Research report
Observational learning in C57BL/6j mice
Pascal Carlier
a
,
∗
, Marc Jamon
b
,
1
aEquipe CNRS G´enomique Fonctionnelle Comportements et Pathologies, CNRS—GFCP/P3M/Aix-Marseille Universit´es, 31 Chemin Joseph Aiguier, 13402 Marseille Cedex 20, France
bEquipe CNRS G´enomique Fonctionnelle Comportements et Pathologies, CNRS—GFCP/P3M/Aix-Marseille Universit´es, 31 Chemin Joseph Aiguier, 13402 Marseille Cedex 20, France
Received 8 June 2006; received in revised form 11 July 2006; accepted 13 July 2006 Available online 30 August 2006
Abstract
The ability of mice to solve a complex task by observational learning was investigated with C57BL/6j mice. Four female demonstrators
were trained to reliably perform a sequence that consisted in pushing a piece of food into a tube attached to the side of a puzzle box, and
recovering it by opening a drawer in front of the box. They then performed this sequence in front of naive mice assigned to individual cubicles
in a box with a wire mesh front arranged in a row facing the demonstrators. A total of 25 naive mice (13 males and 12 females) were used.
Fifteen mice observed 14 demonstrations a day for 5 days; 10 control mice were placed in similar cubicles, but behind a plastic screen which
prevented them from observing the demonstrators. The mice were post-tested in the demonstrator situation, and 6 of 15 observers immediately
reproduced the complete task successfully, but none of the naive or control mice were able to solve the task. The observers and controls were
then subjected to a five level individual learning schedule. Observers learned the individual task significantly faster than the controls. No sex
difference was found. These results suggest that observational learning processes at work were based on stimulus enhancement and observational
conditioning.
© 2006 Elsevier B.V. All rights reserved.
Keywords: Mouse; Social learning; Observational learning; Stimulus enhancement; Observational conditioning
1. Introduction
Social animals can adapt their behaviour to environmental
requirements by transmitting individual experience. This “social
transmission” is the by-product of a compromise between
exploratory drives and the avoidance of negative experiences
[4]
and helps improve individual fitness. Famous field observations
of primates and birds
[32]
opened new paths that shed light on
aspects of social transmission in animal behaviour. Gradually,
the contribution of social transmission of behaviour, both within
and between generations, has been identified and/or
demon-strated
[2–4,16,18,26,27,29,54,62,64,68]
.
Social transmission can be defined as a cultural system of
information transfer
[17]
that affects the individual phenotype,
in the sense that part of the phenotype is acquired from other
∗Corresponding author. Tel.: +33 4 91 16 45 89; fax: +33 4 91 77 50 84.
E-mail addresses:[email protected](P. Carlier),
[email protected](M. Jamon).
1 Tel.: +33 4 91 16 43 37; fax: +33 4 91 77 50 84.
individuals. Social transmission can either be a merely social
influence (individual B is influenced by individual A but does
not learn any part of mimicry from A)
[64]
, or can be genuine
social learning, when B learns some part of mimicry from A
[4,17,64]
. Social transmission can be based on various clues,
but visual clues require special attention as social transmission
related to visual observation of peers involves different
lev-els of different cognitive skills. When visually related social
transmission (i.e. “observational social transmission”) occurs,
the processes at work rely on observational social influence
and observational learning, respectively
[64]
. Contrary to social
influence genuine observational learning does not require that
the model be present to work
[27]
. Observational learning has
mainly been documented in vertebrates, with experimental
stud-ies on primates
[5,20,45,54,60,62,63]
, cats
[9,25,33,70]
, and
birds
[6,8,15,21,24,31,38,39,47,70]
, and also in invertebrates:
cephalopods
[19]
, insects (e.g. bumblebees
[66]
, crickets
[12]
.
