by tomato pericarp and strawberry flesh tissue excised from fruits at different developmental stages and exposed, for 3 days, to exogenous air94.5mmol l−1C2H4immediately after H2O or 0.5 mM STS applicationa
STS Air 0.12a 0.84a 0.84a 0.05a
0.04a
C2H4 0.87a 1.52a 1.55a 1.81a
1.57a 1.95a
STS Air 0.86a 0.91a 1.01a 1.91a
1.88a 1.64a
C2H4 0.80a 0.89a 1.04a 1.74a
To test that a C2H4 feedback mechanism is
dependent on C2H4 sensitivity, STS was used to
inhibit C2H4 action before exposing both fruit
tissues either to air or C2H4 atmosphere. Data
presented in Table 2 show C2H4autoinhibition in
immature tomato tissue but C2H4autocatalysis in
mature-green and pink tomato as well as in white and half-coloured strawberry fruit tissues after the
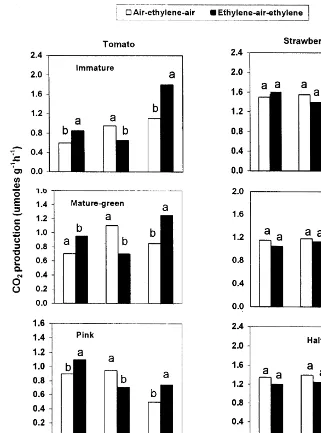
Fig. 5. Effect of transferring tomato and strawberry fruit tissues, excised at different developmental stages from air to ethylene and back to air [air – ethylene – air; starting with 3 days in air (A) to 1 day in ethylene (B) and back to air for another day (C)] or to an opposite sequence of exposure [ethylene – air – ethylene; starting with 3 days in ethylene (D) to 1 day in air (E) and back to ethylene for another day (F)] on the levels of CO2 production at each atmosphere change. Significant differences are presented for each sampling date at the 5% level.
3 days of continuous C2H4 exposure. Both C2H4
autoinhibition and autocatalysis were diminished when STS was used. Riov and Yang (1982) re-ported that silver ion blocked the autocatalytic
effect of C2H4. Inhibiting C2H4 action in the
Lallu, 1994; Tian et al., 1997a), while opposite results were obtained in the nonclimacteric strawberry fruit (Tian et al., 1997b). It was also evident that C2H4 autoinhibition is shifted to
C2H4 autocatalysis in tomato fruit as ripening is
initiated and progresses by stimulating both ACC synthase and ACO (Nakatsuka et al., 1998; Atta-Aly et al., 2000). Data presented in this work, therefore, indicate that inhibiting C2H4 action using silver ion blocks both C2H4
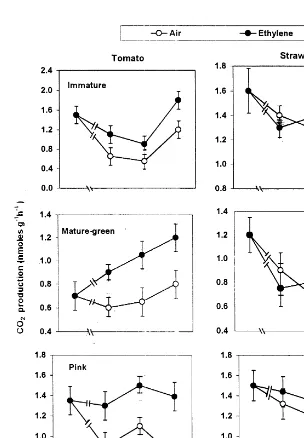
positive and negative feedback mechanisms. Since the pattern of fruit respiration during ripening initiation and development is another strong feature to distinguish between climacteric and nonclimacteric fruit behaviour, CO2
produc-tion therefore, was determined in both fruit tis-sues at three developmental stages during continuous exposure to either air or C2H4
atmo-sphere. Exogenous C2H4 application induced
CO2 production by tomato fruit tissue while it
had no effect on strawberry as ripening pro-gressed (Fig. 4). Inhibiting C2H4 action with
DACP application reduced tomato fruit respira-tion (Sisler and Lallu, 1994; Tian et al., 1997a), while no effect was found in strawberry (Tian et al., 1997b). When both fruit tissues were trans-ferred between air and C2H4 atmospheres, CO2
production by tomato significantly increased in the exogenous C2H4 atmosphere. This increase
diminished upon transfer to air and re-occurred when tissues were returned to the C2H4
atmo-sphere. In strawberry however, none of these differences occurred (Fig. 5). The stimulation of tomato fruit respiration caused by exogenous C2H4 application did not occur when C2H4
ac-tion and subsequently its feedback mechanism was blocked by STS application (Table 2). This means that during tomato fruit ripening there was a positive correlation between a positive C2H4 feedback mechanism and fruit climacteric
respiration. This correlation was absent in the nonclimacteric strawberry fruit.
In conclusion, it is suggested that a negative C2H4 feedback mechanism may be the reason
for the nonclimacteric behaviour of strawberry fruit and immature tomato fruit, while a posi-tive C2H4 feedback mechanism is the reason for
tomato fruit climacteric behaviour during
ripen-ing initiation and development. ACC formation is possibly the limiting step for either positive or negative C2H4 feedback mechanisms, since
ex-ogenous C2H4 treatment induced ACO activity
regardless of fruit species and physiological age.
References
Abeles, F.B., 1973. Ethylene in Plant Biology. Academic Press, New York, p. 302.
Archbold, D.D., Dennis, F.G., 1985. Strawberry receptacle growth and endogenous IAA content as affected by growth regulator application and achene removal. J. Am. Soc. Hort. Sci. 110, 816 – 820.
Atta-Aly, M.A., 1992. Ethylene production by different tomato fruit tissues at different ripening stages. Egypt. J. Hort. 19, 137 – 147.
Atta-Aly, M.A., Saltveit, M.E., Hobson, G.E., 1987. Effect of silver ions on ethylene biosynthesis by tomato fruit tissue. Plant Physiol. 83, 44 – 48.
Atta-Aly, M.A., Brecht, J.K., Huber, D.J., 2000. Ripening of tomato locule gel tissue in response to ethylene, Posthar-vest Biol. Technol. 19 (3), 239 – 244.
Biale, J.B., Young, R.E., 1981. Respiration and ripening in fruits retrospect and prospect. In: Friend, J., Rhodes, M.J. (Eds.), Recent Advances in the Biochemistry of Fruits and Vegetables. Academic Press, New York, pp. 1 – 40. Burg, S.P., Burg, E.A., 1965. Ethylene action and the ripening
of fruit. Science 148, 1190 – 1196.
Hoffman, N.E., Yang, S.F., 1980. Changes inL -aminocyclo-propane-L-carboxylic acid content in ripening fruits in relation to their ethylene production rates. J. Am. Soc. Hort. Sci. 105, 492 – 495.
Knee, M., Sargent, J.A., Osborne, D.J., 1977. Cell wall metabolism in developing strawberry fruit. J. Exp. Bot. 28, 377 – 396.
Little, T.M., Hills, F.J., 1978. Agricultural Experimentation. Wiley, New York, pp. 31 – 52.
Lizada, C., Yang, S.F., 1979. A simple and sensitive assay for
L-amino cyclopropane-L-carboxylic acid. Anal. Biochem. 100, 140 – 145.
McMurchie, E.J., McGlasson, W.B., Eaks, I.L., 1972. Treat-ment of fruit with propylene gives information about the biogenesis of ethylene. Nature (Lond.) 235, 237.
Nakatsuka, A., Murachi, S., Okunishi, H., Shiomi, S., Nakano, R., Kubo, Y., Inaba, A., 1998. Differential ex-pression and internal feedback regulation of 1-aminocyclo-propane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 118, 1295 – 1305.
Riov, J., Yang, S.F., 1982. Autoinhibition of ethylene produc-tion in citrus peel disks: suppression of L
-aminocyclo-propane-L-carboxylic acid synthesis. Plant Physiol. 69, 687 – 690.
Saltveit, M.E., 1982. Procedures for extracting and analyzing internal gas samples from plant tissue by gas chro-matograph. HortScience 17, 878 – 881.
Saltveit, M.E., Dilley, D.R., 1978. Rapidly induced wound ethylene from excised segments of etiolatedPisum sati6um
L. cv Alaska. II. Oxygen and temperature dependency. Plant Physiol. 61, 675 – 679.
Sisler, E.C., Lallu, N., 1994. Effect of diazocyclopentadiene (DACP) on tomato fruits harvested at different ripening stages. Postharvest Biol. Technol. 4, 245 – 254.
Tian, M.S., Bowen, J.H., Bauchot, A.D., Gong, Y.P., Lallu, N., 1997a. Recovery of ethylene biosynthesis in diazocyclopen-tadiene (DACP)-treated tomato fruit. Plant Growth Regul. 22, 73 – 78.
Tian, M.S., Gong, Y.P., Bauchot, A.D., 1997b. Ethylene
biosynthesis and respiration in strawberry fruit treated with diazocyclopentadiene and IAA. Plant Growth Regul. 23, 195 – 200.
Yang, S.F., 1980. Regulation of ethylene biosynthesis. HortScience 15, 238 – 243.
Yang, S.F., Hoffman, N.E., 1984. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35, 155 – 189.
Zauberman, G., Fuchs, Y., 1973. Ripening processes in avocado stored in ethylene atmosphere in cold storage. J. Am. Soc. Hort. Sci. 98, 477 – 480.
Zeroni, M., Galil, I., Ben-Yehoshua, S., 1976. Autoinhibition of ethylene formation in nonripening stages of the fruit of sycamore fig (Ficus sycomorusL.). Plant Physiol. 57, 647 – 650.