It has been extensively studied in the rat; see, for example
[13,14,28,30,35,36,40,41,46,48,49,51,65,69]
, while mice were
less studied than rats. Social transmission based on olfactory
clues has been demonstrated consistently in mice tested for
learned food preferences, both in adults (preference for a food
after an interaction with a recently fed conspecific
[57]
) and
with mother and pup interaction
[44,59]
; the effect of the age
of the demonstrator has also been tested
[10]
. Observational
social transmission, based on visual observation of peers, has
been investigated, testing mice with tasks that consisted in
open-ing baited puzzle boxes
[42,43,55,56,58]
, experiments where
the observers could interact freely with the demonstrator. In
these situations, the visual component of social transmission
was combined with other senses, involving olfactory and tactile
skills, making it impossible to assess the exact contribution of
observational clues to performance. These experiments,
there-fore, could not provide a clear-cut demonstration of the type of
observational transmission at work. One such study
[56]
listed
several mechanisms influencing the social context to explain the
results: social exposure
[64]
, a case of social influence (e.g. B,
placed together with A, is exposed to the same environment and
learns to respond to the environment in a way that matches A’s
response), stimulus enhancement
[22,27,52,64]
which is a case
of social learning
[17,62,64]
(e.g. B learns from A to what object
to orient its behaviour), and trial-and-error learning, explaining
the performance of mice observing the activities of a
demon-strator. In one experiment
[11]
, observers and demonstrators
had limited contact, being separated by a wire mesh. The task
consisted in opening a swinging pendulum door, having to push
either on the right or the left to get a reward. The observers
per-formed significantly better than the controls that were behind
a visual barrier and could not see the demonstrator. The study
showed there was observational learning ability in mice, but the
task was simple, requiring only one action to be conducted in a
single location.
We are proposing a more detailed analysis of the mechanisms
involved in observational learning, using a new paradigm based
on conditional sequences of actions in specific locations. To
break down the complexity of the problem, we defined a linear
task: action A in location
␣
, followed by action B in location

. The spatial dissociation of the two actions was designed to
produce a clearer understanding of what was being learnt, i.e.
actions and/or locations where the actions occurred, so as to
separate the actions and locations, and determine whether the
animal could repeat a complete sequence, a sequence in order
(in time and/or space), or had simply focused on certain locations
of the actions (e.g. the first or last). The experimental design did
not allow for any close interaction between demonstrators and
observers, to ensure that the information related to the task itself
was obtained visually.
This preliminary study showed that there was a significant
improvement in the performance of observer mice after
observ-ing the behaviour of demonstrators. Some of the observers
successfully reproduced the task. For all the observers, the
obser-vation of demonstrators enhanced the subsequent learning in
a progressive learning program. These results are discussed
in relation to the cognitive skills of rodents, focusing on the
prospects of this new paradigm for investigating social
cogni-tion in mice.
2. Materials and methods
2.1. Animals
C57BL/6j mice were used for the study because they are not visually impaired and are proficient for both observational learning [11] and spa-tial learning[1,34,37,50,61]. Thirty-one C57BL/6j mice, from Charles River France, were housed in same-sex groups, in standard cages (30 cm long×12 cm high×18 cm wide), and in a room at a constant temperature of 21◦C, with
30–40% relative humidity, and a 12/12 h light–dark cycle. Food pellets and water were availablead libitum. Before the experiment, the mice were divided in two groups:
• Six females, aged 20 weeks, were used as demonstrators. Females were selected because they are less absent-minded than males tend to be in the presence of females[11].
• Twenty-five mice (13 males and 12 females) aged between 6 and 12 weeks were used as learners. The 25 learners were randomly allocated 15 observers (8 males and 7 females) and 10 controls (5 males and 5 females).
From the beginning of the experimental sessions, the observers and controls were housed in single cages 30 cm long×12 cm high×18 cm wide.
2.2. Set-up
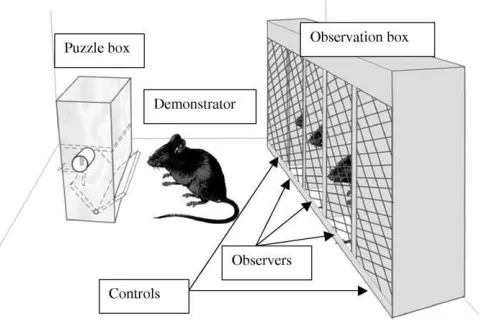
The set up consisted in a puzzle box and an observation box (Fig. 1). The puzzle box was a translucent Plexiglas parallelepiped (7.5 cm wideY(Y10Ycm highY(Y4Ycm deep) with an open tube (inside diameter 0.7 cm) extending 0.5 cm and positioned 6 cm from the bottom, plus a drawer made of two pieces of Plexiglas (6.5 cm wide×2.5 cm high) glued together at right angles to form a drawer, positioned in front at the bottom, and attached so that it could be tipped over towards the front (Fig. 1). An electric remote control was used by the experimenter to lock and unlock the drawer. A small movable metal tab in the tube prevented the mice from pulling out the food (a piece of dry cookie) that was placed inside it. To get the food, the mouse first had to go to the side of the box and push the metal tab with a front paw; this made the food fall into the drawer. The mice then had to go to the front of the device and open the drawer to recover the food.
The observation box (outside dimensions: 28 cm wide×15 cm high×6 cm deep) had a wire mesh front, and was divided into five individual cubicles (inside dimensions: 5 cm wide×5 cm deep×15 cm high) (Fig. 1). The par-titions between the cubicles and the back wall were made of white Plexiglas so that the observers had no visual contact with each other. The front wall, 26 cm wide×13 cm high, was covered with wire mesh and the observers had a clear view of the set-up. The two end cubicles, one on the left and one on the
right, were covered with white opaque Plexiglas for the controls. The puzzle box and observation box were placed in a Plexiglas arena (36 cm long×30 cm wide×15 cm high) opposite each other at a distance of 15 cm. The mice were monitored throughout the experiment via a video camera (Ikegami ICD 47 E) attached to a metal bracket above the arena. The experiments were videotaped for additional analysis.
2.3. Experimental procedure
2.3.1. Shaping demonstrators
The demonstrators were trained over 4 weeks, 3 days a week, following a weekly schedule. Day 1: food deprived (18 h); days 2–4: training (the mice were fed 3 g per day to maintain 85–90% of their normal body weight); days 5–7: rest (free access to usual diet). The shaping schedule had five graded levels (based on a previous study[7]).
• Level 1. The mouse has to get the food placed on the outside edge of the tube protruding on the left side of the puzzle box; i.e. “one action—one location.”
• Level 2. The mouse has to get the food placed further inside the tube, but still outside the metal tab; “one action—one location.”
• Level 3. The mouse has to get the food from inside the tube; the food is on the inside of the metal tab which is unlocked in the open position; “one action—one location.”
• Level 4. The mouse has to push the food into the drawer by pushing the metal tab inside the tube. If the mouse succeeds in doing this, the experimenter immediately opens the drawer and the mouse can go to the front and retrieve the food inside the open drawer; “one action—two locations.”
• Level 5. The mouse has to push the food into the drawer by pushing the metal tab inside the tube. If the mouse succeeds, the experimenter immediately unlocks the drawer. The mouse has to go to the front side and to tip up the drawer to get the food; “two actions—two locations.”
The daily shaping sessions lasted 30 min per mouse. The mouse performed the following trial as soon as the previous one had been successfully completed (consumption of food) or, if unsuccessful (after 5 min). A minimum of two consecutive trials had to be successful at each level before moving to the next level. After two failures at a given level, the mouse was taken back to the previous level and had to succeed at least once before moving on again to the next level. If the mouse also failed the previous level, it was taken back to the one before, and so on. The demonstrators had to be able to carry out the task without any error or hesitation. Each level could therefore be started over again until it was carried out perfectly in the training sessions. At level 5 the mice had to perform perfect sequences with a short latency period (<1 s) between the two actions. The two least efficient mice were discarded, and the remaining four were trained for a further 6 weeks until they were perfectly fluent in their movements when carrying out the task. Once the four demonstrators selected had reached the required learning level, and before the demonstration phase, they were trained to perform the task over three consecutive days in front of five naive observers (not used in the demonstration phase) so as to reduce any disturbance caused by carrying out the task “in public”.
2.3.2. Subject learning
2.3.2.1. Familiarisation. Before the experimental session, the observer and control mice were familiarized with the experimental device with a 30-min session with groups of cage mates (2–4 mice) so as to prevent neophobia. Pieces of cookie were placed around and inside the device to make it easier to explore the set-up.
2.3.2.2. Observation sessions. During the observation sessions, demonstrators, observers and controls were fed 3 g per day to maintain 85–90% of their normal body weight. The mice were divided into five teams, each with three observers and two controls; two teams were all male, and two were all female, and one mixed team had three males and two females. Individual mice were randomly assigned to one of the wire-fronted cubicles opposite the device. The two controls were placed in the end cubicles, one on the right and the other on the left. After the subjects settled in, a demonstrator was placed in the arena and left for 2 min to interact with the observers and controls behind the wire mesh. The two control
cubicles were then covered with white opaque Plexiglas. The demonstration phase began when the experimenter placed the puzzle box in the arena. Observers and controls had social contact with the demonstrators, but only the observers could see the sequences performed by the demonstrators. Each team observed the demonstrators’ performance for a total of 70 display sessions, scheduled as 14 daily sessions, with a 2-min inter-session pause, over five consecutive days. Two teams of two demonstrators were used, with two alternating each day, which meant that every observer saw all four demonstrators perform the task.
2.3.2.3. Test sessions.
Pre-test session. Before the observation phase, each subject (observer and control) was individually presented with the experimental task for a 10-min period (device closed and piece of cookie inside the tube) to check that none could solve the task by chance.
Post-test session. After the observation phase, each subject was individually presented with the same task for a 10-min period. When a mouse solved the task, its allocated time was extended to 20 min or three consecutive successes, to check that the performance was repeatable and did not occur by chance. Mice that passed the task at least once were tested again the next day to confirm the performance.
2.3.2.4. Individual learning. Twenty-four hours after the post-test session, every subject had to complete the individual learning programme, working through the five training levels described above. The subjects had to get the piece of cookie twice at levels 1–4, and pass level 5 three times to validate their learning achievement. The best learning performance therefore took only 11 trials. Each trial ran for a maximum of 5 min, with a 2-min inter-trial break. The maximum number of trials per day was 11. The maximum number of sessions was 4.
2.4. Statistical analyses
Four dependent variables were analysed:
• Observational learning phase (pre- and post-test sessions):
◦ Latencies to explore the tube and the drawer. Exploration was defined as contact by a paw or the muzzle.
◦ Number of subjects able to perform the task.
• Individual learning phase:
◦ Number of subjects passing level 1 on the first trial.
◦ Number of trials needed to validate a given level.
Given the sample size and the differences in mice’s behavioural profiles, non-parametric tests were chosen. However, it is worth noting that parametric tests provided the same results.
For observational learning, Fischer exact probability tests were conducted to compare the results of subjects that successfully learned by observation with the results of subjects that failed. Latencies were compared using the Wilcoxon test for paired data (e.g. pre-test versus post-test) and with Mann–Whitney test for independent data (e.g. status and sex).
For individual learning, the non-parametric Fischer exact probability test compared the number of observer and control mice passing level 1 on the first trial. Non-parametric ANOVA Kruskal–Wallis analysis on observers versus con-trols and males versus females compared the pooled number of trials required to solve a given level. APvalue of <0.05 was considered significant. All the analyses were performed with STATISTICA (data analysis software, Version 7.1. StatSoft France 2005).
3. Results
3.1. Observational learning phase
Fig. 2. Observers vs. controls for pre-test vs. post test latencies to explore the drawer and to explore the tube Males and females were pooled.lat. draw.
pret.;lat. draw. post.;△lat. tube pret.;lat. tube post. Medians±centiles
(whiskers) 25–75% are indicated. Wilcoxon tests were carried out to compare drawer and tube latencies at pre-test vs. post-test both for observers (n = 15) and controls (n = 10); significant comparisons (p < 0.05) are indicated.
0.014, respectively,
n
= 15, Wilcoxon test,
Fig. 2
). In control
mice, the latency to explore the drawer and tube did not differ
significantly between pre- and post-test (
P
= 0.798 and 0.959,
respectively,
n
= 10, Wilcoxon test,
Fig. 2
). While the six mice
that successfully performed the post-test may account for some
of the difference, it should be noted that the observers’
laten-cies in pre-test versus post-test were still significantly different
after the six observers that passed the post-test were factored out
(
P
= 0.050 and 0.038, respectively,
n
= 9, Wilcoxon test).
Whatever the status of the subjects (observers and
con-trols) and the test session (pre- and post-test), the latency to
explore the tube was always significantly longer than the latency
to explore the drawer (pre-test: observers
P
= 0.0006,
n
= 15,
controls
P
= 0.005,
n
= 10, Wilcoxon test; post-test: observers
P
= 0.0006,
n
= 15, controls
P
= 0.005,
n
= 10). For all subjects,
the Mann–Whitney tests did not reveal any significant
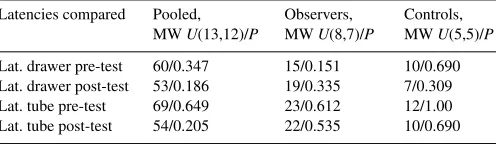
differ-ence between males and females for latencies in either the
pre-or post-test (
Table 1
).
3.2. Individual learning phase
All 15 observers successfully completed level 1 the first trial
(getting the food from the outside edge of the tube on the left
side of the device) (
P
= 0.0047, Fischer exact probability test),
Table 1
Males vs. females comparisons for the latencies to explore the tube or the drawer: Mann–Whitney tests are used to compare the latencies
Latencies compared Pooled, MWU(13,12)/P
Observers, MWU(8,7)/P
Controls, MWU(5,5)/P
Lat. drawer pre-test 60/0.347 15/0.151 10/0.690 Lat. drawer post-test 53/0.186 19/0.335 7/0.309
Lat. tube pre-test 69/0.649 23/0.612 12/1.00
Lat. tube post-test 54/0.205 22/0.535 10/0.690
Fig. 3. Observers vs. controls pooled trials number required to pass each learn-ing level Males and females were pooled.L1;L2;△L3;•L4;L5
Medians±centiles (whiskers) 25–75% are indicated. Table 2
Observers vs. controls—comparisons for each learning level: the groups were compared with a non-parametric Kruskall–Wallis ANOVA on pooled trials
Levels KWH(1,25) P
L1 8.8933 0.0029**
L2 10.8951 0.0010**
L3 5.8385 0.0157*
L4 4.1563 0.0415*
L5 5.4077 0.0200*
* P<0.05 ** P<0.01
but only 5 of the 10 controls succeeded in level 1 on the first
trial. The analysis of the performance at the successive levels
showed that controls needed more trials than observers to learn
the task (
Fig. 3
). The Kruskall–Wallis ANOVA performed on
pooled trials showed significant differences between observers
and controls for all levels (
Table 2
): the observers needed less
trials than the controls to learn the task.
When the statistical analysis factored out the six males that
passed the post-test, the difference between observers and
con-trols for the number of trials required to learn the task was
only significant for levels 1 and 2 (
H
9,10= 5.658,
P
= 0.017 and
H
9,10= 8.945,
P
= 0.002, respectively)
No significant male–female difference was detected for any
status or level.
4. Discussion
The results presented in this paper provide evidence that mice
can reproduce a complex task by observational learning only,
i.e. with no physical contact with the demonstrator during the
demonstration of the task. This shows that visual clues can
sup-port the social transmission, as was seen in earlier study
[11]
study, it may be that the males were more attentive to the female
demonstrators than the female observers were
[11]
. Other
eli-gible causes such as males higher level of activity or different
spatial skills should be accurately investigated in a further study.
The comparison of performance achievements shown by
demonstrators, successful observers, unsuccessful observers,
and controls provides information on cognitive processes
under-lying learning in mice. The reference performance was by
demonstrators (but their learning procedure may be
qualita-tively different). The four demonstrators over-learned the task,
and accurately performed the sequence (1) tube, (2) drawer, and
seem to have learned the order for the actions. We would
sug-gest a more parsimonious hypothesis, i.e. they learnt an overall
movement going from the tube to the drawer and requiring
two specific actions. While successful observers reproduced
the task correctly, they did not find the fastest way of
obtain-ing the food, which would have been true imitation
[27,53]
or
goal emulation
[54,64]
, and which would have led the mice to
explore the tube first (in an imitation process, B learns part of a
given behaviour pattern from A
[17,23,27,64,67]
; in goal
emu-lation, B learns the goal to pursue from A
[54,64]
. However, the
observers had longer latencies to explore the tube and shorter
latencies to explore the drawer. It could be suggested that the
observers explored the drawer first because they had observed
the demonstrator consuming the food there, but the drawer
pref-erence was also found with all observers and controls, both
pre-and post-test. The geometrical properties of the drawer (larger,
lower, and more accessible than the tube) probably induce
spon-taneous preferential attraction, but the observers did establish
the link between the reinforcement and both the tube and the
drawer.
Even the observers that did not successfully perform the task
test explored the drawer and tube more quickly in the
post-test period compared to the pre-post-test period. No such difference
was found with the controls. In addition, the observers needed
fewer trials than controls for the individual learning of the task
in stages. Social influence can be ruled out as an explanation for
this social transmission, as the post-test was deferred. A process
of social learning based on stimulus enhancement
[22,52]
(the
observers found the drawer and tube more attractive because
they observed the demonstrators carrying out actions there) is
a more probable explanation for the fact that observers focused
on the relevant parts of the puzzle-box more than controls, even
though all were unable to mimic the spatial association between
them. All observers, regardless of whether they passed the
post-test, leant on the tube on the left at the very first trial (level 1),
but only half of the controls did so. This means that the tube
was a relevant stimulus for all observers. In the individual
learn-ing phase, however, the observers that did not pass the post-test
did not differ from controls from level 3 on, yet pre- and
post-test differences in latencies to explore the tube and/or drawer
were still significant. This suggests that the observers unable
to pass the post-test learned the two relevant locations by
stim-ulus enhancement, but failed to associate them in a temporal
sequence such as “drawer-tube-drawer” or “tube-drawer”). An
observational conditioning process is needed to establish this
relationship
[62,64]
.
The individual learning phase highlights the different social
learning processes involved in the observation phase. Initially,
the mice learned to focus on the tube, instead of the drawer
which was the most spontaneously attractive element, and did
this through a stimulus enhancement process. This tallies with
the observations of the simple task performed in the previous
study and shows observational learning
[11]
. Observers learned
the two relevant locations by stimulus enhancement, regardless
of whether they eventually passed the post-test. From level 4 on,
it was difficult to grasp the food and pull it out of the tube, so
the task was more efficiently solved by pushing the food into
the drawer. This stage was solved more quickly by the observers
that passed the post-test, as they had learned a sequence such as
“drawer-tube-drawer” requiring a more complex observational
conditioning process.
In conclusion, this experiment showed that mice were able to
learn by observing conspecifics carry out a complex task
involv-ing two different actions performed in two parts of a puzzle-box.
The social learning process involved stimulus enhancement and
observational conditioning. It will be interesting to investigate
this further by studying a larger sample and analysing the
modi-fications in learner strategies in relation to the training provided.
This will be done with more complex tasks, in a sequence of three
actions-three locations and behavioural responses. The order for
the actions to be reproduced, as related to the level of
train-ing, will be used to assess the cognitive processes involved in
observational learning. Looking beyond investigations of
cog-nitive processes in mice, the new protocol could be used to
design discriminatory tests to evaluate the cognitive impairment
in genetically modified mice used as models of human mental
disorders such as Rett or Down syndromes, schizophrenia, or
Alzheimer diseases.
References
[1] Ammassari-Teule M, Hoffmann HJ, Rossi-Arnaud C. Learning in inbred mice: strain-specific abilities across three radial maze problems. Behav Genet 1993;23:405–12.
[2] Avital E, Jablonka E. Animal traditions: behavioral inheritances in evolu-tion. Cambridge: Cambridge University Press; 2000, 446 pp.
[3] Bonner JT. The evolution of culture in animals. Princeton: Princeton Uni-versity Press; 1980, 232 pp.
[4] Boyd R, Richerson PJ. Culture and the evolutionary process. Chicago: University of Chicago Press; 1985, 340 pp.
[5] Bugnyar T, Huber L. Push or pull: an experimental study on imitation in marmosets. Anim Behav 1997;58:817–31.
[6] Campbell FM, Heyes CM, Goldsmith AR. Stimulus learning and response learning by observation in the European starling, in a two-object/two-action test. Anim Behav 1999;58:151–8.
[7] Carlier P, Lefebvre L. Differences in individual learning between group-foraging and territorial Zenaida doves. Behaviour 1996;133:1197–207. [8] Carlier P, Lefebvre L. Ecological differences in social learning
between adjacent, mixing populations of Zenaida doves. Ethology 1997;103:772–84.
[9] Chesler P. Maternal influence in learning by observation in kittens. Science 1969;166:901–3.
[10] Choleris E, Guo C, Liu H, Mainardi M, Valsecchi P. The effect of demon-strator age and number on duration of socially-induced food preferences in house mouse (Mus domesticus). Behav Process 1997;41:69–77. [11] Collins RL. Observational learning of a left-right behavioral asymmetry in
[12] Coolen I, Dangles O, Casas J. Social learning in noncolonial insects? Curr Biol 2005;15:1931–5.
[13] Del Russo JE. Observational learning of discriminative avoidance in hooded rats. Anim Learn Behav 1975;3:76–80.
[14] Denny MR, Clos CF, Bell RC. Learning in the rat of a choice response by observation of S–S contingencies. In: Zentall TR, Galef BG, editors. Social learning: psychological and biological perspectives. Hillsdale, NJ: Lawrence Erlbaum; 1988. p. 207–23.
[15] Dolman C, Templeton J, Lefebvre L. Mode of foraging competi-tion is related to tutor preference in Zenaida aurita. J Comp Psychol 1996;110:45–54.
[16] Dugatkin LA. The imitation factor: evolution beyond the gene. New York: Free Press; 2000, 256 pp.
[17] Dugatkin LA. Principles of animal behavior. New York, London: W.W. Norton & Company; 2004, 596 pp.
[18] Dunbar R, Knight C, Power C. The evolution of culture: an interdisciplanary view. New Brunswick, NJ: Rutgers University Press; 1999, 272 pp. [19] Fiorito G, Scotto P. Observational learning inOctopus vulgaris. Science
1992;256:545–6.
[20] Fragaszy DM, Visalberghi E. Social influences on the acquisition of tool-using behaviors in tufted capuchin monkeys (Cebus apella). J Comp Psy-chol 1989;103:159–70.
[21] Fritz J, Kotrschal K. Social learning in common ravens,Corvus corax. Anim Behav 1999;57:785–93.
[22] Galef BG. Imitation in animals: history, definition and interpretation of data from the psychological laboratory. In: Zentall TR, Galef BG, editors. Social learning: psychological and biological perspectives. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 3–28.
[23] Galef BG. Recent progress in studies of imitation and social learning in ani-mals. In: Saborin M, Craik F, Robert M, editors. Advances in psychological science. Hove: Psychology Press; 1998. p. 275–300.
[24] Galef BJJ, Manzig LA, Field RM. Imitation learning in budgerigars: Daw-son and Foss (1965) revisited. Behav Process 1986;13:191–202. [25] Herbert MJ, Harsh CM. Observational learning by cats. J Comp Psychol
1944;37:81–95.
[26] Heyes CM. Imitation, culture and cognition. Anim Behav 1993;46: 999–1010.
[27] Heyes CM. Social learning in animals: categories and mechanisms. Biol Rev Camb Philos Soc 1994;69:207–31.
[28] Heyes CM, Dawson GR. A demonstration of observational learning in rats using a bidirectional control procedure. Q J Exp Psychol 1990;42B:59–71. [29] Heyes CM, Galef BGJ. Social learning in animals: the roots of culture.
London: Academic Press; 1996, 411 pp.
[30] Heyes CM, Jaldow E, Dawson GR. Imitation in rats: conditions of occur-rence in a bidirectional control procedure. Learn Motiv 1994;25:276–87. [31] Heyes CM, Saggerson A. Testing for imitative and nonimitative social
learning budgerigar using a two-object/two action test. Anim Behav 2002;64:851–9.
[32] Hinde R, Fischer J. Further observations on the opening of milk bottles by birds. Br Birds 1951;44:393–6.
[33] John ER, Chesler P, Bartlett F, Victor I. Observational learning in cats. Science 1968;159:1489–91.
[34] Klapdor K, van der Staay FJ. The Morris water-escape task in mice: strain differences and effects of intramaze contrast and brightness. Physiol Behav 1996;60:1247–54.
[35] Kohn B. Observation and discrimination learning in the rats: effects of stimulus substitution. Learn Motiv 1976;7:303–12.
[36] Kohn B, Dennis M. Observation and discrimination learning in the rat: specific and nonspecific effects. J Comp Physiol Psychol 1972;78: 292–6.
[37] Koopmans G, Blokland A, Nieuwenhuijzen PV, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav 2003;79:683–93.
[38] Lefebvre L, Palameta B, Hatch KK. Is group-living associated with social learning? A comparative test of a gregarious and a territorial columbid. Behaviour 1996;133:1–21.
[39] Lefebvre L, Templeton J, Brown K, Koelle M. Carib grackles imitate con-specific and Zenaida dove tutors. Behaviour 1997;134:1003–17.
[40] Levine JM, Zentall TR. Effect of conspecific’s presence on deprived rats performance: social facilitation vs. distraction/imitation. Anim Learn Behav 1974;2:119–22.
[41] Lore R, Blanc A, Suedfeld P. Empathic learning of a passive avoid-ance response in domesticatedRattus norvegicus. Anim Behav 1971;19: 112–4.
[42] Mainardi D, Mainardi M. Culture and genetics in the house mouse. In: Zentall TR, Galef BG, editors. Social learning psychological and biolog-ical perspectives. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 239–52.
[43] Mainardi D, Mainardi M, Pasquali A. Genetic and experiential features in a case of cultural transmission in the house mouse (Mus musculus). Ethology 1986;72:191–8.
[44] Mainardi M, Poli P, Valsecchi P. Ontogeny of dietary selection in wean-ing mice: effects of early experience and mother’s milk. Biol Behav 1989;14:185–94.
[45] Mineka S, Cook M. Social learning and the acquisition of snake fear in monkey. In: Zentall TR, Galef BG, editors. Social learning: psychological and biological perpectives. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 51–73.
[46] Oldfield-Box H. Comments on two preliminary studies of “observation” learning in the rat. J Genet Psychol 1970;116:45–51.
[47] Palameta B, Lefebvre L. The social transmission of a food-feeding tech-nique in pigeons: what is learned? Anim Behav 1985;33:892–6. [48] Powell RW, Burns R. Visual factors in observational learning with rats.
Psychon Sci 1970;21:47–8.
[49] Powell RW, Saunders D, Thompson W. Shaping, autoshaping, and obser-vational learning with rats. Psychon Sci 1969;13:167–8.
[50] Roullet P, Lassalle JM. Radial maze learning using exclusively distant visual cues reveals learners and nonlearners among inbred mouse strains. Physiol Behav 1995;58:1189–95.
[51] Sanavio E, Savardi U. Observational learning of a discriminative shuttlebox avoidance by rats. Psychol Rep 1980;44:1151–4.
[52] Spence KW. The differential response in animals to stimuli varying within a single dimension. Psychol Rev 1937;44:430–44.
[53] Thorpe WH. Learning and instinct in animals. Cambridge, MA: Harvard University Press; 1963, 558 pp.
[54] Tomasello M, Kruger AC, Ratner HH. Cultural learning. Behav Brain Sci 1993;16:495–552.
[55] Valsecchi P, Bosellini I, Mainardi D, Mainardi M. Further analysis of a case of social learning in the house mouse (Mus domesticus). Ethol Ecol Evol 1993;5:121–5.
[56] Valsecchi P, Bosellini I, Sabatini F, Mainardi M, Fiorito G. Behavioral analysis of social effects on the problem-solving ability in the house mouse. Ethology 2002;108:1115–34.
[57] Valsecchi P, Galef BG. Social influences on the food preferences of house mice (Mus musculus). J Comp Psychol 1989;2:245–56.
[58] Valsecchi P, Mainardi M, Mainardi D, Bosellini I. On the role of the demon-strator for the solution of a problem in the house mouse. Ethol Ecol Evol 1989;1:213–6.
[59] Valsecchi P, Moles A, Mainardi M. Does mother’s diet affect food selection of weanling wild mice. Anim Behav 1993;46:827–8.
[60] Visalberghi E, Fragaszy DM. Do monkeys ape? In: Parker ST, Gibson KR, editors. Language and intelligence in monkeys and apes Compara-tive developmental perspecCompara-tives. Cambridge: Cambridge University Press; 1990. p. 247–73.
[61] Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav 2001;72:271–81.
[62] Whiten A. Primate culture and social learning. Cogn Sci 2000;24:477– 508.
[63] Whiten A, Custance DM. Studies of imitation in chimpanzees and children. In: Heyes CM, Galef BGJ, editors. Social learning in animals: the roots of culture. London: Academic Press; 1996. p. 291–318.
[65] Will B, Pallaud B, Soczka M, Manikowski S. Imitation of lever pressing “strategies” during the operant conditoning of albino rats. Anim Behav 1974;22:664–71.
[66] Worden BD, Papaj DJ. Flower choice copying in bumblebees. Biol Lett 2005;1:504–7.
[67] Zentall TR, Akins C. Imitation in animals: evidence, function, and mecha-nisms. In: Cook RG, editor. Avian visual cognition. Comparative Cognition Press; 2001., http://www.pigeon.psy.tufts.edu/avc/zentall/.
[68] Zentall TR, Galef BG. Social learning: psychological and biologi-cal perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988, 368 pp.
[69] Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science 1972;178:1220–1.